Abstract
Transcallosal inhibitory interactions between proximal representations in the primary motor cortex remain poorly understood. In this study, we used transcranial magnetic stimulation to examine the ipsilateral silent period (iSP; a measure of transcallosal inhibition) in the biceps and triceps brachii during unilateral and bilateral isometric voluntary contractions. Healthy volunteers performed 10% of maximal isometric voluntary elbow flexion or extension with one arm while the contralateral arm remained at rest or performed 30% of maximal isometric voluntary elbow flexion or extension. The iSP was measured in the arm performing 10% contractions, and electromyographic (EMG) recordings were comparable across conditions. The iSP onset and duration in the biceps and triceps brachii were comparable. In both muscles, the iSP depth and area were increased during bilateral contractions of homologous agonist muscles (extension-extension and flexion-flexion) compared with a unilateral contraction, whereas during bilateral contractions of nonhomologous antagonist muscles (extension-flexion and flexion-extension), the iSP depth and area were decreased compared with a unilateral contraction, and sometimes facilitation of EMG was seen. This effect was never observed during bilateral activation of homologous muscles. The size of responses evoked by cervicomedullary electrical stimulation in the arm that made 10% contractions remained unchanged across conditions. Thus transcallosal inhibition targeting triceps and biceps brachii is upregulated by voluntary contraction of the contralateral agonist muscle and downregulated by voluntary contraction of the contralateral antagonist muscle. We speculate that these reciprocal task-dependent interactions between bilateral flexor and extensor arm regions of the motor cortex may contribute to coupling between the arms during motor behavior.
Keywords: primary motor cortex, interhemispheric inhibition, transcallosal pathways, transcranial magnetic stimulation, force
early studies in monkeys showed an uneven distribution of callosal connections between primary motor cortices and suggested that distal forelimb representation lacked direct connections between homologous areas (Pandya and Vignolo 1971). Later studies identified that direct callosal connections were present for some of the multiple representations of the distal forelimb but suggested that connections between trunk and shoulder areas were more numerous, as were transcallosal connections between supplementary motor areas (Gould et al. 1986; Jenny 1979; Rouiller et al. 1994). Thus primary motor cortex devoted to proximal limb movements has interhemispheric callosal connections that are at least as strong as the area devoted to hand movements, but their functional role remains poorly understood.
In humans, transcranial magnetic stimulation (TMS) has been used to demonstrate transcallosal facilitation and, more commonly, inhibition between areas in the motor cortex (Bäumer et al. 2006; Ferbert et al. 1992). Most studies have concentrated on distal upper limb muscles, whereas less attention has been given to interhemispheric interactions between proximal upper limb muscles. Conditioning TMS over the motor cortex of one hemisphere can reduce the size of a test motor evoked potential (MEP) elicited from the other hemisphere after shorter (10 ms) or longer (40 ms) intervals. This suppression is known as interhemispheric inhibition (Ferbert et al. 1992). It has been reported that the biceps brachii have interhemispheric inhibition similar to or slightly less than that of an intrinsic hand muscle (Ferbert et al. 1992; Harris-Love et al. 2007), whereas that for triceps brachii is appreciably reduced. Single suprathreshold TMS can also suppress ongoing voluntary electromyographic (EMG) activity to give an ipsilateral silent period (iSP), another measure of interhemispheric inhibition (Ferbert et al. 1992). The iSP may be more difficult to elicit without a preceding facilitatory response in biceps brachii compared with hand or wrist muscles (Ferbert et al. 1992; Zjidewind et al. 2006), whereas reliable inhibition of triceps brachii is reported with strong ipsilateral stimulation during moderate contraction (Ziemann et al. 1999). It is not known how interhemispheric interactions between proximal muscles are influenced by voluntary contraction. However, studies that have compared forces produced in bilateral and unilateral contractions allow some predictions. Force is reduced during bilateral contractions for the elbow flexors and elbow extensors (Ohtsuki 1983; Seki and Ohtsuki 1990). Reductions occur with maximal or submaximal efforts and appear to be as great as or greater than the deficits seen with bilateral handgrip or thumb adduction (Herbert and Gandevia 1996; Seki and Ohtsuki 1990). In contrast, when elbow flexion of one arm is combined with elbow extension of the other arm, force is not reduced compared with the unilateral efforts (Ohtsuki 1983). Furthermore, when asymmetric forces are produced, the weaker force is affected more than the stronger. Thus voluntary contraction of either the elbow flexors or extensors might be expected to increase interhemispheric inhibition to the homologous muscles of the other arm. However, this increase would not be expected with contraction of flexors of one arm and extensors of the other.
To examine interhemispheric inhibition during bilateral contractions of proximal arm muscles, we elicited iSPs in the elbow extensor and flexor muscles during voluntary contractions of the homologous and antagonist muscles of the other arm. We demonstrate that bilateral activation of antagonist proximal muscles results in disinhibition or facilitation at the cortical level, whereas in contrast, bilateral activation of homologous proximal muscles results in pronounced inhibition.
METHODS
Subjects.
Twenty-two healthy volunteers (10 women, 12 men; 3 left handed) with an average age of 28.0 ± 7.6 yr participated in the study. All subjects gave their informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. Subjects were preselected from a total of 45 subjects who were screened to ensure that they showed the iSP in the biceps and/or triceps brachii during unilateral voluntary contraction; this allowed us to measures changes in the iSP magnitude during our different experimental conditions. Fifteen of the 22 subjects showed a visible iSP in the triceps during unilateral elbow extension, and 15 subjects showed a visible iSP in the biceps during unilateral elbow flexion. Eight individuals showed the iSP in both muscles tested. Fifteen subjects, who were known to show iSPs in biceps or triceps, participated in control studies. Eight had participated in the main study reported here.
Experimental sessions.
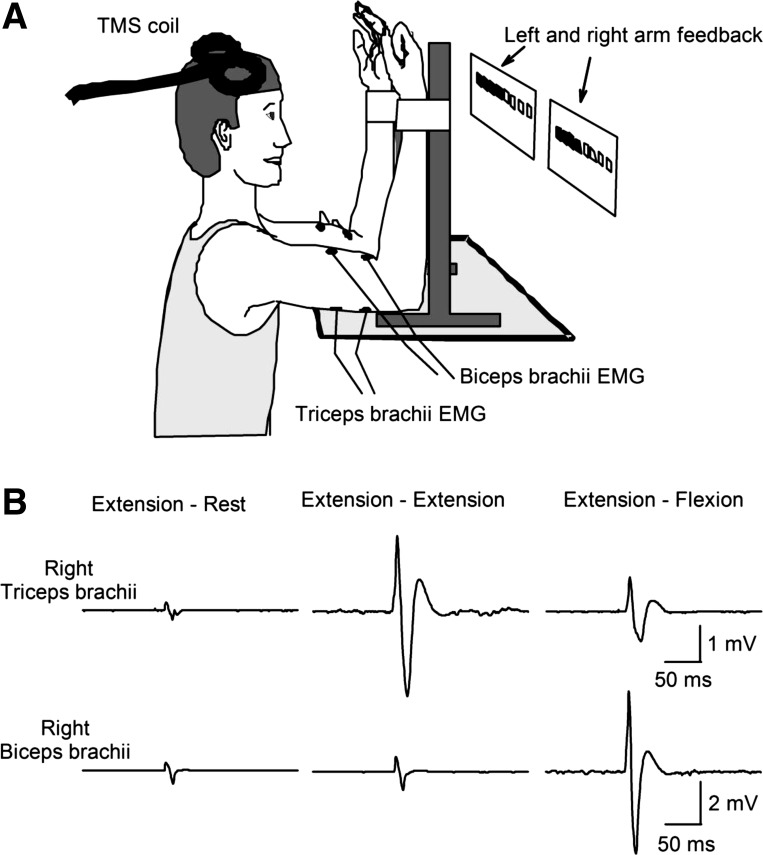
Subjects were seated with both shoulders and elbows flexed at 90° (Fig. 1A). Each arm was held in an isometric myograph with a strap at the wrist. At the start of the experiment, all subjects performed two to three brief maximal voluntary contractions (MVCs; 3–5 s) of the elbow flexors or extensors with each arm separately and with 30 s of rest between contractions. The maximal forces were used to set targets for subsequent submaximal contractions. During maximal contractions, subjects were verbally encouraged to perform maximally, and visual feedback was provided (Gandevia 2001). Subjects participated in two randomized testing sessions where we assessed the onset, duration, depth, and area of the iSP in the biceps and triceps brachii during unilateral and bilateral elbow flexion and extension isometric voluntary contractions. The iSP was always assessed in the nondominant arm, since previous evidence has suggested that the magnitude of interhemispheric inhibition is stronger from the dominant to the nondominant primary motor cortex (Bäumer et al. 2007; Netz et al. 1995). In one session (Fig. 1B, elbow extension session) subjects performed 10% of MVC into elbow extension with the nondominant arm while the contralateral arm remained at rest (extension-rest) or performed 30% of MVC into elbow extension (extension-extension) or flexion (extension-flexion). In the other session (elbow flexion session) subjects performed 10% of MVC into elbow flexion with the nondominant arm while the contralateral arm remained at rest (flexion-rest) or performed 30% of MVC into elbow flexion (flexion-flexion) or extension (flexion-extension). In each session subjects performed 10 sets of 3 contractions. A set consisted of 10- to 15-s contractions of each of the three conditions separated by 10 s of rest. During each contraction, TMS was delivered three times at 3.5- to 4-s intervals over the dominant hemisphere to give a total of 30 trials of each condition. Custom software was written to acquire signals from a load cell to display visual feedback corresponding to 10% and 30% of MVC into elbow flexion and extension forces with the nondominant and dominant arm in real time, respectively (LabView, San Jose, CA). Subjects were asked to move one (unilateral) or two (bilateral) independently controlled cursors on a computer monitor to a target line displaying the force to be exerted. During elbow flexion, the visual feedback was provided toward the right side of the screen, and during elbow extension, the visual feedback was provided toward the left side of the screen. Therefore, during bilateral activation of homologous muscles, the visual feedback was presented toward the same direction (in parallel), whereas during bilateral activation of nonhomologous muscles, the visual feedback was presented in opposite directions. In an additional control experiment (n = 8 for elbow extension and n = 8 for elbow flexion), visual feedback for the nondominant arm was provided as described above, whereas feedback for the dominant arm was provided toward both the right and left side of the screen in each condition in separate blocks of 30 trials in a pseudorandomized order. Additional verbal feedback was provided to the subjects to ensure that both arms performed the correct task at all times.
Fig. 1.
A: schematic illustration of the experimental setup. Note that feedback of elbow flexion and extension force for the left and right arm (indicated by arrows) was presented on a display in front of the subject. During elbow flexion the visual feedback was provided toward the right side of the screen, and during elbow extension the visual feedback was provided toward the left side of the screen. TMS, transcranial magnetic stimulation. B: electromyography (EMG) of the right biceps and triceps brachii, which were contralateral to the stimulated motor cortex in the elbow extension session. Traces show the average unrectified EMG data in 30 trials. In the left column, recordings are shown during 10% maximal extension on the left (not shown) while the right arm remained at rest (extension-rest). In the middle column, recordings are shown during 10% maximal extension on the left (not shown) and 30% maximal extension with the right arm (extension-extension). In the right column, recordings are shown during 10% maximal extension on the left (not shown) and 30% maximal flexion with the right arm (extension-flexion).
EMG recordings.
EMG was recorded from biceps and triceps brachii by surface electrodes secured to the skin over the belly of each muscle (Ag-AgCl, 20-mm diameter). The signals were amplified, filtered (16–1,000 Hz), and collected at 2 kHz for off-line analysis using customized software (CED 1401 with Signal software; Cambridge Electronic Design, Cambridge, UK). Forces exerted at the elbow were measured bilaterally by two load cells (Honeywell; range ±111.2 N, voltage ±5 V, high-sensitivity transducer 0.045 V/N). Force was sampled at 200 Hz and stored on a computer for off-line analysis.
Transcranial magnetic stimulation.
A figure-eight coil was oriented with the handle pointing posterolaterally at around 45° to the midline with the induced current in the cortex flowing posterior to anterior across the motor strip. With this coil position the induced current in the brain flowed in an anterior-medial direction and probably produced D and early I wave activation of corticospinal neurons (Di Lazzaro et al. 2004). The hot spot was defined as the optimal position in which TMS (Magstim 200; Magstim, Dyfed, UK) evoked the largest MEP in the dominant biceps or triceps brachii and was determined for each subject using suprathreshold intensity (50 to 100% of maximum stimulator output). The TMS coil was held to the head of the subject by one of the experimenters or by a coil holder while the head was firmly secured to a headrest by straps to limit head movements. The resting motor threshold (RMT) was calculated for each muscle and defined as the lowest intensity of TMS output required to evoke MEPs of at least 50 μV in peak-to-peak amplitude in at least three of five consecutive trials (Rothwell et al. 1999).
Ipsilateral silent period.
The iSP was measured in the biceps and triceps brachii in the nondominant arm while the dominant arm was at rest or performing a flexion or extension muscle contraction of 30% maximum. In each session, the intensity of TMS was adjusted in each subject to produce a visible iSP without a previous facilitation during unilateral elbow flexion or extension. On the basis of previous literature we started the test with intensities 10 or 20% above the RMT. If the iSP was unclear, the stimulus intensity was increased in small steps until the iSP was present without evoking a short-latency facilitation. TMS was applied at the same intensity in all conditions tested in one session. For the 30 trials in each subject and each condition, the EMG from biceps or triceps brachii of the nondominant arm was rectified and averaged about the stimulus. The mean of the ongoing EMG was calculated as the mean rectified EMG for a period of 90 ms before TMS. The iSP was measured following a previously standardized method (Trompetto et al. 2004). This method is similar to the automated method described by Chen et al. (2003). The onset of the iSP was determined by visual inspection and by using a horizontal cursor showing the mean rectified EMG prior to the TMS artifact as a reference. iSP onset was defined as the time point when the EMG dropped below the mean (minimal duration of 10 ms) and the end of the iSP as the time point when the EMG returned through this level. The duration of the silent period was measured as the interval between the onset and offset of the iSP [iSP duration = (time of iSP end) − (time of iSP onset)]. The depth of the iSP was calculated from the mean EMG activity during the iSP, expressed as a percentage of the mean of the prestimulus EMG, and was then subtracted from 100 (Jung and Ziemann 2006). To ensure that changes in the iSP depth were not influenced by variation in the duration of the iSP, the area was also calculated. The area of the iSP was calculated using the following formula: [iSP area = (mean prestimulus EMG) × (iSP duration) − (au_iSP)], where au_iSP is the area under the rectified iSP. The iSP area was then normalized against the level of contraction [iSP area normalized to contraction = iSP area/(area under mean EMG preceding stimulus); Trompetto et al. 2004]. Prestimulus EMG area was calculated by multiplying the prestimulus mean EMG calculated over 90 ms by the mean duration for all subjects of iSPs measured in both muscles and all conditions (22.5 ms). During bilateral extension-flexion and flexion-extension contractions several subjects showed facilitation, and it was not possible to precisely estimate the beginning of the iSP. Therefore, in those subjects the iSP was measured using the same window as previously determined by the onset and offset times obtained in the extension-extension and flexion-flexion conditions. In an additional analysis in all conditions in all subjects, the iSP was measured using the same window as determined by the onset and offset times obtained from the condition with the largest iSP. Analyses based on both individual iSP timing and fixed windows across conditions allow us to ensure that our results were less likely influenced by small changes in the onset and offset times of the iSP. In cases where facilitation was observed, we also examined the onset, height, and duration of the peak. The beginning of the facilitation was defined as 2 SD above the mean rectified EMG calculated from a period of 90 ms before the TMS and the end as the time point when the EMG dropped below the mean. In addition, the peak-to-peak amplitude and latency of the MEPs evoked by TMS in the contralateral biceps and triceps brachii were quantified.
Cervicomedullary MEPs.
Cervicomedullary motor evoked potentials (CMEPs) are not affected by changes in cortical excitability and are sensitive to changes in motoneuronal excitability. To examine CMEPs, the corticospinal tract was stimulated at the cervicomedullary level by a high-voltage electrical current (200-μs duration; Digitimer DS7AH) passed between adhesive Ag-AgCl electrodes fixed to the skin behind the mastoid process (Taylor and Gandevia 2004). CMEPs were measured during the elbow extension (n = 4) and flexion (n = 7) sessions in the triceps and biceps brachii in the nondominant arm while the dominant arm was at rest or performing a flexion or extension muscle contraction of 30% maximum. The stimulation intensity (186.4 ± 22.3 mA for biceps and 198.7 ± 38.8 mA for triceps) was set to elicit a CMEP of around 10% of the maximal M wave (Mmax) for the biceps and triceps muscles during unilateral contractions. Ten to 15 CMEPs were tested during each condition. At the beginning of the testing, the iSP was tested in each muscle using the same methodology described above. Later, CMEPs were elicited in each subject in the middle part of the iSP by giving electrical pulses to the cervicomedullary junction around 25 to 35 ms after the TMS pulse used to elicit the iSP. The following conditions were tested: CMEP alone or preceded by a TMS pulse during unilateral flexion and extension, and CMEP preceded by a TMS pulse during bilateral contractions of agonist and antagonistic muscles.
Data analysis.
Repeated-measures ANOVAs were performed to determine the effect of condition in the elbow extension (extension-rest, extension-extension, extension-flexion) and elbow flexion sessions (flexion-rest, flexion-flexion, flexion-extension) on the onset, duration, depth, and area of the iSP in the biceps and triceps brachii. Repeated-measures ANOVAs were also used to compare prestimulus mean EMG and MEP size in all sessions tested. A two-way ANOVA was used to examine the effect of session (elbow extension vs. elbow flexion) and condition in the depth and area of the iSP and in MEP size. The same analysis was used to determine the effect of the direction of visual feedback (parallel vs. opposite) and condition in the elbow extension and elbow flexion sessions on the depth and area of the iSP in the biceps and triceps brachii. Tukey post hoc analysis was used to test for significant comparisons. Friedman repeated-measures ANOVA on ranks was used to examine the effect of condition in CMEP size. Significance was set at P < 0.05. Paired t-tests were used to compare the iSP onset and duration between conditions. Pearson correlation analysis was used as needed. Group data are presented as means ± SD in the text and as means ± SE in the figures.
RESULTS
Elbow extension session.
Fifteen participants tested in the study showed a visible iSP in the triceps muscle during unilateral elbow extension and therefore participated in the elbow extension session. In these subjects, RMT in the dominant triceps muscle was 62.5 ± 9.9 and the mean intensity of TMS used to examine the iSP was 73.0 ± 10.5% of the maximum stimulator output (range from 56 to 90%) or 117.0 ± 10.2% of the RMT. Mean rectified EMG activity in the nondominant triceps, in which the iSP was measured, was similar before TMS across conditions (F = 2.1, P = 0.13). The mean onset latency and duration of the iSP were similar across conditions (see Table 1).
Table 1.
| 10% MVC Extension of Target Arm iSP in Triceps Brachii |
10% MVC Flexion of Target Arm iSP in Biceps Brachii |
|||||
|---|---|---|---|---|---|---|
| Extension Rest | Extension Extension | Extension Flexion | Flexion Rest | Flexion Flexion | Flexion Extension | |
| Onset, ms | 25.9 ± 5.3 | 24.5 ± 5.1 | 24.5 ± 5.2 | 28.6 ± 6.4 | 28.5 ± 6.6 | 28.8 ± 6.8 |
| Duration, ms | 22.1 ± 6.8 | 24.1 ± 5.6 | 24.0 ± 5.5 | 21.1 ± 4.3 | 22.6 ± 6.5 | 21.4 ± 7.3 |
| Depth, %prestimulus EMG | 23.7 ± 9.4 | 33.6 ± 13.9 | 13.8 ± 15.9 | 26.3 ± 17.2 | 35.6 ± 17.9 | 16.3 ± 23.9 |
| iSP area normalized to contraction | 0.19 ± 0.12 | 0.29 ± 0.16 | 0.09 ± 0.12 | 0.11 ± 0.08 | 0.18 ± 0.07 | 0.03 ± 0.1 |
Values are means ± SD and were determined during 10% maximal voluntary contraction (MVC) extension or flexion of target arm ipsilateral silent period (iSP) in triceps brachii (n = 15) or biceps brachii (n = 15), respectively, in the conditions indicated.
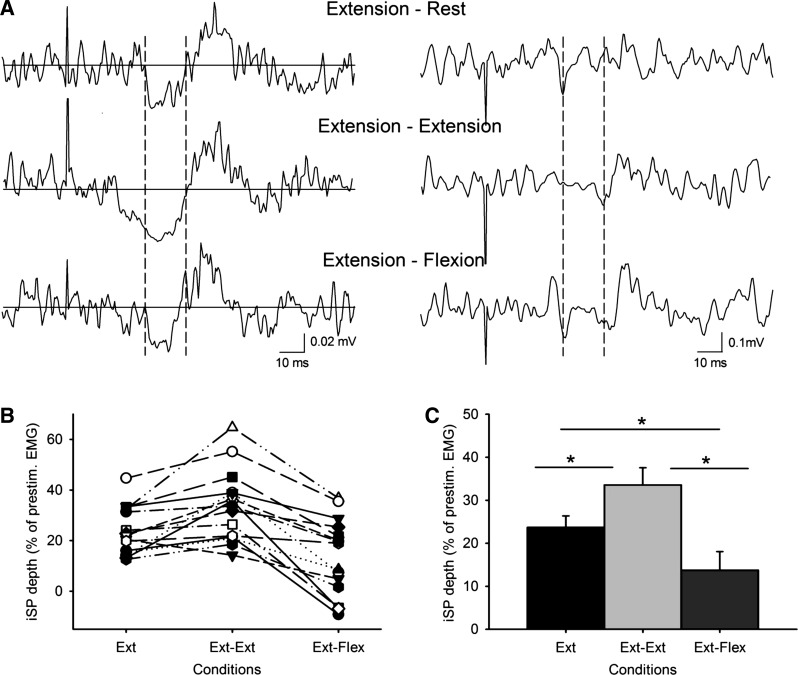
Figure 2A shows raw traces of the iSP in the triceps brachii during all conditions tested in a representative subject. These indicate that the depth and area of the iSP were increased during bilateral activation of homologous muscles (extension-extension) and decreased during bilateral activation of antagonist muscles (extension-flexion) compared with unilateral elbow extension. These observations were confirmed by repeated-measures ANOVA, which showed an effect of condition during the elbow extension session on the depth (F = 26.9, P < 0.001; Fig. 2, B and C) and area (F = 12.9, P < 0.001) of the iSP. Post hoc testing showed that for each condition, iSP depth and area (see Table 1) were significantly different from those in the two other conditions. The iSP was larger during bilateral than during unilateral elbow extension (depth, P = 0.003; and area, P = 0.02) and smaller during elbow flexion of the other arm than during unilateral elbow extension (depth, P = 0.003; and area, P < 0.001). When individual subjects were considered, 12 of 15 subjects showed an increase in the depth and area of the iSP during bilateral compared with unilateral elbow extension, whereas 13 of 15 subjects showed a decrease in the depth and area of the iSP during extension-flexion compared with unilateral elbow extension. Of these 13 subjects, 5 showed a peak of facilitation (147.1% of the mean prestimulus EMG) 4.1 ms earlier than the iSP onset during extension-flexion contraction (see Fig. 4, A and C). This facilitation was never observed in the other conditions. When the same window for analysis was used across conditions, repeated-measures ANOVA showed an effect of condition during the elbow extension session on the depth (F = 27.7, P < 0.001) and area (F = 12.5, P < 0.001) of the iSP. In the control experiment, repeated-measures ANOVA showed no effect of the direction of visual feedback during the elbow extension session on the depth (F = 2.3, P = 0.36) and area (F = 1.8, P = 0.25) of the iSP.
Fig. 2.
A: raw EMG traces recorded from the triceps brachii muscles in a representative subject during all conditions tested (extension-rest, extension-extension, and extension-flexion) with TMS applied to the ipsilateral motor cortex. Traces show the average rectified (left column) and unrectified (right column) EMG data in 30 trials. The onset and offset of the ipsilateral silent period (iSP) are shown between broken lines. The same time period is also shown on the unrectified data. Notice that the subject shows more inhibition of the EMG during bilateral extension-extension and less inhibition of the EMG during bilateral extension-flexion compared with unilateral elbow extension. B shows individual subject data in all conditions tested; in C, group data (n = 15) show the depth of the iSP in all conditions tested. The abscissa shows the conditions tested, and the ordinate shows the depth of the iSP calculated from the mean EMG activity during the iSP divided by the mean of the prestimulus EMG and subtracted from 100. Note the increase in the iSP during bilateral activation of agonist muscles (extension-extension) and a decrease during bilateral activation of antagonist muscles (extension-flexion) compared with a unilateral contraction. Error bars indicate SE. *P < 0.05. Ext, extension; Flex, flexion.
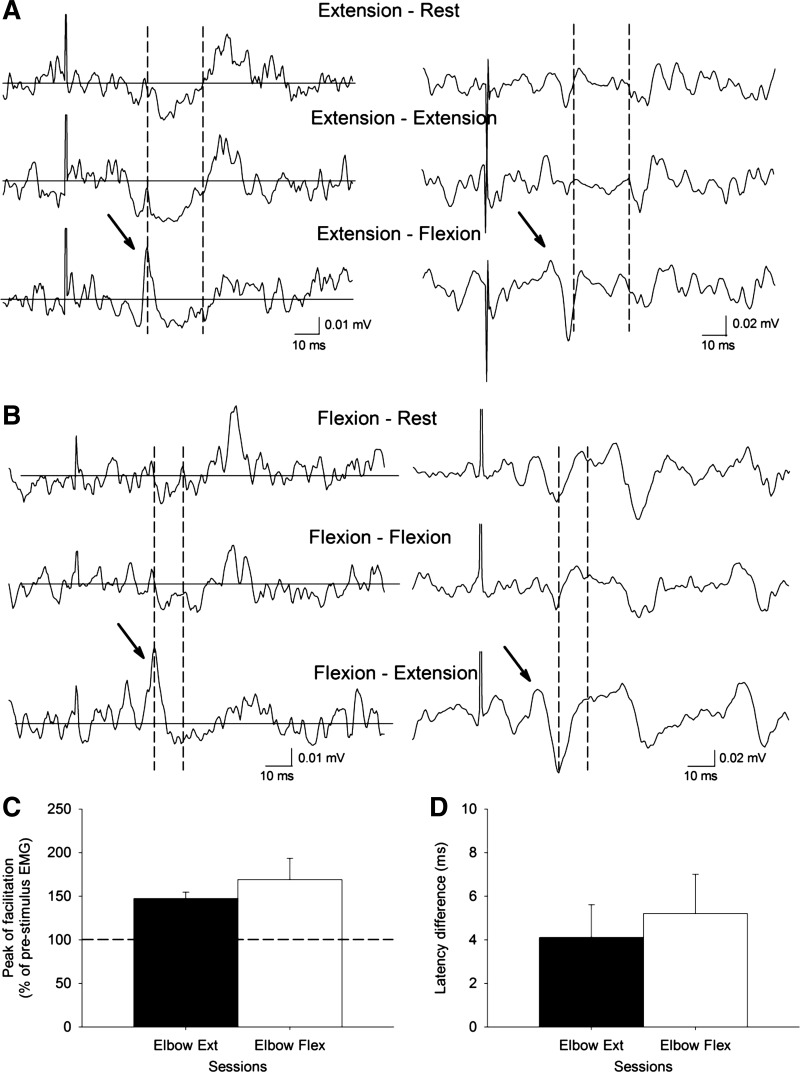
Fig. 4.
A and B: raw EMG data in 2 representative subjects showing the peak of facilitation (indicated by arrow) preceding the EMG inhibition in the extension-flexion (measured in triceps; A) and flexion-extension conditions (measured in biceps; B). The peak is not present in the other conditions tested. C: the abscissa shows the sessions tested (elbow extension, n = 5; elbow flexion, n = 4) and the ordinate shows the peak of facilitation (expressed as a percentage of the prestimulus EMG). D: the abscissa shows the sessions tested (elbow extension, n = 5; elbow flexion, n = 4) and the ordinate shows the latency of the facilitation (beginning defined as 2 SD above the mean rectified EMG for a period of 90 ms before the TMS) minus the latency of the iSP. Note that the magnitude and the latency of the peak of facilitation observed during bilateral activation of antagonist muscles were similar in both sessions. Error bars indicate SE. *P < 0.05.
Elbow flexion session.
Fifteen participants tested in the study showed a visible iSP in the biceps muscle during unilateral elbow flexion. These participants completed the elbow flexion session. RMT in the biceps muscle was 59.1 ± 7.6%. The mean stimulus intensity was 69.6 ± 9.9% of the maximum stimulator output (range from 55 to 93%) or 118 ± 10.5% of the RMT. Mean prestimulus rectified EMG activity in the biceps in which the iSP was measured was similar across conditions (F = 1.6, P = 0.2). The mean onset latency (P = 0.9) and the duration of the iSP (P = 0.4) were similar across conditions (see Table 1).
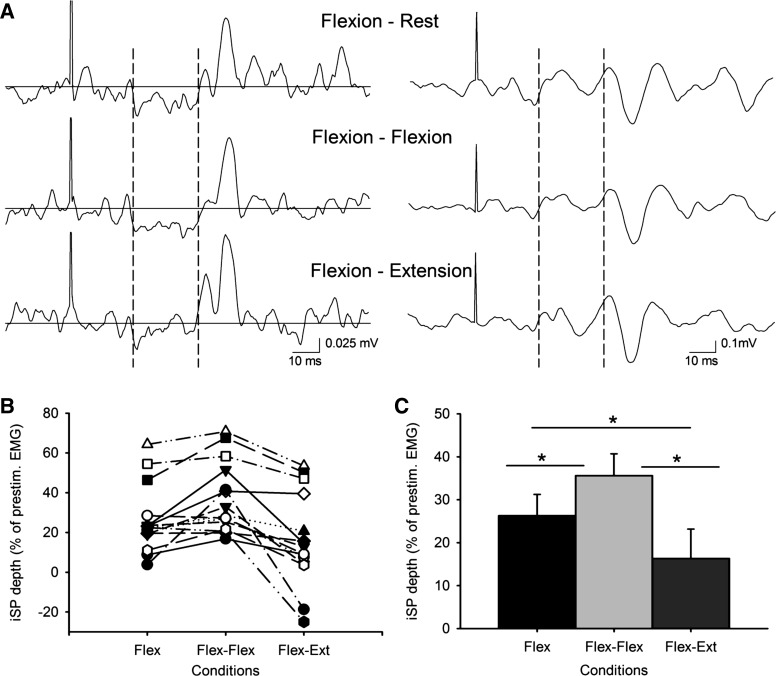
Figure 3A shows an example of the iSP in the biceps brachii in a representative subject. As reported for the triceps, we observed that the depth and area of the iSP were increased during bilateral activation of homologous muscles (flexion-flexion) and decreased during bilateral activation of antagonist muscles (flexion-extension) compared with unilateral elbow flexion. Repeated-measures ANOVA showed an effect of condition during the elbow flexion session on the depth (F = 15.8, P < 0.001; Fig. 3, B and C) and area (F = 10.8, P < 0.001) of the iSP. Post hoc testing showed significant increases in the iSP depth (P = 0.02) and area (P = 0.01) during bilateral compared with unilateral elbow flexion and significant decreases (depth, P = 0.03; and area, P = 0.02) during bilateral flexion-extension compared with unilateral flexion (see Table 1). For individuals, 11 of 15 subjects showed a larger iSP during bilateral compared with unilateral elbow extension, and 11 of 15 subjects showed a decrease in the iSP during flexion-extension compared with unilateral elbow flexion. During flexion-extension, four of these subjects showed a peak of facilitation (169.0% of the mean prestimulus EMG) 5.2 ms earlier than the iSP onset during flexion-extension contraction (Fig. 4, B and D). The facilitation was never observed in the other conditions. Using the same window for analysis across conditions, repeated-measures ANOVA showed the same significant effects of condition during the elbow flexion session on the depth (F = 23.0, P < 0.001) and area (F = 15.6, P < 0.001) of the iSP. In the control experiment, repeated-measures ANOVA showed no effect of the direction of visual feedback during the elbow flexion session on the depth (F = 2.0, P = 0.19) and area (F = 1.2, P = 0.30) of the iSP.
Fig. 3.
A: raw EMG traces recorded from the biceps brachii muscles in a representative subject during all conditions tested (flexion-rest, flexion-flexion, and flexion-extension) with TMS applied to the ipsilateral motor cortex. Traces show the average rectified (left column) and unrectified (right column) EMG data in 30 trials. The onset and offset of the iSP is shown between broken lines. The same time period is also shown on the unrectified data. Notice that the subject shows more inhibition in the EMG during bilateral flexion-flexion and less inhibition in the EMG during flexion-extension compared with unilateral elbow flexion. B shows individual subject data in all conditions tested; in C, group data (n = 15) show the depth of the iSP in all conditions tested. The abscissa shows the conditions tested, and the ordinate shows the depth of the iSP. Similar to what was observed in the triceps, note here the increase in the iSP during bilateral activation of agonist muscles (flexion-flexion) and the decrease during bilateral activation of antagonist muscles (flexion-extension) compared with a unilateral contraction. Error bars indicate SE. *P < 0.05.
Elbow extension session vs. flexion session.
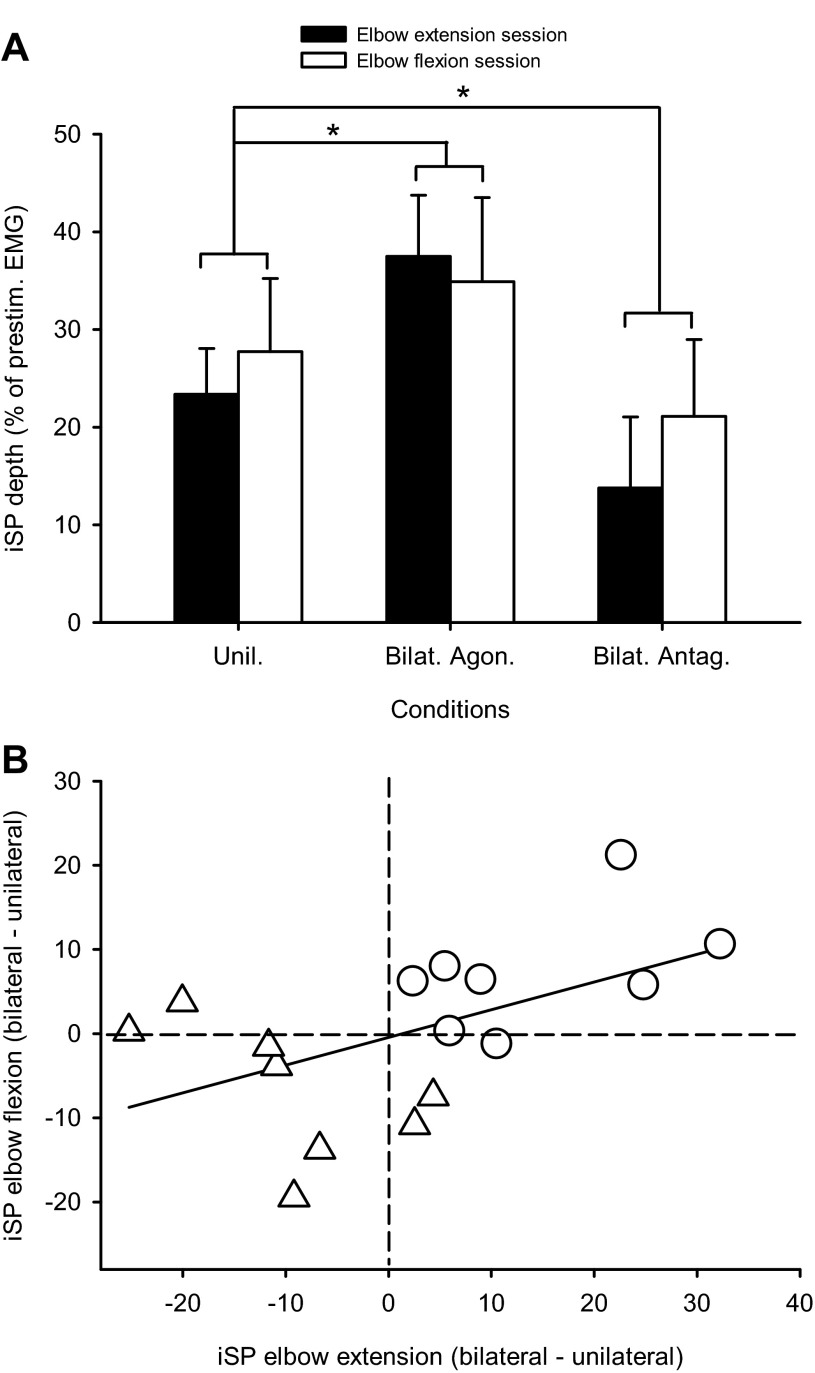
Eight of the participants tested in the study showed visible iSPs in both triceps and biceps brachii. For these subjects, the depth (23.4 ± 11.7% and 27.7 ± 18.7%, respectively; P = 0.6) and area (0.19 ± 0.07 and 0.16 ± 0.04, respectively; P = 0.4) of the iSP were not different during unilateral extension and flexion contractions. We found an effect of condition (F = 32.4, P < 0.001) but not session (F = 0.1, P = 0.7), nor their interaction (F = 2.6, P = 0.1), in the iSP depth in triceps and biceps brachii. The iSP depth was significantly increased during bilateral activation of homologous muscles (P < 0.001; Fig. 5A) and decreased during bilateral activation of antagonist muscles (P < 0.001; Fig. 5A), regardless of whether subjects performed the elbow extension (filled bars) or flexion (open bars) session. Significant changes also occurred in the iSP area (P < 0.001 for both comparisons). Figure 5A shows the relation in single subjects between the changes in iSP depth during bilateral contractions relative to unilateral activation of the triceps and biceps brachii; the different symbols indicate bilateral activation of homologous and antagonist muscles compared with unilateral contractions. There was a positive correlation (r = 0.52, P = 0.03), indicating that individuals showed similar changes in the iSP depth whether iSPs were elicited in elbow extension or flexion. Changes in iSP area during bilateral contractions relative to unilateral activation of the triceps and biceps brachii were also correlated (r = 0.6, P = 0.01).
Fig. 5.
A: a comparison of the iSP in the triceps and biceps measured in the same subjects (n = 8) in all conditions tested. The abscissa shows the conditions tested (Unil, unilateral; Bilat. Agon., bilateral activation of agonist muscles; Bilat. Antag., bilateral activation of antagonist muscles) during the elbow extension (filled bars) and flexion sessions (open bars). The ordinate shows the depth of the iSP. Note the increase in the iSP during bilateral activation of agonist muscles and a decreased during bilateral activation of antagonist muscles compared with a unilateral contraction regardless of the elbow session tested. Error bars indicate SE. *P < 0.05. B: a correlation analysis between flexion and extension sessions of the changes in the iSP depth during bilateral (△, agonist; ○, antagonist) compared with unilateral contractions (a negative number indicates more inhibition and a positive number indicates less inhibition during the unilateral compared with bilateral condition). Each subject is represented by 2 points: one for bilateral agonist and one for bilateral antagonist contractions. Note that subjects who showed more pronounced inhibition in the elbow extension session also showed a similar trend in the elbow flexion session.
MEPs.
MEPs were measured in the triceps and biceps brachii in the side contralateral to TMS stimulation during all conditions tested. The peak-to-peak amplitude of the triceps MEP elicited in the dominant contralateral arm was larger during extension-extension (4.0 ± 2.6 mV) compared with unilateral elbow extension (0.37 ± 0.4 mV, P < 0.001) and extension-flexion (0.89 ± 0.5 mV, P < 0.001). The triceps MEP latency in the dominant arm was longer in the unilateral extension (13.3 ± 1.3 ms) compared with extension-extension (10.2 ± 0.9 ms, P < 0.001) and extension-flexion (10.5 ± 1.2 ms, P < 0.001). The peak-to-peak amplitude of the biceps MEPs elicited in the dominant contralateral arm was larger during bilateral flexion (5.9 ± 4.9 mV) compared with unilateral flexion (0.78 ± 0.9 mV, P < 0.001) and bilateral flexion-extension (1.4 ± 1.6 mV, P < 0.001). The biceps MEP latency in the dominant arm was longer in the unilateral flexion (13.4 ± 1.3 ms) compared with bilateral flexion-flexion (10.6 ± 1.6 ms, P < 0.001) and flexion-extension (12.6 ± 1.8 ms; P = 0.02). Repeated-measures ANOVA showed that the MEPs in biceps and triceps were the same size (F = 2.2, P = 0.16).
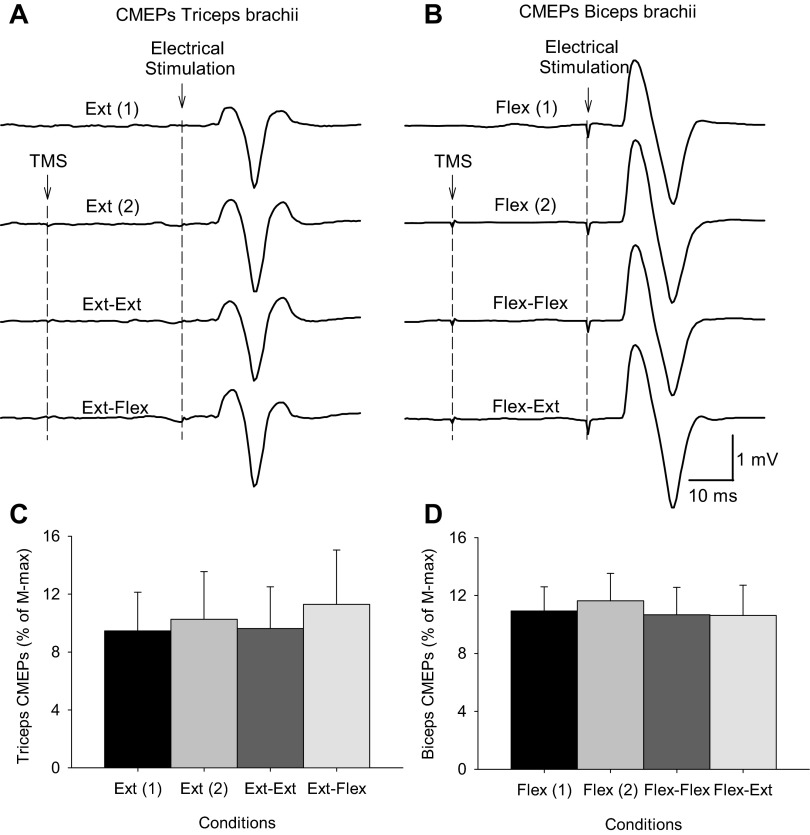
CMEPs.
The size of CMEPs in the triceps (9.4 ± 5.3% of Mmax) and biceps (10.9 ± 4.5% of Mmax) brachii were similar during unilateral elbow extension and flexion (P = 0.6), respectively. The latencies of the CMEPs were 9.0 ± 0.7 ms in the triceps and 8.4 ± 0.4 ms in the biceps brachii (P = 0.45). Figure 6 shows an example of the CMEPs in the triceps and biceps brachii in two representative subjects. Note that the size of the CMEPs elicited during the iSP remained unchanged during bilateral activation of homologous and nonhomologous muscles compared with unilateral contractions in the elbow extension and elbow flexion tasks. Friedman repeated-measures ANOVA showed no effect of condition on the size of CMEPs in the triceps (χ2 = 0.3, P = 0.99) and biceps brachii (χ2 = 2.1, P = 0.54).
Fig. 6.
A and B: raw EMG data in 2 representative subjects showing motor evoked responses to electrical cervicomedullary stimulation (cervicomedullary motor evoked potentials, CMEPs) recorded from the triceps (A) and biceps brachii (B) in all conditions tested in the elbow extension [Ext (1), extension-rest, CMEPs alone; Ext (2), extension-rest, CMEPs preceded by TMS; Ext-Ext, extension-extension, CMEPs preceded by TMS; Ext-Flex, extension-flexion, CMEPs preceded by TMS] and flexion sessions [Flex (1), flexion-rest, CMEPs alone; Flex (2), flexion-rest, CMEPs preceded by TMS; Flex-Flex, flexion-flexion, CMEPs preceded by TMS; flex-Ext, flexion-extension, CMEPs preceded by TMS]. Ten traces were average in each set. Because the stimulation artifact in the triceps brachii is small, the time of stimulation is indicated by dashed lines. C and D: in the graphs, the abscissa shows the conditions tested and the ordinate shows the size of CMEPs as a percentage of the maximal M wave (Mmax) in the triceps (C) and biceps brachii (D). Note that the size of CMEPs in triceps and biceps remained similar across conditions in both sessions. Error bars indicate SE. *P < 0.05.
DISCUSSION
In the present study, we examined the iSP in the triceps and biceps brachii in one arm while the contralateral arm remained at rest or performed an extension or flexion isometric voluntary contraction. In both muscles the iSP depth and area were increased during bilateral contractions of the elbow extensors for triceps or of the elbow flexors for biceps compared with unilateral contractions. In contrast, when subjects performed bilateral contractions of flexor muscles on one side and extensor muscles on the other, either the depth and area of the iSP were decreased or facilitation was present compared with bilateral activation of homologous muscles and to unilateral contractions. Subcortically evoked CMEPs tested during the time at which the iSP was present remained unchanged during bilateral compared with unilateral contractions. The present data show a distinct pattern of reciprocal interactions between bilateral flexor and extensor arm regions of the motor cortex.
Triceps and biceps iSP during unilateral contractions.
We had difficulty in eliciting inhibition in the triceps and biceps brachii during a unilateral contraction. In fact, from 45 subjects screened we were able to detect a visible iSP with no short-latency facilitation in either muscle in around half of all subjects. In about half of those, an iSP was seen in both muscles. This is in agreement with Ferbert et al. (1992), who reported similar problems and identified clear iSPs in the biceps brachii in only three of nine subjects. When we compared the depth and area of the iSP in the two muscles in subjects in whom it occurred in both muscles, we found that this was not different. A previous study demonstrated a less prominent interhemispheric inhibition in the triceps compared with the biceps brachii (Harris-Love et al. 2007). The differences with our results are likely to be due to differences in methodology. In the study by Harris-Love et al. (2007), measurements were taken at rest and interhemispheric inhibition was tested with a paired-pulse protocol. Previous studies have suggested that the iSP and the inhibition measured by a paired-pulse protocol provide information that is complementary, but their mechanisms might differ (Chen et al. 2003; Ferbert et al. 1992; Perez and Cohen 2009).
An intriguing question is, why was it in general difficult to elicit an iSP in the triceps and biceps brachii? The representations of triceps and biceps brachii in the motor cortex are close in monkeys (Kwan et al. 1978) and humans (Penfield and Boldrey 1937). Previous evidence has shown that during a unilateral voluntary contraction, high-intensity TMS applied over the ipsilateral motor cortex frequently elicited ipsilateral MEPs in the biceps, and lower intensity stimulation could elicit them during bilateral phasic contractions (Bawa et al. 2004; Ziemann et al. 1999). Brinkman and Kuypers (1973) also suggested the presence of both direct and indirect ipsilateral pathways from the cortex to the biceps brachii. Since an ipsilateral pathway is one of the possible routes contributing to ipsilateral responses (Carson 2005; Jankowska and Edgley 2006), it is possible that in the biceps brachii ipsilateral excitatory pathways are more readily accessed than in the hand muscles in which iSPs have been more often studied. Direct ipsilateral excitation of the motoneurons could mask the disfacilitation resulting from transcallosal inhibition of contralateral descending drive. In contrast, direct ipsilateral facilitation has not been reported for triceps; rather, inhibition of EMG occurred in most subjects (Ziemann et al. 1999). However, as noted above, interhemispheric inhibition is less prominent for triceps than for biceps or for distal muscles (Harris-Love et al. 2007). Another possibility is that the ability of eliciting the iSP in proximal arm muscles is related to the characteristics of the motor task. A recent study demonstrated that large iSPs can be recorded in the biceps brachii when measured during fast ballistic compared with slower self-paced elbow flexion movements (Tazoe and Perez 2013).
Triceps and biceps bilateral contractions of homologous and antagonistic muscles.
In both muscles the iSP was largest during bilateral contractions of homologous muscles. This is consistent with previous results showing that during bilateral isometric contractions of homologous finger muscles transcallosal inhibition increased compared with unilateral contractions (Soteropoulos and Perez 2011; Yedimenko and Perez 2010). Similarly, iSPs evoked during maximal efforts of first dorsal interosseous were increased by weak, or even imagined, contraction of muscles of the other hand (Giovanelli et al. 2009). Surprisingly, iSPs evoked during 20% of MVC were not altered by contralateral steady or sinusoidal force production (Fling and Seidler 2012), but this may reflect a floor effect given the deep suppression of EMG (∼90%) in the unimanual condition.
Although the iSP is thought to be mediated by axons passing through the corpus callosum (Boroojerdi et al. 1996; Meyer et al. 1995), an iSP also can be obtained from stimulation of subcortical pathways (Compta et al. 2006; Gerloff et al. 1998). However, our CMEPs results indicate that it is less likely that subcortical pathways contributed to the observed changes in the iSP magnitude. The magnitude of short-interval intracortical inhibition (SICI) is reduced by muscular activity (Ortu et al. 2008), and in the presence of less SICI the magnitude of interhemispheric inhibition to the contralateral hemisphere increases (Lee et al. 2007; Perez and Cohen 2008). Therefore, these factors could contribute to increase the iSP magnitude during bilateral contractions of homologous muscles compared with unilateral contractions.
In contrast, we observed in both muscles that during bilateral contractions of different muscles in the two arms, the depth and area of the iSP were decreased, and in some subjects EMG was facilitated compared with the other conditions. This is a surprising finding because other studies involving hand muscles reported increases in the iSP or in paired-pulse interhemispheric inhibition with bilateral contractions of nonhomologous muscles (Giovanelli et al. 2009; Yedimenko and Perez 2010). However, these studies also indicate that interhemispheric inhibitory interactions between hand muscles are more independent of muscular constraints, which is unclear for proximal muscles. Our current and previous findings suggest that interhemispheric inhibitory interactions involving proximal arm muscles have a more widespread effect to nonhomologous muscles (Soteropoulos and Perez 2011).
The iSP methodology does not allow dissection of the source of the differences in homologous and nonhomologous agonist-antagonist bilateral contractions, but some factors can be considered. First, the decrease in iSP and the variable facilitation with agonist-antagonist contractions could represent activation of an excitatory ipsilateral descending pathway. This seems unlikely because ipsilateral MEPs are common in biceps but are not reported for triceps (Ziemann et al. 1999), yet here facilitation occurred equally in both muscles. Alternatively, the latency of the facilitation in our study is consistent with a possible contribution from interhemispheric facilitation, which is observed 4–6 ms earlier than inhibition (Bäumer et al. 2006; Hanajima et al. 2001). Second, are the important changes in the stimulated hemisphere or in the hemisphere where the transcallosal signal acts? Although the increase was greater in the agonist muscle, MEPs in both biceps and triceps muscles increased during both kinds of bilateral contractions, and stimulation of muscle afferents from a flexor muscle can reduce intracortical inhibition measured in the antagonist muscle, suggesting a pattern of cortical “reciprocal facilitation” in the same limb (Aimonetti and Nielsen 2001). Thus, with increased corticospinal excitability and decreased inhibition likely in the stimulated hemisphere in both kinds of bilateral contractions, it seems unlikely that these mechanisms resulted in the differential changes during activation of homologous or nonhomologous muscles. Changes in excitability and intracortical inhibition are reported in the hemisphere ipsilateral to voluntary contractions (Perez and Cohen 2009; Soteropoulos and Perez 2011). Such changes may be caused by increased transcallosal signals between primary motor areas but may be mediated through other pathways (Bestmann et al. 2008; Liuzzi et al. 2010; Münchau et al. 2002).
In humans, motor tasks that require flexion of one arm and extension of the other arm are produced in a less stable and consistent fashion compared with simultaneous patterns in which both limbs flex or extend together (Carson et al. 2000; Kelso 1995). It is tempting to speculate that the facilitatory effect and the decreased inhibition observed in our study during asymmetric bilateral muscle contractions might contribute to decouple arm movements or, on the contrary, might reflect a neural correlate of our inability to completely decouple our arms. However, previous evidence has shown that interactions between actively moving arms and those obtained when both arms are engaged in isometric contractions differ (Carson 1995). Thus caution must be taken in extrapolation of the present results to a more dynamic task. In terms of isometric tasks, our findings are consistent with studies that demonstrate deficits in force production during bilateral elbow flexion or extension but not with flexion of one arm and extension of the other (Ohtsuki 1983; Seki and Ohtsuki 1990), and also with evidence that iSPs are larger with in-phase compared with out-of-phase bilateral isometric contractions (Tazoe et al. 2013).
Limitations of the study.
Methodological factors that might influence interhemispheric interactions include the level of muscle contraction, the type and direction of visual feedback, and the stimulus intensity (Byblow et al. 1999; Carson and Ruddy 2012; Perez and Cohen 2008). In our study, the strength of muscle contraction exerted by elbow flexor and extensor muscles in the arm contralateral to the TMS was similar across conditions and the iSP was modulated to a similar extent regardless of the direction of the visual feedback. Therefore, it is unlikely that these aspects contributed to our findings. Furthermore, the same stimulus intensity was used across conditions, whereas the iSP was differentially modulated by both bilateral motor tasks, making it less likely that this factor affected our results.
GRANTS
This work was supported by funding from the National Health and Medical Research Council of Australia, National Institute of Neurological Disorders and Stroke Grant R01 NS076589, and Department of Veterans Affairs Grant 3397626.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.P., J.E.B., and J.L.T. conception and design of research; M.A.P., J.E.B., and J.L.T. performed experiments; M.A.P., J.E.B., and J.L.T. analyzed data; M.A.P., J.E.B., and J.L.T. interpreted results of experiments; M.A.P., J.E.B., and J.L.T. prepared figures; M.A.P., J.E.B., and J.L.T. drafted manuscript; M.A.P., J.E.B., and J.L.T. edited and revised manuscript; M.A.P., J.E.B., and J.L.T. approved final version of manuscript.
REFERENCES
- Aimonetti JM, Nielsen JB. Changes in intracortical excitability induced by stimulation of wrist afferents in man. J Physiol 534: 891–902, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, Münchau A. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol 572: 857–868, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Dammann E, Bock F, Klöppel S, Siebner HR, Münchau A. Laterality of interhemispheric inhibition depends on handedness. Exp Brain Res 180:195–203, 2007 [DOI] [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res 158: 385–390, 2004 [DOI] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex 18: 1281–1291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 144: 160–170, 1996 [DOI] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain 96: 653–674, 1973 [DOI] [PubMed] [Google Scholar]
- Byblow WD, Summers JJ, Semjen A, Wuyts IJ, Carson RG. Spontaneous and intentional pattern switching in a multisegmental bimanual coordination task. Motor Control 3: 372–393, 1999 [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S, Smethurst CJ, Párraga JF, Byblow WD. Neuromuscular-skeletal constraints upon the dynamics of unimanual and bimanual coordination. Exp Brain Res 131: 196–214, 2000 [DOI] [PubMed] [Google Scholar]
- Carson RG, Ruddy KL. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J Neurosci 32: 646–652, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev 49: 641–662, 2005 [DOI] [PubMed] [Google Scholar]
- Carson RG. The dynamics of isometric bimanual coordination. Exp Brain Res 105: 465–476, 1995 [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 89: 1256–1264, 2003 [DOI] [PubMed] [Google Scholar]
- Compta Y, Valls-Solé J, Valldeoriola F, Kumru H, Rumià J. The silent period of the thenar muscles to contralateral and ipsilateral deep brain stimulation. Clin Neurophysiol 117: 2512–2520, 2006 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115: 255–266, 2004 [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Seidler RD. Task-dependent effects of interhemispheric inhibition on motor control. Behav Brain Res 226: 211–217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 510: 249–259, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol 587: 5393–5410 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247: 297–325, 1986 [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol 531: 849–859, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Love ML, Perez MA, Chen R, Cohen LG. Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. J Neurophysiol 97: 2511–2515, 2007 [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Muscle activation in unilateral and bilateral efforts assessed by motor nerve and cortical stimulation. J Appl Physiol 80: 1351–1356, 1996 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12: 67–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol 188: 137–145, 1979 [DOI] [PubMed] [Google Scholar]
- Jung P, Ziemann U. Differences of the ipsilateral silent period in small hand muscles. Muscle Nerve 34: 431–436, 2006 [DOI] [PubMed] [Google Scholar]
- Kelso JA. Dynamic Patterns: the Self-Organization of Brain and Behavior. Cambridge, MA: MIT Press, 1995 [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41: 1120–1131, 1978 [DOI] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol 580: 1021–1032, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi G, Hörniss V, Hoppe J, Heise K, Zimerman M, Gerloff C, Hummel FC. Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb Cortex 20: 1323–1331, 2010 [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, von Grafin EH, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118: 429–440, 1995 [DOI] [PubMed] [Google Scholar]
- Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22: 554–561, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104:527–533, 1995 [DOI] [PubMed] [Google Scholar]
- Ohtsuki T. Decrease in human voluntary isometric arm strength induced by simultaneous bilateral exertion. Behav Brain Res 7: 165–178, 1983 [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586: 5147–5159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Res 26: 217–233, 1971 [PubMed] [Google Scholar]
- Penfield WG, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443, 1937 [Google Scholar]
- Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 587: 725–726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 97–103, 1999 [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 102: 227–243, 1994 [DOI] [PubMed] [Google Scholar]
- Seki T, Ohtsuki T. Influence of simultaneous bilateral exertion on muscle strength during voluntary submaximal isometric contraction. Ergonomics 33: 1131–1142, 1990 [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Perez MA. Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J Neurophysiol 105: 1594–1602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol 96: 1496–1503, 2004 [DOI] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Speed-dependent contribution of callosal pathways to ipsilateral movements. J Neurosci 33: 16178–16188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Sasada S, Sakamoto M, Komiyama T. Modulation of interhemispheric interactions across symmetric and asymmetric bimanual force regulations. Eur J Neurosci 37: 96–104, 2013 [DOI] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res 158: 133–140, 2004 [DOI] [PubMed] [Google Scholar]
- Yedimenko JA, Perez MA. The effect of bilateral isometric forces in different directions on motor cortical function in humans. J Neurophysiol 104: 2922–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wasserman EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175: 526–535, 2006 [DOI] [PubMed] [Google Scholar]