Abstract
Basal ganglia-thalamocortical circuits are multistage loops critical to motor behavior, but the contributions of individual components to overall circuit function remain unclear. We addressed these issues in a songbird basal ganglia-thalamocortical circuit (the anterior forebrain pathway, AFP) specialized for singing and critical for vocal plasticity. The major known afferent to the AFP is the premotor cortical nucleus, HVC. Surprisingly, previous studies found that lesions of HVC alter song but do not eliminate the ability of the AFP to drive song production. We therefore used this AFP-driven song to investigate the role of basal ganglia and thalamus in vocal structure, tempo, and initiation. We found that lesions of the striatopallidal component (Area X) slowed song and simplified its acoustic structure. Elimination of the thalamic component (DLM) further simplified the acoustic structure of song and regularized its rhythm but also dramatically reduced song production. The acoustic structure changes imply that sequential stages of the AFP each add complexity to song, but the effects of DLM lesions on song initiation suggest that thalamus is a locus of additional inputs important to initiation. Together, our results highlight the cumulative contribution of stages of a basal ganglia-thalamocortical circuit to motor output along with distinct involvement of thalamus in song initiation or “gating.”
Keywords: zebra finch, Area X, DLM, lesion
basal ganglia-thalamocortical circuits are complex recurrent loops critical for learning and carrying out motor programs. Damage to human basal ganglia-thalamocortical circuits, such as that caused by stroke or neurodegenerative disease, has devastating effects including both hypo- and hyperkinesis, difficulty with movement control and amplitude, and problems with gesture initiation, including speech (Bruce et al. 2004; Turner and Desmurget 2010; Wichmann and Dostrovsky 2011; Zaidel et al. 2009). However, damage due to disease or stroke is seldom localized to a specific region, especially for thalamus, and motor phenotypes are often complicated by cognitive and behavioral effects of damage (Carrera and Bogousslavsky 2006; Merello et al. 2001). Thus it is difficult in humans to determine the contribution of individual nuclei in these circuits to overall circuit function. Studies of animal models of basal ganglia and its disorders have suggested that the basal ganglia is important for gain control and sequence learning, but there is still controversy about whether it is important for sequence performance after learning or inhibition of competing movements. The thalamus has been particularly difficult to study in humans because of the lack of focal lesions and because of the extensive interconnection of the thalamus with all parts of the cortex.
Songbirds provide a simple but potentially powerful model for understanding neural mechanisms of basal ganglia circuit function because they have a discrete basal ganglia-thalamocortical circuit, the anterior forebrain pathway (AFP; Fig. 1, A and E, in red), specialized for the learning and production of song. The AFP interconnects the two major nuclei of the direct motor pathway, essential for song production throughout life (Fig. 1, A and E, in black): the cortical premotor nucleus, HVC (used as proper name), and the robust nucleus of the arcopallium (RA), which receives input from HVC and in turn projects to motor neurons that control vocal and respiratory musculature. In addition to innervating RA, HVC projects to the first step in the AFP, Area X, a nucleus that contains striatal neurons intermingled with pallidal neurons (Carrillo and Doupe 2004; Farries and Perkel 2002; Reiner et al. 2004). The pallidal output neurons of Area X provide inhibitory input to neurons in a medial portion of the dorsolateral thalamus (DLM), which also receive a sparse projection from RA (Fig. 1E; Goldberg and Fee 2012). The thalamic DLM neurons project to the lateral portion of the magnocellular nucleus of the anterior neostriatum (LMAN), which not only provides excitatory input forward to motor nucleus RA, but also sends a recurrent connection back to Area X, thus completing a cortical-basal ganglia-thalamocortical loop (LMAN-Area X-DLM-LMAN).
Fig. 1.
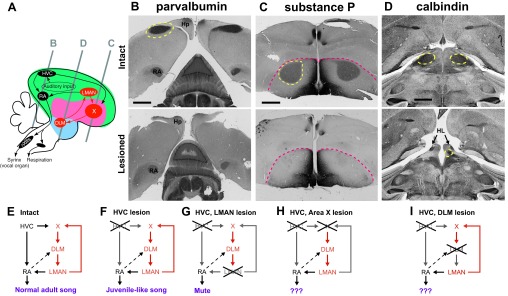
Schematics of lesion experiments. A: diagram showing the approximate location of the major nuclei of the song system relative to each other in a composite sagittal plane of the zebra finch brain. Colored areas indicate the division of the forebrain into pallium (“cortex”; green), striatum/pallidum (pink), and thalamus (blue). Gray lines represent the approximate location of coronal sections shown in B–D. B–D contain representative sections for comparison of intact brains (top) with lesioned brains (bottom). Dotted yellow lines indicate the nucleus of interest. X, Area X (a nucleus that contains striatal neurons intermingled with pallidal neurons); nXIIts, tracheosyringeal part of nucleus XII. B: parvalbumin immunostaining highlights the robust nucleus of the arcopallium (RA) in both sections, but there is no staining of the cortical premotor nucleus, HVC, in the lesioned bird (bottom). Hp, hippocampus. C: Area X is visualized with substance P immunostaining. Although there is the usual dark substance P staining on the medial border of the striatum in the lesioned bird (bottom), there is no visible staining of Area X. Pink dashed line indicates division between pallium (top) and striatum (bottom). D: calbindin immunostaining identifies the medial portion of the dorsolateral thalamus (DLM). This region is entirely absent on the left side of the DLM-lesioned bird, but a small amount (∼15% of intact) remains on the right side. The arrow indicates the lateral habenula (HL; Pinaud et al. 2007), which is displaced anteriorly due to the missing space created by the DLM lesion. E: schematic of song control nuclei. When both the ventral motor pathway and the anterior forebrain pathway (AFP) are intact, birds produce stereotyped adult song. F: when HVC is lesioned in adult birds, song reverts to a state of juvenile-like babbling that is controlled by the AFP. G: when both HVC and the lateral portion of the magnocellular nucleus of the anterior neostriatum (LMAN) are silenced, thus stripping RA of its major inputs, birds become “mute” for learned song, although they can still produce calls (Aronov et al. 2008). This study investigates the differences in adult song produced solely by the intact AFP (F) vs. song produced when the AFP loop is disrupted at the basal ganglia (Area X; H) or when the loop is disrupted at the thalamus (DLM; I). Scale bars = 1 mm.
Insults to the AFP affect singing but leave other motor programs intact. In adult birds, the AFP is activated during singing, including the induction of immediate early genes (Hessler and Doupe 1999; Jarvis and Nottebohm 1997; Jarvis et al. 1998; Jin and Clayton 1997; Kao et al. 2008), and drives variability in song via its projections to the premotor nucleus RA (Hampton et al. 2009; Kao and Brainard 2006; Kao et al. 2005; Stepanek and Doupe 2010). The outflow of the AFP is also required for adult plasticity (Andalman and Fee 2009; Brainard and Doupe 2000; Charlesworth et al. 2012; Nordeen and Nordeen 2010; Thompson et al. 2007; Warren et al. 2011; Williams and Mehta 1999).
Strikingly, in adult birds, elimination of the premotor cortical nucleus HVC, which is the major afferent input to the AFP as well as to nucleus RA, disrupts song structure but does not eliminate the ability of the AFP to generate activity that can drive song production (Fig. 1F; Aronov et al. 2008; Nottebohm et al. 1976). We therefore used the AFP-dependent vocalizations of HVC-lesioned adult birds as a simplified behavioral readout of the function of the AFP in driving and shaping singing. Any changes in this vocal output due to subsequent manipulations of stages in this basal ganglia-thalamocortical circuit should teach us how each stage contributes to the function of the entire adult loop. We hypothesized that lesions of the vocal striatopallidum (Area X) would have different effects on AFP-driven song than lesions of the vocal thalamus (DLM). In juvenile birds, in which circuits are still under development, lesions of the different components of the AFP clearly have different effects (Bottjer et al. 1984; Goldberg and Fee 2011; Olveczky et al. 2005; Scharff and Nottebohm 1991; Sohrabji et al. 1990). The results here indicate that the adult striatopallidum and thalamus make distinct contributions to motor behavior driven by the basal ganglia-thalamocortical loop for song, with a centrally important function of the thalamus in the ability of the AFP to generate song.
MATERIALS AND METHODS
Subjects
We used 14 adult (>100 days posthatch) male zebra finches (Taeniopygia guttata) for lesion experiments. Birds were raised in our colony and then isolated in a small cage inside a sound-attenuating chamber (Acoustic Systems) for song recording. All procedures were performed in accordance with protocols approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Surgical Procedures
Birds were anesthetized with equithesin as previously described (Kao et al. 2008). All surgeries were performed with the animal placed in a stereotaxic apparatus with a beak angle of 50° relative to vertical. All stereotaxic coordinates are measured from the posterior divergence of the central sinus. HVC lesions were made by passing 100 μA of current for 60 s through a tungsten electrode. Lesions were made in a 3 × 5 grid centered at 2.4 mm lateral to the central sinus with a spacing of 0.3 mm between lesions and a depth of 0.5 mm below the surface of the brain. Area X and DLM lesions were both made using the excitotoxin ibotenic acid to spare fibers of passage. Area X lesions were made by pressure injection of 0.6 μl of 1% ibotenic acid divided between four injection locations. Two injections were made at a 20° angle aimed laterally from vertical (0.3 mm lateral for both; 4.6 mm rostral, 3.7 mm deep and 5.0 mm rostral, 4.0 mm deep) to miss LMAN. Forty-eight hours later, two more injections were made at a 45° angle aimed medially from vertical (4.0 mm lateral for both; 4.6 mm rostral, 3.0 mm deep and 5.0 mm rostral, 3.4 mm deep). The location of DLM was identified using a tungsten electrode to map characteristic high-frequency firing (Kojima and Doupe 2009; Person and Perkel 2007) using a starting location of 1.2 mm lateral, 1.3 mm rostral, and 4 mm deep. Subsequently, 0.15 μl of 1% ibotenic acid was injected into the electrophysiologically determined coordinates through a glass capillary tube.
Six of seven HVC, X-lesioned birds received HVC lesions first. One bird was lesioned in reverse order to ensure that lesion order did not affect the results of these experiments: this bird's song was normal after Area X lesion alone, as in previous studies (Scharff and Nottebohm 1991; Sohrabji et al. 1990). After subsequent HVC lesions, this bird's songs resembled the other six HVC, X-lesioned birds' songs in structure and tempo. All seven HVC, DLM-lesioned birds received DLM lesions first. This order was necessary because the firing properties of DLM were used to identify the lesion site, and these properties might change after HVC lesion. Moreover, we monitored song after DLM lesions, which allowed us to confirm that the DLM lesion had not inadvertently damaged projection fibers from RA to lower motor neurons. As was true for songs of adult zebra finches after Area X lesions, songs of birds with DLM lesions alone appeared normal in both structure and sequence. Lesions to different brain regions were made ≥8 days apart to allow time for song recording in between lesions.
Song Recording
Birds' vocalizations were recorded as previously described (Kao and Brainard 2006). Birds were recorded for ≥2 days in the week before their first lesions. Birds were recorded again within a few days after surgery and then continuously until they started to produce song (usually 1–2 wk after their 2nd surgical procedure) and until enough song was collected within 1 day to perform analysis (1–5 wk after their 2nd surgical procedure). We continued monitoring song at regular intervals until birds were perfused (weeks to months after lesions).
Song Analysis
Definition of song.
Adult zebra finch song motifs are defined as stereotyped sequences of syllables (individual song elements separated by silent intervals ≥5 ms). Song “bouts” usually consist of several repeated introductory elements followed by one or more motifs separated by intervals of ≥500 ms (example, Fig. 2A). Individually identifiable syllables and motif structure are lost following bilateral HVC lesions, and subsequent Area X or DLM lesions further degrade song structure. Therefore, we defined song postlesion as a group of three or more song syllables separated from each other by <500 ms. Calls were defined as in Zann (1996). Most birds produced identifiable distance calls (or “long calls”) and an abundance of “tet” calls after lesion (Simpson and Vicario 1990). Tet calls could further be differentiated from song as they were often produced at intervals of ≥1 ms.
Fig. 2.
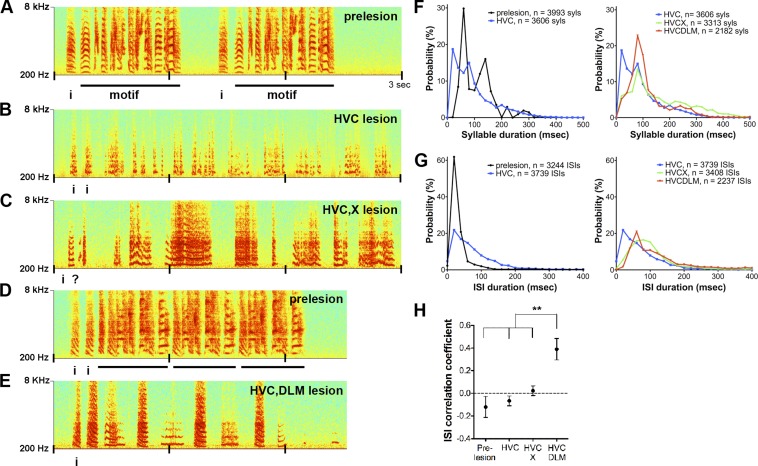
Comparison of songs produced after lesions to different nuclei of the AFP. A–E: examples of song spectrograms. A: 1 bout of song before lesion. Two motifs are shown; “i” refers to introductory notes (Supplemental Audio S1). B: song of same bird as in A following bilateral HVC lesions (histology shown in Fig. 1B; Supplemental Audio S2). Syllables from the bird's prelesion motif are no longer identifiable. There are many short syllables, and overall the song structure is noisier. C: song of bird in A and B, following bilateral Area X lesions (histology, Fig. 1C; Supplemental Audio S3). The bird has lost the short-duration syllables and intervals present in B and produces some syllables that are much longer than those of the prelesion song. D: song of a different bird prelesion (Supplemental Audio S4). E: song following HVC and DLM lesions (Supplemental Audio S5). Postlesions, the song has more regular syllable and interval durations than in B or C and a striking loss of frequency modulation (FM). F: distributions of syllable durations for each lesion group. Left: prelesion song (black) has several peaks in syllable duration, reflecting the average of many stereotyped syllable durations across birds and a maximum syllable duration of 360 ms. HVC-lesioned song (blue) has a peak ∼20 ms that does not exist in the other groups and has otherwise shifted toward an exponential distribution, reflecting the loss of syllable duration stereotypy in all birds. Right: the same data from HVC-lesioned birds plotted with data from double-lesioned birds. The distribution of syllables of HVC, X-lesioned song (green) has a long tail, indicating the presence of unusually long syllable durations in this group. HVC, DLM-lesioned song (red) has a peak at ∼100 ms and has few very short or very long syllables (syls). G: distributions of intersyllable interval (ISI) durations for each lesion group. Left: prelesion song has a sharp peak at 40 ms. ISIs produced by HVC-lesioned birds have a broader distribution, including both short and long ISIs. For HVC, X-lesioned birds and HVC, DLM-lesioned birds (right), the curves shift toward longer ISIs, indicating a slowing in song tempo. H: the correlation coefficient of consecutive ISIs for each group of birds. HVC, DLM-lesioned birds have significantly more reliable spacing between syllables than the other birds (P = 0.003, ANOVA; prelesion vs. HVC, DLM-lesioned, P < 0.001; HVC- and HVC, X-lesioned vs. HVC, DLM, P < 0.01, Tukey-Kramer posttest for n = 6 prelesion, 6 HVC-lesioned, 7 HVC, X-lesioned, and 6 HVC, DLM-lesioned birds). **P < 0.01. Error bars indicate SE.
Amount of singing.
To quantify amount of singing, we counted the number of seconds of singing within a 1-h session starting when the bird began singing in the morning. To analyze amount of singing post-HVC or -X lesion, we selected a period for analysis between 7 and 17 days, after the bird began singing with some regularity. Post-DLM lesion, we selected a period for analysis between 7 and 58 days. Two HVC, DLM-lesioned birds did not sing during this time period. One of the two never sang, and the other was not observed singing until ∼6 mo following lesions.
Song feature analysis.
Vocalizations were segmented into syllables and calls using semiautomated, amplitude-based, custom-written code in MATLAB (MathWorks). Experimenters verified boundaries for song elements by examining plots of frequency modulation (FM) vs. time. A total of 13,093 syllables (≥300 syllables from each bird in each condition) and 1,044 calls were used for audio analysis. For each song element, Sound Analysis Pro for MATLAB (SA+; Tchernichovski et al. 2000) was used to compute 4 acoustic feature time series: amplitude modulation [AM(t)], FM [FM(t)], entropy(t), and amplitude(t). These time series were then used to calculate mean AM, SD of AM, mean FM, SD of FM, mean entropy, SD of entropy, and coefficient of variation (CV) of amplitude. Duration of the syllable was added as the 8th acoustic feature. Therefore, each syllable was represented as a vector of 8 acoustic features. To extract the predominant acoustic variation across all of the syllables and birds in different experimental groups, principal component analysis (PCA) was performed on all of the acoustic feature vectors after normalization of each individual feature. If each acoustic feature contributed equally to the acoustic variation across all syllables and all birds, then every principal component would have the same magnitude for each of the eight acoustic features, i.e., ±0.354, so this value was used as a significance threshold for a bootstrapping test. To evaluate the statistical significance of each principal component, confidence intervals of principal components were calculated by bootstrapping, i.e., sampling birds with replacement.
Determination of Lesion Size
At the end of experiments, birds were intracardially perfused with 3.7% formaldehyde. Brains were cryoprotected, and 40-μm coronal or sagittal sections were cut on a freezing microtome. Each brain was divided into three sets with each set to be stained with a different antibody. Parvalbumin (PV) was used to identify HVC (Heyers et al. 2008). Sections were incubated in 1:10,000 mouse anti-PV (Sigma P3171). Rat anti-substance P was used at a dilution of 1:10,000 to identify any remaining Area X (Accurate Chemical and Scientific; Carrillo and Doupe 2004). Calbindin was used as a marker for DLM as well as HVC in some birds. Sections were incubated in 1:4,000 mouse anti-chicken calbindin (Sigma C8666). Although different antibodies raised against chicken or bovine calbindin were used in previous studies, we observed the same pattern of staining in unlesioned zebra finches as previously reported (Heyers et al. 2008; Pinaud et al. 2007; Wild et al. 2005). To ensure that LMAN was not damaged by lesions to more ventral brain structures, calcitonin gene-related peptide (CGRP) was used to identify LMAN (Bottjer et al. 1997). We incubated sections in a 1:10,000 dilution of rabbit anti-CGRP (Chemicon). Secondary antibodies (biotinylated anti-rat or anti-mouse IgG) were used at a 1:200 dilution for 1 h followed by detection using an ABC kit (Vector Laboratories, Burlingame, CA) and nickel-enhanced diaminobenzidine (see Carrillo and Doupe 2004 for details). Some sections were counterstained with cresyl violet acetate. In two of the DLM-lesioned birds, retrograde tracer was injected into LMAN to label any remaining DLM projection neurons (see below).
HVC was lesioned in 14 birds. In 11 of these birds, we were able to estimate lesion size, which ranged from 84 to 100% (example lesion in Fig. 1B). In the remaining 3 birds, we did not observe staining for HVC, but the caudal-most sections of the brain were lost during the histology procedures, so we were missing ∼200 μm of brain that might contain HVC. The small extension of HVC known as paraHVC (pHVC) may have remained partially intact in some birds, because it extends medial and deep to our HVC electrolytic lesion grid (Johnson and Bottjer 1995), but there was nothing obvious in our Nissl staining. Lesion size ranged from 70 to 100% in the 7 Area X-lesioned birds (example in Fig. 1C) and 85 to 100% in the 7 DLM-lesioned birds (example in Fig. 1D). Retrograde tracer injection into LMAN after DLM lesions did not label any cell bodies in the area of DLM, although we did observe labeled terminals in RA in these 2 birds (see results; Fig. 3, B–F). As our goal was to eliminate all of DLM, we also inadvertently lesioned the posterior portion of the dorsomedial thalamic nucleus (DMP) in most birds as well (see discussion). The thalamic nuclei ovoidalis and uvaeformis were spared based on histology and tests of birds' ability to hear following DLM lesions. CGRP staining indicated that LMAN was also spared in all lesioned birds (Fig. 3A).
Fig. 3.
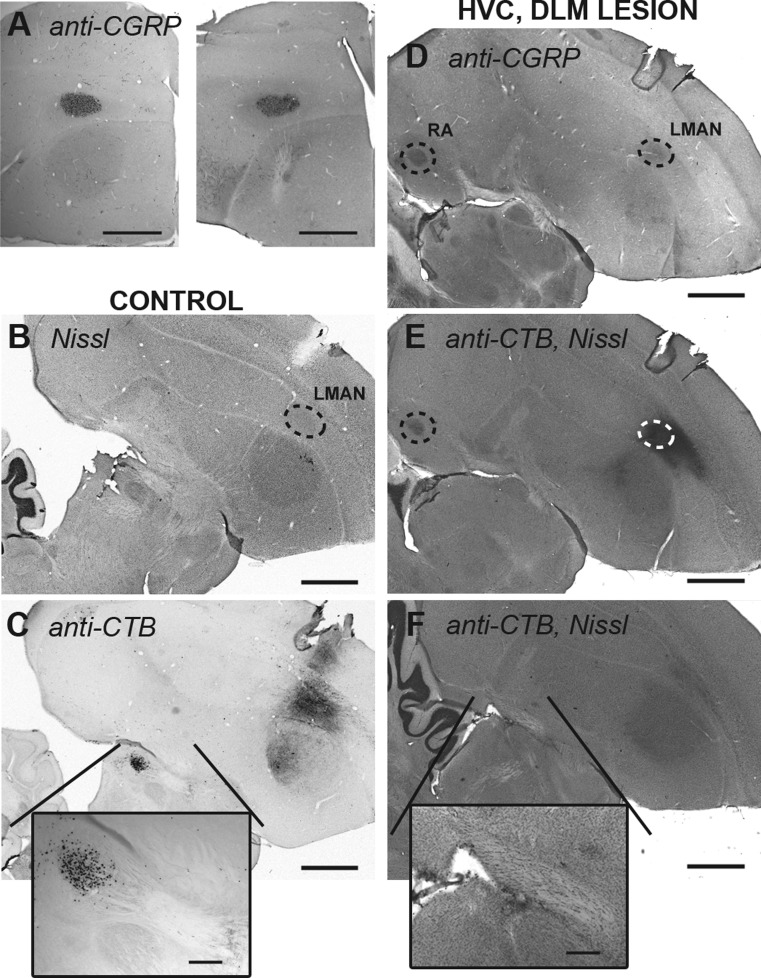
A: LMAN is spared by ibotenic acid lesions of Area X. Coronal sections of 1 hemisphere of a control bird (left) and a HVC, X-lesioned bird (right) show that LMAN is similarly stained by an anti-calcitonin gene-related peptide (anti-CGRP) antibody in both birds. B–F: cholera toxin B (CTB) tracing to verify DLM lesions. B: Nissl-stained sagittal section of a control bird. Dotted circle indicates LMAN. C: adjacent serial section of the control bird. Immunostaining for CTB shows the injection site (LMAN) and cell bodies retrogradely filled with CTB in DLM. Box shows magnified image of DLM. D: sagittal section of a HVC, DLM-lesioned bird stained for CGRP. Dotted circles indicate LMAN and CGRP-positive terminals in RA. E: adjacent serial section of the lesioned bird. Immunostaining for CTB shows the injection site (LMAN) and terminals in RA. F: section of lesioned bird's brain ∼300 μm medial to the section shown in E. No CTB-filled cells are visible in the thalamic region of this section or any adjacent section, suggesting that DLM was completely lesioned. The HVC, DLM-lesioned bird's brain was not cut at quite the same angle as the control bird; thus the region of thalamus that should contain DLM is medial to LMAN (note that RA does not appear in the control sections due to the different sectioning angle). Scale bars = 1 mm in A–F, 200 μm in magnifications.
RESULTS
To dissect the contributions of the striatopallidum and the thalamus to song in adult zebra finches, we first removed HVC, the major cortical input to the premotor nucleus RA (Fig. 1B). The surgical removal of HVC allowed us to examine song driven solely by the basal ganglia-thalamocortical circuit (AFP) outflow to the motor cortical nucleus RA (Fig. 1, E vs. F). If both HVC and the AFP outflow are disrupted, birds are mute (Fig. 1G; Aronov et al. 2008). When only HVC is bilaterally lesioned, however, birds still produce strings of song syllables, but these are no longer the highly stereotyped sequences of acoustically complex, identifiable syllables seen when HVC drives song (Aronov et al. 2008; compare Fig. 2, A vs. B and Supplemental Audio S1 vs. S2, available in the data supplement online at the Journal of Neurophysiology web site). In addition, the distribution of syllable durations changes from a distribution with many peaks (reflecting the average of many stereotyped syllable durations across birds) to a broad, unimodal distribution (Aronov et al. 2008; Fig. 2F, left). Along with these known effects of HVC lesions, we also found that HVC-lesioned birds produced many unusually short syllables, which are not observed in normal adult song [percentages of syllables with duration <35 ms: prelesion, 0.9 ± 0.7% (SE) vs. HVC-lesioned, 20.8 ± 7.9% in 6 birds; P = 0.0043, Wilcoxon rank sum test]. We used the data collected from HVC-lesioned birds, representing song driven by the intact AFP, as a baseline for our subsequent experiments on the role of different stages in the pathway.
Song Production
We then examined the contributions of Area X and DLM to AFP-mediated song production by performing bilateral lesions of Area X (Fig. 1, C and H) or DLM (Fig. 1, D and I) in HVC-lesioned birds. We verified that LMAN did not sustain damage from lesions to more ventral brain structures by staining for CGRP in all lesioned birds (see example, Fig. 3A). In addition to Nissl and calbindin staining in all DLM-lesioned birds, we verified the success of DLM lesions in two birds by injecting the retrograde tracer cholera toxin B into the target of DLM (LMAN) and confirming the absence of retrograde filling in DLM (Fig. 3, B–F; see materials and methods).
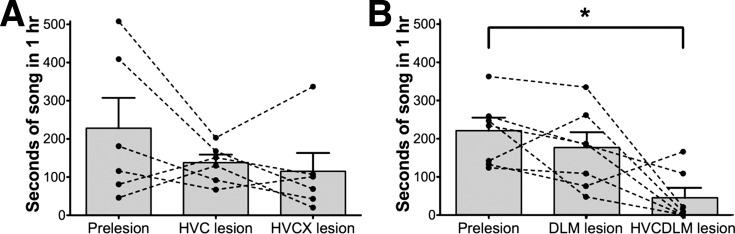
A recent study in juvenile birds suggested that DLM, but not Area X, was required for the generation of juvenile AFP-driven song (Goldberg and Fee 2011). Consistent with this finding, HVC, X-lesioned birds lesioned as adults also continued to sing robustly despite developmental differences in circuit connectivity (Fig. 4A). In contrast, HVC, DLM-lesioned birds lesioned as adults sang very little. They continued to produce distance calls and tet calls, and some birds produced syllables that resembled introductory notes (Zann 1996). These birds did not appear completely mute for song, however, because they occasionally produced strings of vocalizations that had features of song in several respects (Fig. 2E and Supplemental Audio S5). For one, these strings of vocalizations were often preceded by soft introductory notes. Moreover, they were sung at a slightly faster tempo, and with more variation in amplitude between individual vocalizations, than strings of tet calls, which are sung with stable amplitude and are generally separated by around 1 s or more. We thus defined all vocalizations that contained three or more vocal elements (excluding distance calls) with intersyllable interval (ISI) durations <500 ms as song (this criterion was used for all postlesion vocalizations described in this paper). Even with this broad definition of song, singing behavior was significantly impaired in HVC, DLM-lesioned birds (Fig. 4B). After lesions, HVC, DLM-lesioned birds sang on average 44 ± 25 s of song in 1 h compared with the 215 ± 33 s these same birds sang before lesions (n = 7 birds; P < 0.05, Dunn's multiple comparisons test). Four of seven birds sang <10 s of song in 1 h. In contrast, all six HVC, X-lesioned birds sang >10 s of song in 1 h, and on average these birds sang as much as they did before Area X lesions (Fig. 4A). Note that the six HVC, X-lesioned birds shown here received HVC lesions first, so we compared the amount of singing after HVC lesions alone with the amount of singing after double lesions and did not find a difference. HVC, DLM-lesioned birds received DLM lesions first (see materials and methods), so we do not have data for HVC lesions alone in these birds, but we did not see a decrease in singing after DLM lesions alone (Fig. 4B). The continued production of song after HVC and Area X double lesions demonstrates that, despite the striking recurrent structure of the AFP, the entire loop is not necessary for vocal production in adult birds. Rather, subsonglike vocalizations continued to be produced by the remaining DLM → LMAN → RA pathway. However, even using a broad definition of song, we found birds with double lesions of HVC and DLM were greatly inhibited in song production relative to birds with double lesions of HVC and Area X. Thus our finding suggests that, in adults as in juveniles, song thalamus, but not striatopallidum, strongly influences initiation of AFP-driven song.
Fig. 4.
The amount of song produced by birds with HVC and DLM double lesions is dramatically reduced. A: the average amount of song produced in 1 h by 6 birds prelesion, after HVC lesions alone, and after HVC, Area X lesions [mean for each group: prelesion = 224 ± 78 s (SE); HVC-lesioned = 135 ± 20 s; HVC, X-lesioned = 113 ± 47 s]. Although there was a tendency for birds to sing less after HVC lesions, the amount of singing across birds was not significantly different between conditions (P = 0.2522, Friedman test). B: in contrast, the average amount of song produced in 1 h dropped significantly across birds after HVC, DLM lesions, although not after DLM lesions alone (mean for each group: prelesion = 215 ± 33 s; DLM-lesioned = 171 ± 39 s; HVC, DLM-lesioned = 44 ± 25 s; P = 0.0272, Friedman test; prelesion vs. HVC, DLM-lesioned, P < 0.05, Dunn's multiple-comparison posttest; n = 7 birds). *P < 0.05. Error bars indicate SE.
Song Tempo
Although double-lesioned birds continued to sing after Area X or DLM lesions, these vocalizations were very different from the vocalizations they produced after HVC lesions alone. Strikingly, one effect of Area X lesions was the lengthening both of many syllables, as well as of ISI durations, compared with prelesion and HVC-lesion song, resulting in slowed tempo (Fig. 2C vs. Fig. 2, A and B). After HVC and Area X double lesions, the distribution of syllable durations shifted to longer durations (Fig. 2F, right), and the percentage of syllables >300 ms was significantly greater than in HVC-lesioned birds (HVC, X-lesioned = 12.8 ± 4.1%, n = 7 birds, vs. HVC-lesioned = 2.3 ± 1.1%, n = 6 birds; P = 0.035, Wilcoxon rank sum test). In addition, the distribution of ISI durations was also shifted to longer durations (Fig. 2G, right), and the mean ISI duration was significantly greater in HVC, X-lesioned birds than in HVC-lesioned birds [HVC, X-lesioned = 107.2 ± 76.9 ms (SD) vs. HVC-lesioned = 79.8 ± 76.9 ms; P < 0.001, Dunn's multiple-comparison test].
Songs of HVC, DLM-lesioned birds were also slowed, with even longer ISI durations [Fig. 2G; HVC, DLM-lesioned = 130.8 ± 99.6 ms (SD) vs. HVC, X-lesioned; P < 0.001, Dunn's multiple-comparison test]. The slow song they produced, however, no longer included any of the extremely long syllables (>300 ms) observed in the HVC, X-lesioned birds (HVC, DLM-lesioned = 3.0 ± 2.2% of syllables, n = 6 birds; P < 0.05 vs. HVC-X lesioned birds; Fig. 2F, right). In addition to the slowing of their tempo, songs produced by HVC, DLM-lesioned birds had a striking regularity of tempo that is unusual for zebra finches. To quantify this apparent regularity, we first calculated the CV of ISIs for each bird. The CV of ISIs had a wide range in unlesioned birds, from 0.66 to 1.48. The CV of ISIs had a smaller range in HVC, DLM-lesioned birds, from 0.48 to 0.91. Although the mean CVs were significantly different between groups (prelesion = 1.06 ± 0.11 vs. HVC, DLM-lesioned = 0.67 ± 0.07; P = 0.0182; n = 6 birds), we did not feel that this measurement fully captured the tempo regularity that we observed within individual songs. The CV of ISIs compares the variability of ISIs across all of the songs that a bird sings. HVC, DLM-lesioned birds did not necessarily have ISIs of the same duration from song to song (if so, the CV of ISIs would be close to 0). Therefore, we also calculated the correlation coefficient between pairs of consecutive ISIs. The mean ISI correlation coefficient was close to 0 in HVC- and HVC, X-lesioned birds, indicating that the duration of consecutive gaps between syllables was unrelated and thus variable in these birds. In contrast, the correlation coefficient increased to ∼0.40 in songs of HVC, DLM-lesioned birds (Fig. 2H). Thus DLM appears to be important for both tempo and the normal variability in spacing between syllables of AFP-mediated song.
Song Acoustic Structure
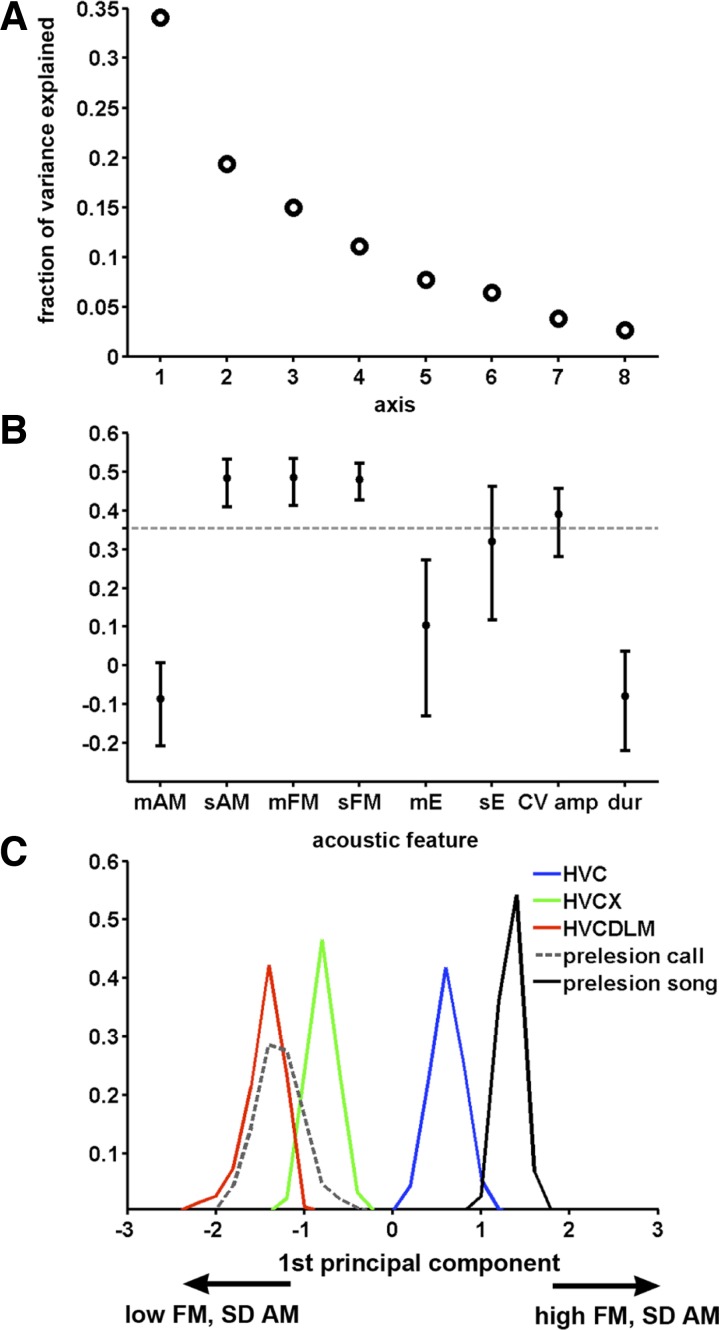
In addition to effects on amount of song production and song tempo, lesions to the AFP affected the acoustic structure of song. Although these lesions eliminated the normal adult stereotypy of syllables, experimenters could still distinguish lesion groups by qualitative differences in songs from different groups. For example, song from HVC-lesioned birds generally had a rapidly varying, noisy quality (compare Supplemental Audio S1 vs. S2) that became much less apparent after Area X was also lesioned, due to a decrease in the modulation of acoustic structure within syllables (Fig. 2, B vs. C, and Supplemental Audio S2 vs. S3). This effect was even stronger in birds with lesions closest to the outflow of the AFP (i.e., DLM lesions) so that birds that sustained HVC and DLM lesions produced strings of vocalizations almost call-like in character (Fig. 2E and compare Supplemental Audio S5 with S4). To capture these differences quantitatively, we performed a PCA in which each syllable was represented as a vector of 8 features, including mean and SD of AM, mean and SD of FM, mean and SD of entropy, CV of amplitude throughout the syllable, and syllable duration (note that SD here is measuring variation of the feature between 8-ms windows within 1 syllable rather than trial-to-trial variation of the feature between different syllables). Syllables were taken from 5 experimental groups: songs from prelesion birds, songs from HVC-, HVC, X- and HVC, DLM-lesioned birds, and calls from prelesion birds. The 1st principal axis (PC1) explained 34% of the variance between the 5 groups (Fig. 5A; see materials and methods). A permutation test on PC1 indicated that SD of AM, mean and SD of FM, and, to a lesser extent, the CV of amplitude were the important distinguishing features (Fig. 5B). In contrast, the other principal components each explained <19% of the acoustic variation, and they did not have acoustic features for which the magnitudes exceeded the significance threshold (see materials and methods).
Fig. 5.
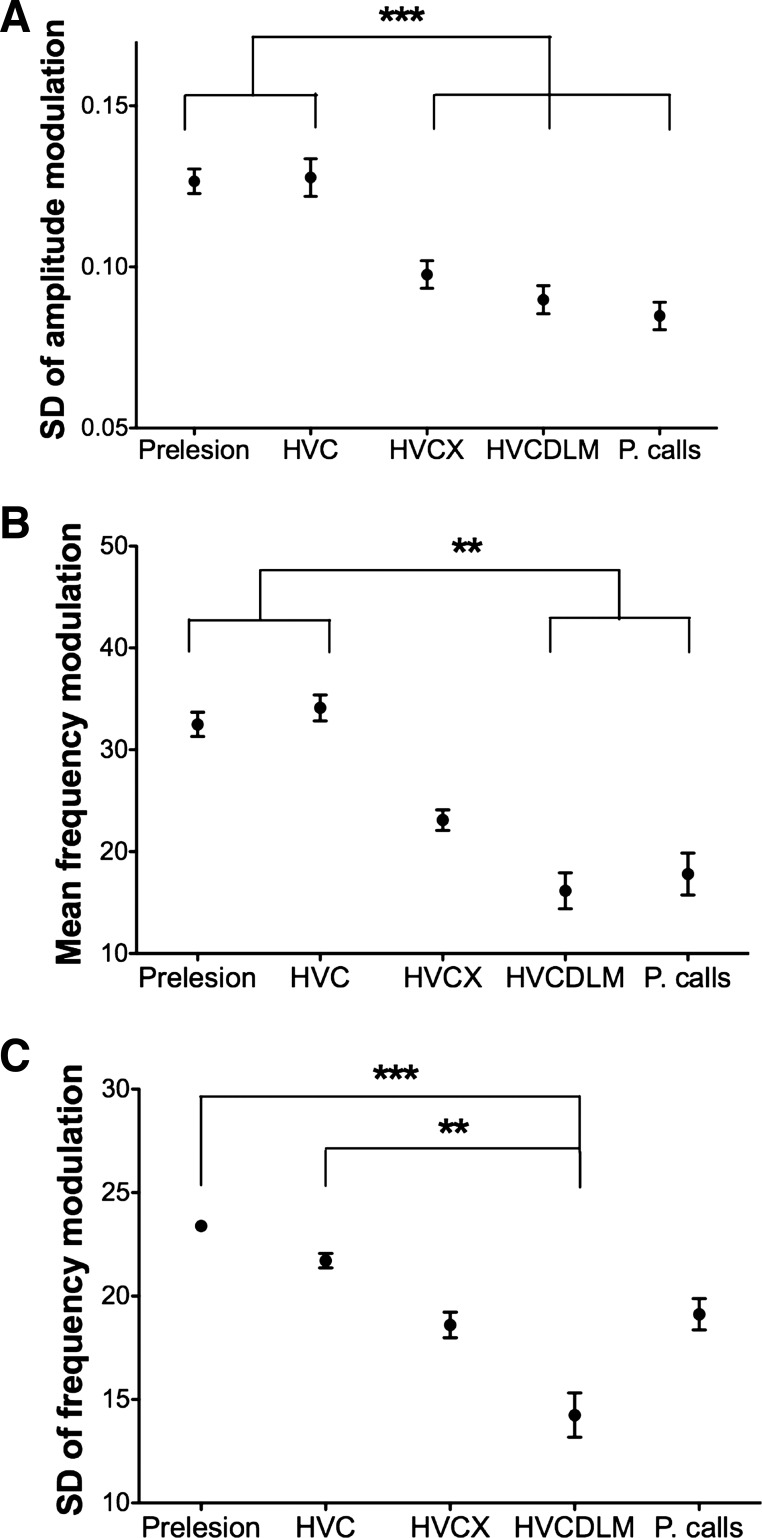
Vocalizations become more call-like as more of the AFP is eliminated. A: the fraction of variance explained by each principal component. The 1st principal component (PC1) explains 34% of the variance. B: graphic representation of PC1 (normalized). Points indicate median, and error bars indicate 95% confidence interval for each feature from bootstrapping on birds. Features with values >0.354 (points above the dashed line) contributed the most to the acoustic variation along the axis. For PC1, these were SD of amplitude modulation (sAM), mean FM (mFM), SD of FM (sFM), and coefficient of variation of amplitude (CV amp), and they are positively correlated with each other along PC1. mAM, mean AM; mE, mean entropy; sE, SD of entropy; dur, duration. C: bootstrap estimation of the median location of the syllable projections for each group onto PC1. For each group, the median location of the projection is represented by a distribution that shows the best estimate (peak) and confidence interval (spread). Birds sustaining only HVC lesions (blue) produce syllables most similar to song syllables (black), whereas birds with both HVC and DLM lesions (red) produce syllables most like prelesion calls (dotted). Birds with both HVC and X lesions (green) produce syllables with characteristics falling between these 2 groups. The positive direction of the x-axis indicates high SD of AM and high mean and SD of FM.
PCA revealed clear differences between each lesion group when the population of syllables from each group was projected onto PC1. To ensure that syllables from one bird did not skew the overall distribution, we recalculated the distribution of the projections by resampling (with replacement) the experimental birds included in the PCA. The medians of these projections are plotted as peaks in Fig. 5C, with the statistical spread of the median (confidence intervals) shown by the distribution around each peak. This plot reveals a systematic change in song structure with each lesion. The syllables of prelesion songs and calls have a characteristic location along the projection axis: song syllables from prelesion birds are distributed to the far right side of the axis (indicating high SD of AM and mean and SD of FM), whereas calls from prelesion birds, with their simple harmonic structure, are distributed to the leftmost side of the axis. Song syllables from HVC-lesioned birds lie closest in the distribution to those of prelesion birds. When Area X is lesioned in addition to HVC, the distribution of song syllables shifts substantially leftward from that of HVC-lesioned birds, indicating that syllables produced after Area X lesions have less AM and FM. Syllables produced by HVC, DLM-lesioned birds have a distribution that is shifted even further leftward, essentially overlapping with the distribution of calls from unlesioned birds.
We then examined separately the three song features that emerged as most important for differentiating lesion groups in the PCA (SD of AM and mean and SD of FM). All three of these features capture structural aspects of syllables. The SD of AM describes time-varying changes in loudness during a syllable. FM describes changes in frequency during a syllable; stacklike syllables have low FM, whereas syllables for which frequencies change from high to low (or vice versa) over time have high FM. The SD of FM captures changes in FM; for instance, it would be high for syllables that sweep first up and then down in frequency or that waver in pitch. In HVC-lesioned birds, individual syllables were modulated in the amplitude and frequency domains to the same extent as prelesion syllables (Fig. 6, A–C). Thus the AFP on its own can produce syllables that are in many ways as structurally complex as those of normal adult birds. In contrast, HVC, X-lesioned birds had significantly lower SD of AM within syllables than prelesion and HVC-lesioned birds (Fig. 6A). SD of AM did not further decrease in HVC, DLM-lesioned birds. Thus lesions of Area X in addition to HVC especially affected the time-varying AM of song. Lesions of DLM, on the other hand, stripped song of FM. HVC, DLM double-lesioned birds had significantly lower mean and SD of FM than birds before lesion or HVC-lesioned birds (Fig. 6, B and C). The songs of HVC, Area X-lesioned birds had measures of FM that fell between HVC, DLM-lesioned birds and controls (Fig. 6, B and C).
Fig. 6.
FM and AM are affected by Area X and DLM lesions. A: mean of SD of AM for each group of birds. Syllables from HVC, X- or HVC, DLM-lesioned birds, and prelesion calls (P. calls) have significantly lower variability in AM than syllables from prelesion and HVC-lesioned birds (P < 0.001, prelesion or HVC-lesioned vs. HVC, X-lesioned, HVC, DLM-lesioned, or prelesion calls, Tukey-Kramer posttest. No other pairs were significantly different). B: mean FM for each group of birds. Syllables from HVC, DLM-lesioned birds and prelesion calls have significantly less FM than syllables from prelesion and HVC-lesioned birds (P < 0.01, prelesion or HVC-lesioned vs. HVC, DLM-lesioned or prelesion calls, Dunn's posttest). Syllables from HVC, X-lesioned birds show a nonsignificant decrease in FM compared with prelesion syllables. C: mean of SD of FM for each group of birds. Syllables from HVC, DLM-lesioned birds have significantly less SD of FM than syllables from both prelesion (P < 0.001, Dunn's posttest) and HVC-lesioned birds (P < 0.01). Syllables from HVC, X-lesioned birds have significantly lower variability in FM than syllables from prelesion birds (P < 0.05) but are not significantly different from syllables from HVC-lesioned birds. For A–C, number of birds: n = 6 prelesion, n = 6 HVC, n = 7 HVC, X, n = 6 DLM, HVC, and n = 5 prelesion calls. **P < 0.01; ***P < 0.001. Error bars indicate SE.
In sum, AFP-driven song was similar to normal adult song as measured by the degree of FM and AM within syllables. However, this within-syllable modulation began to diminish as stages of the AFP circuit were removed. In particular, AM was greatly diminished following removal of basal ganglia alone (with no further reduction following thalamic lesions). Both mean FM and variability of FM also decreased following the loss of the basal ganglia but decreased much further following the loss of the thalamus: deafferented LMAN input to RA was not capable of inducing AM and FM beyond the level of normal calls. Taken together, the PCA and individual feature analysis describe a continuum of syllable complexity phenotypes ranging from normal adult song to calls.
DISCUSSION
This study demonstrates that the vocal striatopallidum and thalamus are important for different aspects of AFP-driven song production. Specifically, lesions of the striatopallidum (Area X) resulted in songs that were slowed in tempo and had less modulation of acoustic structure, especially time-varying AM. Disruptions of the thalamic nucleus DLM, on the other hand, dramatically affected AFP-dependent song initiation. Moreover, the very few songs that HVC, DLM-lesioned birds could produce were strikingly regular in tempo with very simple acoustic structure and invariant pitch. The marked difference between the effects of basal ganglia and thalamic lesions suggests that the vocal thalamus does not just passively transmit signals from the basal ganglia to the cortex in adulthood. Rather, it may be an important entry point into the AFP for signals critical to song initiation.
Our finding that lesions of the striatopallidum (Area X) affect both the acoustic structure and tempo of AFP-driven song contrasts with a recent study suggesting little effect of Area X lesions on juvenile song (Goldberg and Fee 2011). However, that work focused largely on differences in the structure of syllables between separate renditions of a bird's song. Here, we measured acoustic variability within single syllables to assess their structural complexity. The study of Area X lesions in juveniles also assessed tempo but noted that results were hard to interpret because, in contrast to our adult birds' stable AFP-mediated song, syllable durations were changing in the days before lesions in juveniles (Goldberg and Fee 2011). The reduced AM and tempo of song that we observed after striatopallidal lesions is reminiscent of the reduced AM of movements (including low speech volume) and bradykinesia observed in human basal ganglia disorders (Mazzoni et al. 2007; Trail et al. 2005; Viviani et al. 2009). These convergent phenotypes of striatopallidal damage in birds and humans suggest that the striatopallidum is normally important for modulating the amplitude and tempo of movements.
Surprisingly, the disruption of the AFP loop at the level of the striatopallidum did not significantly affect the generation of AFP-driven adult song. This result eliminates the possibility that AFP-driven song is generated by intrinsic Area X activity, by reverberant activity within the loop via the recurrent projection from LMAN, or by other known inputs to Area X, such as the ventral tegmental area. Instead, song production was severely reduced after HVC, DLM double lesions, a finding similar to that observed in juvenile birds (Goldberg and Fee 2011). In addition, both our adult experiments and the juvenile lesion experiments indicate that DLM contributes to rhythm. The similar results of studies in juvenile and adult birds suggest that the DLM lesion effects are not a consequence of disrupted development and provide strong support for the hypothesis that the avian thalamus is central to the contribution of the AFP to song initiation and rhythm. These findings raise the possibility that in mammals as well, the thalamus may be especially important in movement initiation and pacing. Although strokes rarely involve just the thalamus, it has been noted that thalamic damage can have effects on speech, including aphasia and even akinetic mutism (Carrera and Bogousslavsky 2006; De Witte et al. 2011; Nagaratnam et al. 1999; Shinoda et al. 1993).
The dorsal thalamus of songbirds contains several areas related to song. In particular, there is a more anterior portion that projects to LMAN and shows specific induction of the IEG dusp1 during singing (Horita et al. 2012) and a more posterior dorsomedial thalamic nucleus (DMP) that projects to medial magnocellular nucleus of the anterior neostriatum (MMAN). Our HVC, DLM-lesioned birds also sustained damage to DMP (Bottjer et al. 2000; Foster et al. 1997; Vates et al. 1997). However, DMP damage is unlikely to have caused the differences in song phenotypes observed between Area X- and DLM-lesioned birds. DMP projects via MMAN to HVC and to pHVC, a thin strip of estrogen-concentrating neurons lining the lateral ventricle (Bottjer et al. 1989; Foster and Bottjer 2001; Foster et al. 1997; Gahr and Metzdorf 1997; Nordeen et al. 1987). As we bilaterally lesioned HVC in all of our birds, information could not travel from DMP to RA via HVC. Moreover, pHVC has few or no direct projections to RA but primarily projects to Area X (Foster and Bottjer 1998). Thus, in HVC, Area X-lesioned birds, there is no route for information from DMP/MMAN to affect song. All differences between the HVX, Area X and HVC, DLM double-lesion groups were therefore due to differing effects of Area X and DLM lesions.
The deficit in song initiation and the stripping away of acoustic complexity after loss of the thalamus, but not the striatopallidum, could result from differing effects on cortical activity after loss of these two nuclei. Consistent with this possibility, Area X inactivation, which eliminates Area X inhibitory outputs to thalamus, is known to increase acutely both DLM and LMAN activity (Kojima and Doupe 2009), which could drive song. In contrast, the inputs of DLM to LMAN are excitatory, and the loss of these might eliminate any motor-related activity in the cortical nucleus, pushing the bird toward song simplification and mutism. An additional possibility is that the DLM lesions eliminate inputs to DLM, for which the importance to singing has been overlooked thus far. One obvious possibility is the previously described input from RA to DLM, which is presumed to be glutamatergic and could carry an efference copy from motor and premotor areas (Vates et al. 1997; Wild 1993). In juvenile birds, this input seems to drive song-locked rate modulations in DLM (Goldberg and Fee 2012), consistent with recent recordings of activity during singing in adult LMAN after complete, bilateral Area X lesions (Kojima et al. 2013), although the LMAN singing-related activity after Area X lesions was notable for being tonic without much song-locking. Notably, in the experiments here, HVC is eliminated, so the additional activity important to song initiation would of necessity come from RA without any premotor contribution from HVC. The RA-DLM input, however, is noted to be sparse in adult birds (Vates et al. 1997; Wild 1993), and we, too, have found very few RA neurons retrogradely labeled from DLM in unlesioned adult birds (J. R. Chen, L. Stepanek, and A. J. Doupe, unpublished observations). Nonetheless, this sparse input might provide enough excitatory drive to DLM to initiate song or might increase in strength after DLM loses its input from Area X.
The highlighting of different contributions of thalamus and striatopallidum, and the further identification of the vocal thalamus as a key locus for the generation or transmission of signals that influence song production, not only should stimulate further understanding of vocal production and plasticity, but also could provide insights of very general relevance to mammalian systems. HVC-lesioned birds may serve as a useful model for examining the relationship between striatopallidal neural activity and control of movement amplitude and tempo. Songbirds may also provide a particularly tractable system in which to study initiation of motor programs, as neural activity can be recorded or manipulated in a thalamic subregion that is specific to a particular motor behavior.
GRANTS
This work was supported by National Institutes of Health Grants MH-55897 and RC2-NS-069350 to A. J. Doupe and a Brain & Behavior Research Foundation NARSAD Distinguished Investigator Award (A. J. Doupe).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R.C. and A.J.D. conception and design of research; J.R.C. performed experiments; J.R.C. and L.S. analyzed data; J.R.C. and L.S. interpreted results of experiments; L.S. prepared figures; L.S. drafted manuscript; L.S. and A.J.D. edited and revised manuscript; J.R.C., L.S., and A.J.D. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Arteseros and S. Kojima for technical assistance and M. S. Brainard, M. H. Kao, H. Mehaffey, and T. L. Warren for critical reading of the manuscript.
REFERENCES
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA 106: 12518–12523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320: 630–634, 2008 [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol 420: 244–260, 2000 [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol 279: 312–326, 1989 [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901–903, 1984 [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Roselinsky H, Tran NB. Sex differences in neuropeptide staining of song-control nuclei in zebra finch brains. Brain Behav Evol 50: 284–303, 1997 [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766, 2000 [DOI] [PubMed] [Google Scholar]
- Bruce BB, Foote KD, Rosenbek J, Sapienza C, Romrell J, Crucian G, Okun MS. Aphasia and thalamotomy: important issues. Stereotact Funct Neurosurg 82: 186–190, 2004 [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology 66: 1817–1823, 2006 [DOI] [PubMed] [Google Scholar]
- Carrillo GD, Doupe AJ. Is the songbird Area X striatal, pallidal, or both? An anatomical study. J Comp Neurol 473: 415–437, 2004 [DOI] [PubMed] [Google Scholar]
- Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature 486: 251–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Marien P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex 47: 273–319, 2011 [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster EF, Bottjer SW. Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male zebra finches. J Comp Neurol 397: 118–138, 1998 [PubMed] [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol 46: 142–165, 2001 [DOI] [PubMed] [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol 382: 364–381, 1997 [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull 44: 509–517, 1997 [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nat Neurosci 15: 620–627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. Vocal babbling in songbirds requires the basal ganglia-recipient motor thalamus but not the basal ganglia. J Neurophysiol 105: 2729–2739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton CM, Sakata JT, Brainard MS. An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song. J Neurophysiol 101: 3235–3245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci 19: 10461–10481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyers D, Manns M, Luksch H, Gunturkun O, Mouritsen H. Calcium-binding proteins label functional streams of the visual system in a songbird. Brain Res Bull 75: 348–355, 2008 [DOI] [PubMed] [Google Scholar]
- Horita H, Kobayashi M, Liu WC, Oka K, Jarvis ED, Wada K. Specialized motor-driven dusp1 expression in the song systems of multiple lineages of vocal learning birds. PLoS One 7: e42173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA 94: 4097–4102, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21: 775–788, 1998 [DOI] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19: 1049–1059, 1997 [DOI] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Differential estrogen accumulation among populations of projection neurons in the higher vocal center of male canaries. J Neurobiol 26: 87–108, 1995 [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006 [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638–643, 2005 [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28: 13232–13247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Doupe AJ. Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning. J Neurosci 29: 4782–4793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kao MH, Doupe AJ. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci USA 110: 4756–4761, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci 27: 7105–7116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merello M, Starkstein S, Nouzeilles MI, Kuzis G, Leiguarda R. Bilateral pallidotomy for treatment of Parkinson's disease induced corticobulbar syndrome and psychic akinesia avoidable by globus pallidus lesion combined with contralateral stimulation. J Neurol Neurosurg Psychiatry 71: 611–614, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaratnam N, McNeil C, Gilhotra JS. Akinetic mutism and mixed transcortical aphasia following left thalamo-mesencephalic infarction. J Neurol Sci 163: 70–73, 1999 [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Deafening-induced vocal deterioration in adult songbirds is reversed by disrupting a basal ganglia-forebrain circuit. J Neurosci 30: 7392–7400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ, Arnold AP. Estrogen accumulation in zebra finch song control nuclei: implications for sexual differentiation and adult activation of song behavior. J Neurobiol 18: 569–582, 1987 [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457–486, 1976 [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: e153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. Pallidal neuron activity increases during sensory relay through thalamus in a songbird circuit essential for learning. J Neurosci 27: 8687–8698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Saldanha CJ, Wynne RD, Lovell PV, Mello CV. The excitatory thalamo-“cortical” projection within the song control system of zebra finches is formed by calbindin-expressing neurons. J Comp Neurol 504: 601–618, 2007 [DOI] [PubMed] [Google Scholar]
- Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J Comp Neurol 469: 239–261, 2004 [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896–2913, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Tsugu A, Oda S, Masuko A, Yamaguchi T, Tsugane R, Sato O. Development of akinetic mutism and hyperphagia after left thalamic and right hypothalamic lesions. Childs Nerv Syst 9: 243–245, 1993 [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci 10: 1541–1556, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53: 51–63, 1990 [DOI] [PubMed] [Google Scholar]
- Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol 104: 2474–2486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000 [DOI] [PubMed] [Google Scholar]
- Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J Neurosci 27: 12308–12320, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson's disease. NeuroRehabilitation 20: 205–221, 2005 [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol 20: 704–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol 380: 275–290, 1997 [PubMed] [Google Scholar]
- Viviani P, Burkhard PR, Chiuvé SC, Corradi-Dell'Acqua C, Vindras P. Velocity control in Parkinson's disease: a quantitative analysis of isochrony in scribbling movements. Exp Brain Res 194: 259–283, 2009 [DOI] [PubMed] [Google Scholar]
- Warren TL, Tumer EC, Charlesworth JD, Brainard MS. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J Neurophysiol 106: 1806–1821, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Dostrovsky JO. Pathological basal ganglia activity in movement disorders. Neuroscience 198: 232–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338: 225–241, 1993 [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Howie GJ, Mooney R. Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J Comp Neurol 483: 76–90, 2005 [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol 39: 14–28, 1999 [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol 22: 387–393, 2009 [DOI] [PubMed] [Google Scholar]
- Zann RA. Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford Univ. Press, 1996 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.