Abstract
The inhibitory circuits of the striatum are known to be critical for motor function, yet their contributions to Parkinsonian motor deficits are not clear. Altered firing in the globus pallidus suggests that striatal medium spiny neurons (MSN) of the direct (D1 MSN) and indirect pathway (D2 MSN) are imbalanced during dopamine depletion. Both MSN classes receive inhibitory input from each other and from inhibitory interneurons within the striatum, specifically the fast-spiking interneurons (FSI). To investigate the role of inhibition in maintaining striatal balance, we developed a biologically-realistic striatal network model consisting of multicompartmental neuron models: 500 D1 MSNs, 500 D2 MSNs and 49 FSIs. The D1 and D2 MSN models are differentiated based on published experiments of individual channel modulations by dopamine, with D2 MSNs being more excitable than D1 MSNs. Despite this difference in response to current injection, in the network D1 and D2 MSNs fire at similar frequencies in response to excitatory synaptic input. Simulations further reveal that inhibition from FSIs connected by gap junctions is critical to produce balanced firing. Although gap junctions produce only a small increase in synchronization between FSIs, removing these connections resulted in significant firing differences between D1 and D2 MSNs, and balanced firing was restored by providing synchronized cortical input to the FSIs. Together these findings suggest that desynchronization of FSI firing is sufficient to alter balanced firing between D1 and D2 MSNs.
Keywords: Parkinson's disease, striatum, medium-spiny neuron, fast-spiking interneuron, gap junctions
dopamine depletion leads to an imbalance in the activation of the direct and indirect pathways of the striatum (Albin et al. 1989; DeLong 1990; Hikosaka et al. 2000; Mallet et al. 2006; Obeso et al. 2004). The direct pathway is composed of striatal medium spiny neurons (MSNs) that express dopamine D1 receptors (D1 MSNs) and projects to both the substantia nigra pars reticulata and the internal segment of the globus pallidus. The indirect pathway is composed of MSNs that express dopamine D2 receptors (D2 MSNs) and projects to the external segment of the globus pallidus. The two populations of MSNs differ in their intrinsic excitability, with D2 MSNs more responsive to somatic current injection (Gertler et al. 2008). Inhibitory circuits of the striatal network, including feedback inhibition from other MSNs and feedforward inhibition from fast-spiking interneurons (FSIs), modulate this excitability (Koós and Tepper 1999; Plenz 2003). The contributions of these circuits to maintaining balanced firing between D1 and D2 MSNs in the network (Cui et al. 2013; Mallet et al. 2006) are not clear.
A strong source of GABAergic inhibition to MSNs is feedforward inhibition from the parvalbumin-positive (PV+) FSIs (Koós and Tepper 1999). FSIs receive convergent input from multiple cortical regions (Ramanathan et al. 2002) and are interconnected by gap junctions that increase synchronicity of FSI firing (Hjorth et al. 2009; Traub et al. 2001). Each FSI in turn projects to over 100 MSNs (Bennett and Bolam 1994; Kita et al. 1990), and therefore synchronous activation of FSIs can have wide-spread effects. Dopamine depletion reduces the immediate-early gene response of PV+ neurons in the striatum (Trevitt et al. 2005), and, therefore, loss of dopamine might release MSNs from normal FSI inhibition, thus affecting the activation of the direct and indirect pathways. The effect of synchronicity of FSI firing, specifically the modulation of gap junctions, on MSN output and its contribution to striatal balance is not known.
Another source of GABAergic inhibition to MSNs is feedback inhibition from other MSNs. One of the differences between MSN and FSI input to MSNs is in their strength of inhibition (Koós and Tepper 1999). Multiple factors contribute to this difference in strength, including the increased level of synchronization between FSIs, and the more proximal distribution of FSI synapses. Since it is difficult to investigate the effects of MSN inhibition experimentally, a realistic network model of the striatum is a plausible alternative approach.
In this paper, a computational model of the striatal network, consisting of multicompartmental models of 1,000 MSNs and 49 FSIs, was used to identify the inhibitory mechanisms critical for striatal balance. Simulation experiments show that network interactions produced balanced firing, which is disrupted with selective removal of FSI input, but not with removal of MSN input. The balancing effect of FSI inhibition is disrupted when gap junctions between FSIs are removed, suggesting that the base level of synchronization that these connections provide could be critical for FSIs to balance striatal firing.
MATERIALS AND METHODS
Ethics statement.
All animal handling and procedures were in accordance with the National Institutes of Health animal welfare guidelines and were approved by the George Mason University institutional animal care and use committee.
D1 and D2 MSN models.
D1 and D2 MSN models were generated by modifying a previously published MSN model (Evans et al. 2012). The morphology of both MSN models was the same as this previous model, except with the spines removed (to improve computational efficiency), and consisted of 189 compartments with 4 primary dendrites which divide into 8 secondary and then 16 tertiary dendrites. Each primary dendrite was 20 μm long, secondary dendrites were 24 μm long, and tertiary dendrites were composed of 11 compartments, each 36 μm long. The kinetics of the channels included in the model were identical to the previous model (Evans et al. 2012). D1 and D2 MSN models were created by changing the maximal conductance of intrinsic and synaptic channels from values used for our previous MSN model (Table 1), based on experimental data measuring the effect of D1 or D2 receptor agonists, as summarized in Nicola et al. (2000) and Moyer et al. (2007). The maximal conductances of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors of both MSN classes were additionally adjusted to maintain the NMDA-to-AMPA ratio of 2.75:1 measured in cortico-striatal terminals from dorsal striatum of adult animals (Ding et al. 2008), as used previously (Evans et al. 2012). Note that this value is considerably greater than the NMDA-to-AMPA ratio measured in the striatum from other striatal regions (ventral −0.22; Popescu et al. 2007) or from younger animals (∼1.0; Logan et al. 2007).
Table 1.
Modulation of channel conductances
| Channels | D1 | D2 |
|---|---|---|
| Fast sodium channel (NaF) | 95 | 110 |
| Slow A-type potassium channel (KAs) | No change | 110 |
| Inward-rectifying potassium channel (KIR) | 125 | No change |
| L-type calcium (CaL1.2) | 200 | No change |
| L-type calcium (CaL1.3) | No change | 75 |
| NMDA (synaptic) | 130 | No change |
| AMPA (synaptic) | No change | 80 |
Values are % conductance value reported in Evans et al. (2012).
For simulations that investigate the contribution of morphology differences between D1 and D2 MSNs in explaining differences in excitability, the number of primary dendrites for D1 MSNs was increased from 4 to 6 based on reconstructions of these neurons (Gertler et al. 2008). The intrinsic channel properties of both MSN classes were set to match those of a D2 MSN for these simulations since this produced frequency-current (F–I) curves that matched experimental results most accurately.
FSI network.
A previously published FSI network model was used (Hjorth et al. 2009) and extended to include chemical synapses, as seen experimentally (Gittis et al. 2010). Each FSI in this network consisted of 127 compartments with a soma and 2 primary dendrites, which divide into 4 secondary and 8 tertiary dendrites. The channels incorporated in this model included a fast-sodium channel (NaF), voltage-dependent K+ (Kv) 3.1, Kv1.3 and slowly inactivating A-type (transient) potassium channel (KAs) (Kotaleski et al. 2006). The gap junction connections between the FSIs were modeled as resistive elements between the primary dendrites, with a conductance of 0.5 nS, coupling coefficient of 25% and probability of gap junction connection between nearby FSIs of 0.3 (Galarreta and Hestrin 2002; Hjorth et al. 2009; Koós and Tepper 1999). Chemical synapses were GABAergic, chloride-permeable channels [rise time constant, 1.33 ms; decay time constant, 4 ms; reversal potential, −60 mV; maximal conductance, 1 nS; (Gittis et al. 2010)]. The probability of chemical synapse connection between FSIs was 0.58 (Gittis et al. 2010) and was independent of the probability of gap junction connection.
Striatal network.
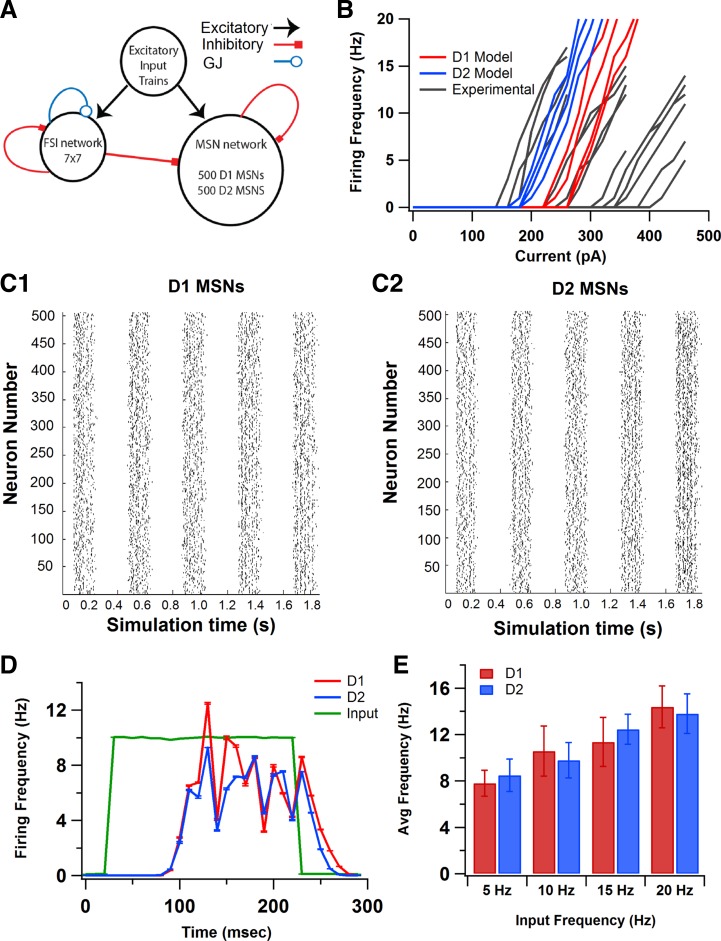
The striatal network consisted of 1,000 MSNs (500 D1 MSNs and 500 D2 MSNs) and 49 FSIs (see Fig. 2A). The MSN-to-FSI ratio was based on experimental observations: each MSN receives input from 55% of nearby striatal FSIs (Tecuapetla et al. 2007), and between 4 and 27 converge on the same MSN (Koós and Tepper 1999). Based on these estimates, the 49 neuron FSI network corresponded to the FSI network seen by 1,000 postsynaptic MSNs. Although the percentage of FSIs is slightly larger than observed experimentally, a smaller number of FSIs would have incorrectly produced homogenous FSI input to each MSN in the network model. A heterogeneous network of neurons was generated by changing the KAs conductance (both MSNs and FSIs) and NMDA channel conductance (MSN only) by ±10%. The range of activity of MSNs used in the network, in response to current injections, was within the range of experimentally observed responses (see Fig. 2B; electrophysiology methods described below). The distance between each MSN soma in the model was 25 μm both in the x-axis and the y-axis based on experimental observations (Tunstall et al. 2002), resulting in a 775 × 775 μm2 grid. At each grid location, the assignment of either D1 or D2 MSNs was random with probability = 0.5.
Fig. 2.
Model of striatal network with 1,000 MSNs and 49 fast-spiking interneurons (FSIs). A: schematic of network model with MSNs receiving inhibitory input from FSIs and other MSNs. Roughly equal numbers of D1 and D2 MSNs are randomly distributed on a regular grid. FSIs receive input from other FSIs through both GABAergic synapses and gap junction (GJ) connections. Excitatory input to both MSNs and FSIs is provided by simulated Poisson trains. B: heterogeneous distribution of MSNs was generated by changing KAs and N-methyl-d-aspartate channel conductances by ±10%. The range of responses of these D1 and D2 MSN model neurons to somatic current injection was within the range of responses seen experimentally. C: raster plots of D1 (C1) and D2 (C2) MSNs in response to synaptic input in the striatal network. D: firing frequencies of D1 and D2 MSN populations during the upstate, calculated from rasters in C by averaging across the neurons within a class and across upstates. Excitatory synaptic input (green) is illustrated to show latency of MSN firing with respect to input. Both MSN classes have similar firing frequencies (D1 MSNs: 10.77 ± 0.60 Hz; D2 MSNs: 9.59 ± 0.13 Hz). E: D1 and D2 MSNs have balanced firing frequencies across a range of cortical input frequencies.
The probabilities of connection of MSN-MSN and FSI-MSN synapses were modeled using a distance-based exponential function, tuned to match experimentally observed probabilities of connections (Gittis et al. 2010; Planert et al. 2010; Plenz 2003; Taverna et al. 2008): where f = 95 μm2. FSIs were connected to MSNs with GABAergic synapses [rise time constant, 0.25 ms; decay time constant, 3.65 ms; reversal potential, −60 mV; maximal conductance, 8.4 nS; (Gittis et al. 2010)], whereas the GABAergic synapses between MSNs had a maximal conductance of 0.75 nS with the same rise and decay times (Koos et al. 2004). Note that GABAergic synapses in MSNs are depolarizing due to the hyperpolarized (−80 to −90 mV) resting membrane potential of mature MSNs (Wilson and Kawaguchi 1996), coupled with GABA responses which reverse between −60 and −50 mV (Kita 1996; Mercuri et al. 1991; Tunstall et al. 2002). The FSI-MSN synapses also were more proximal than MSN-MSN synapses (Oorschot et al. 2010). The probability and strength of connection of MSN-MSN and FSI-MSN synapses in the network model were independent of the type of pre- or postsynaptic MSN (Planert et al. 2010). The transmission delays were distance-based using a conduction velocity of 0.8 m/s for both FSI and MSN synapses (Tepper and Lee 2007; Wilson 1986; Wilson et al. 1990).
Extrinsic synaptic input.
Excitatory input to the striatum comes primarily from the cortex and thalamus. We simulated this glutamatergic input as Poisson distributed spike trains with a minimum time between spikes of 100 μs. Both MSN classes in this model have 360 AMPA and NMDA synapses and 227 GABA synapses. Note that each Poisson train represents activity from more than one cortical neuron, and each synapse represents the population of synapses in a single isopotential compartment. Thus the 100-μs minimum interspike interval is to prevent more than 1 spike per time step to each MSN. Each synaptic channel in the MSN model receives an input of 10 Hz during the upstate, which results in a total input of ∼800 synaptic inputs per second and 1/20 of this input during down states (Blackwell et al. 2003). Each FSI model has 127 AMPA synapses and 93 GABA synapses, resulting in a total AMPA input of 282 Hz and GABA input of 207 Hz, when 2 Hz input is provided to each synapse (Kotaleski et al. 2006). To introduce correlations within both the MSN and FSI input, each spike from the set of spike trains was assigned to more than one synapse, with probability P = 1/n, where , where N = number of synapses, and c = 0.5 (Hjorth et al. 2009). For each neuron, starting from a single mother spike train per neuron, spike trains for each synapse were created by assigning the spike to that synapse if a uniform random number was greater than P. This produced a mean number of synapses activated by each spike of 1.4 for the control simulations. To introduce between-neuron input correlation, an additional shared set of input spikes was generated. The between-neuron input correlation was then adjusted by changing the fraction of input each neuron received from this shared pool (as opposed to the spike trains that were unique to each neuron). Unless otherwise stated, 30% of all excitatory synaptic input to either the FSI or MSN populations was shared; with FSIs and MSNs each having their own sets of shared inputs. This base level of between-neuron correlation was incorporated based on studies that report correlation among the cortical input to the striatum (Krüger and Aiple 1988; Stern et al. 1998; Ts'o and Gilbert 1988). For the case where synchronized cortical input was provided to FSIs to compensate for lack of gap junctions, the correlation value was doubled for FSI inputs only.
The model was implemented in GENESIS (Bower and Beeman 2007), and simulations used a time step of 100 μs. The simulation time was 2 s with five 0.2-s duration upstates separated by 0.2-s duration downstates. Each upstate used a different set of cortical input spikes and thus was an independent observation of the network response. Each network simulation experiment took 3 wk to run. The cortico-striatal Poisson spike trains were generated using MATLAB (version 2007b, MathWorks). The simulation and output processing software, along with the files used for the simulations, are freely available from the authors' website (http://krasnow1.gmu.edu/CENlab/) and modelDB (http://senselab.med.yale.edu/ModelDB/).
Analysis of spikes.
Mean firing frequency during upstates was plotted by averaging across neurons of the same class using 10-ms time bins. The firing frequency was expressed as mean ± SD of values obtained from five different upstates. To investigate the contribution of gap junctions on synchronization, cross-correlograms were constructed for each directly coupled neuron pair in the FSI network and then averaged over the network (Hjorth et al. 2009). Cross-correlograms also were constructed for directly coupled MSN pairs. Correlation was corrected for firing frequency by subtracting the shuffled cross-correlograms for the same network condition. Statistical analyses were performed using SAS. When only two groups were being compared, the procedure TTEST was used. When more than two groups were compared, one-way analysis of variance was performed using the GLM procedure with network condition as the independent variable and difference between D1 and D2 MSN frequencies as the dependent variable. Each of the five upstates was considered as an independent replication, and P < 0.05 was considered significant. Post hoc analyses used Bonferroni correction for multiple comparisons with P < 0.01 considered significant.
Electrophysiology for model tuning.
Patch-clamp recordings were performed to obtain a range of responses of MSNs to somatic current injection (Fig. 2B). C57B6 male and female mice (at least 20 days old) were anesthetized with isoflurane and decapitated. Brains were quickly extracted and placed in oxygenated ice-cold slicing solution (in mM: KCl 2.8, dextrose 10, NaHCO3 26.2, NaH2PO4 1.25, CaCl2 0.5, Mg2SO4 7, sucrose 210). Hemicoronal slices from both hemispheres were cut 350-μm thick using a vibratome (Leica VT 1000S). Slices were immediately placed in an incubation chamber containing artificial cerebrospinal fluid (in mM: NaCl 126, NaH2PO4 1.25, KCl 2.8, CaCl2 2, Mg2SO4 1, NaHCO3 26.2, dextrose 11) for 30 min at 33°C, then removed to room temperature (21–24°C) for at least 90 more minutes before use.
A single hemislice was transferred to a submersion recording chamber (ALA Science) gravity-perfused with oxygenated artificial cerebrospinal fluid containing 50 μM picrotoxin. Temperature was maintained at 30–32°C (ALA Science) and was monitored with an external thermistor. Whole cell patch-clamp recordings were obtained from neurons under visual guidance using infrared differential interference contrast imaging (Zeiss Axioskop2 FS plus). Pipettes were pulled from borosilicate glass on a laser pipette puller (Sutter P-2000) and fire-polished (Narishige MF-830) to a resistance of 3–7 MΩ. Pipettes were filled with a potassium-based internal solution (in mM: K-gluconate 132, KCl 10, NaCl 8, HEPES 10, Mg-ATP 3.56, Na-GTP 0.38, EGTA 0.1, biocytin 0.77) for all recordings. Intracellular signals were collected in current clamp and filtered at 3 kHz using an Axoclamp2B amplifier (Axon Instruments) and sampled at 10–20 kHz using an ITC-16 (Instrutech) and Pulse version 8.80 (HEKA Electronik). Series resistance (6–30 MΩ) was manually compensated.
RESULTS
Changes in channel properties between D1 and D2 MSNs are sufficient to reproduce electrophysiological dichotomy.
Development of a network model of D1 and D2 MSNs required single-neuron models, which have similar intrinsic excitability to that seen experimentally (Gertler et al. 2008). Differences in morphology or intrinsic channel properties (Moyer et al. 2007; Nicola et al. 2000) have been proposed to explain the observation that D2 MSNs are more excitable than D1 MSNs (Gertler et al. 2008; Kreitzer and Malenka 2007). We simulated the response to current injection in D1 and D2 MSNs created two different ways and compared these responses to experimental measurements.
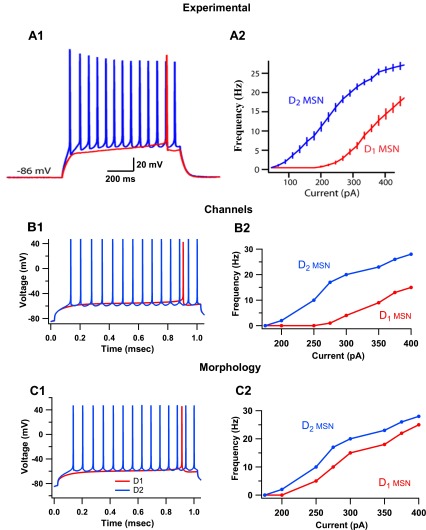
We first evaluated whether the larger number of primary dendrites in D1 MSNs (Gertler et al. 2008) could explain the difference in D1 and D2 MSN excitability. The morphology of both MSN classes was modeled the same, except for the number of primary dendrites, with the D1 MSNs having six and the D2 MSNs having four (Gertler et al. 2008). The intrinsic channel properties were the same between both MSN models (see materials and methods). In response to current injection, the D1 MSN model had significantly greater rheobase current than the D2 MSN model (D1 MSN, 210 pA; D2 MSN, 160 pA; Fig. 1C1). These results match experimental observations (Fig. 1A; Gertler et al. 2008). However, the shape of the F–I curve (Fig. 1C2) did not match experiments, and thus spiking activity differences were not accurately modeled by morphological differences alone.
Fig. 1.
Differences in intrinsic channel properties are sufficient to describe the dichotomy in excitability between D1 and D2 medium spiny neurons (MSNs). A: experimental observations of responses of D1 and D2 MSNs to current injection. [Adapted with permission from Gertler et al. 2008.] A1: D1 MSNs have significantly higher rheobase current (median, 270 pA, n = 35) than D2 MSNs (median, 130 pA, n = 31). A2: frequency-current (F–I) plot of D1 and D2 MSNs demonstrate higher intrinsic excitability in D2 MSNs. B: models of D1 and D2 MSNs constructed with differences in intrinsic excitability reproduce electrophysiological dichotomy. The intrinsic channels with different properties are the L-type calcium channels, fast-sodium channels, slowly inactivating A-type (transient) potassium channel (KAs) and inward-rectifying potassium channels (KIR). B1: D1 and D2 MSN models have rheobase current of 275 pA and 155 pA, respectively. B2: F–I plot of these models reproduce experimentally observed differences in spiking activity. C: simulations suggest that models of D1 and D2 MSNs differentiated based solely on morphological differences do not replicate dichotomy in spiking activity. C1: D1 MSN models constructed with six primary dendrites and D2 MSN models constructed with four primary dendrites have rheobase currents of 210 pA and 160 pA, respectively. C2: the F–I plot of these MSN models does not replicate the dichotomy of D1 and D2 MSN excitability seen experimentally.
Since anatomical differences were not sufficient to accurately describe electrophysiological differences between the D1 and D2 MSNs, the contribution of intrinsic channel differences alone in describing the excitability difference was investigated. The morphology of both neuron models was made the same, with each MSN model containing four primary dendrites. The channel properties were changed based on studies that measured the effects of D1 and D2 agonists on intrinsic and synaptic channels of both types of MSNs (Table 1) (Moyer et al. 2007; Nicola et al. 2000). The differences between D1 and D2 MSN excitability in response to current injection, including the F–I curve, were replicated using these MSN models (Fig. 1B). The rheobase current for D1 MSNs was 275 pA and for D2 MSNs was 155 pA. Intrinsic channel differences between D1 and D2 MSNs were thus sufficient to describe the dichotomy between D1 and D2 MSN spiking activity. This suggests that differences in excitability between D1 and D2 MSNs measured in identified neurons may be due to basal levels of dopamine present in the slice. These models (same morphology, differences in channels) were used for the remainder of the study.
Network inhibition balances excitability between D1 and D2 MSNs.
To investigate the mechanisms that balance activation of the direct and indirect pathways, a striatal network model was developed. We used 500 each of the D1 and D2 MSN models, together with 49 FSIs to create the network (Fig. 2A). A heterogeneous network of these neurons was created using small random changes (within ±10% of original value) in KAs and NMDA channel conductance, and this produced a range in rheobase current comparable to that observed experimentally on unidentified MSNs (Fig. 2B). The probability of connections between MSNs was determined using an exponential distance-based function that matched the probabilities and strength of connections seen experimentally (see materials and methods; Koós and Tepper 1999; Taverna et al. 2008). The probability of FSI-MSN connections also was described using the exponential distance-based function, but these connections were made stronger and more proximal than the MSN inputs (Gittis et al. 2010; Planert et al. 2010). The network (MSNs and FSIs) was activated with excitatory input from Poisson trains that represented cortical input and simulated up and down states (Fig. 2C), as seen in organotypic cocultures and in anesthetized animals. For the simulations that explored single-neuron differences, which were described in the previous section, synaptic channels were not included; however, for all network simulations, the synaptic channels of the MSN classes differed between D1 and D2 models as measured experimentally in response to dopamine agonists (Table 1; Nicola et al. 2000).
Figure 2D shows the firing rates of D1 and D2 MSNs, averaged across neurons and upstates, in response to cortical input and network inhibition. Both MSN classes had similar firing rates when connected in this network (D1 MSN: 10.77 ± 2.16 Hz; D2 MSN: 9.59 ± 1.53 Hz), representing balanced firing. These results, that D1 and D2 MSNs fire at similar frequencies in the network, are consistent with two in vivo studies: one in anesthetized rats (Mallet et al. 2006), and another in awake, behaving mice where spike frequencies were measured between tasks (Cui et al. 2013). Therefore, the imbalance in D1 and D2 MSN firing rates in response to current injection is overcome in the presence of synaptic excitation and inhibition in the network. This balanced firing was observed for a range of cortical input frequencies (Fig. 2E).
Two different variations on inhibitory connectivity were evaluated to verify that the balanced network output does not depend on the specific connectivity results implemented. Some studies have reported slight imbalances in connectivity between D1 and D2 MSNs (Taverna et al. 2008; Tunstall et al. 2002), specifically the higher occurrence of presynaptic D2 MSNs compared with presynaptic D1 MSNs (Taverna et al. 2008). To confirm that the balanced network output was independent of these changes to MSN-MSN connectivity, simulations were repeated in a network with a higher probability of connection from presynaptic D2 MSNs (twice that of presynaptic D1 MSNs), and with stronger connections from presynaptic D2 MSNs (double the strength of connections from presynaptic D1 MSNs). This did not produce significant differences in the firing rates of either MSN class or in the balance produced in the network between the two MSN classes (D1 MSN: 11.66 ± 3.36 Hz; D2 MSN: 10.06 ± 3.12 Hz; P = 0.153). Another possible variation in network connectivity is based on differences in connectivity between FSIs and MSNs. Experimental studies have reported both similar (Planert et al. 2010) and different probabilities of connections (Gittis et al. 2010) from FSI input to the two MSN classes. To confirm that the function of FSI-MSN synapses in balancing firing is independent of differences in connectivity, simulations were repeated with FSI connections to D1 MSNs 15% higher than FSI connections to D2 MSNs (Gittis et al. 2010). These changes did not produce significant changes in the firing rates of either MSN class in the intact network and was similar to that seen in the control condition (D1 MSNs: 10.16 ± 2.35 Hz; D2 MSNs: 9.72 ± 1.31 Hz; P = 0.204). This suggests that the effects of FSI inhibition to striatal balance are independent of slight differences in connectivity from FSIs to the two MSN classes.
In order for network inhibition to balance firing, the intrinsic channels of the MSNs had to be differentially modulated between the MSN classes. When the differential intrinsic excitability of MSNs was produced through a difference in morphology alone (e.g., Fig. 1C), the two MSN classes were imbalanced even in the presence of network inhibition and differences in synaptic channels (D1 MSN: 2.82 ± 0.12 Hz, D2 MSN: 11.65 ± 0.18 Hz; P < 0.01). The network also was imbalanced in the presence of synaptic modulation (Table 1), but no intrinsic channel modulation (D1 MSN: 4.84 ± 1.73 Hz; D2 MSN: 14.35 ± 3.61 Hz). This suggests that intrinsic channel differences might allow D1 and D2 MSNs to be influenced differently by network inhibition. The interaction between network inhibition and intrinsic channels was investigated later in the study.
Inputs from FSIs are responsible for striatal balance.
The contributions of the two main inhibitory circuits of the striatum to balanced MSN firing were studied next. These circuits are the local feedforward inhibition from FSIs to MSNs and the more numerous but weaker feedback inhibitory connections from MSNs to other MSNs. The differences in properties of MSN-MSN and FSI-MSN connections in our model are listed in Table 2. Both types of synaptic inhibition were removed separately to observe their specific roles on MSN firing. Since MSN-MSN synapses were more widespread, two approaches were used to investigate the effect of their removal. In one simulation, removal of MSN-MSN synapses was followed by a replacement of ∼30% of those GABAergic inputs with Poisson distributed input trains that had the same latency and frequency of MSN inputs as seen during the control condition. This was done to maintain the same number of GABAergic input as the FSI removal condition and to prevent a large increase in overall MSN firing from masking the specific effects that feedback inhibition had on the two MSN classes.
Table 2.
Differences between MSN-MSN and FSI-MSN synapse properties
| Synapse property | MSN-MSN | FSI-MSN |
|---|---|---|
| GABA gmax, nS | 0.75 | 8.4 |
| Latency to fire (observed), ms | 75 | 10 |
| Distribution | Distal | Proximal |
gmax, Maximal conductance; MSN, medium spiny neuron; FSI, fast-spiking interneuron.
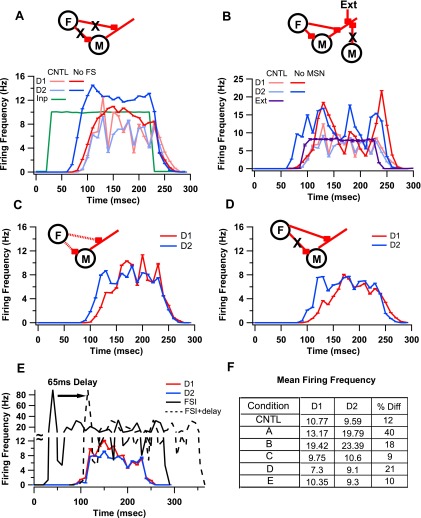
Simulations revealed that FSIs are more significant for balanced firing than MSNs. Removing FSI-MSN input resulted in increased firing in both MSNs, but produced even higher firing frequencies in D2 MSNs compared with D1 MSNs (D1: 13.17 ± 5.96 Hz; D2: 19.79 ± 5.31 Hz; Fig. 3A).
Fig. 3.
Contribution of FSI-MSN and MSN-MSN synapses to striatal balance. A: removing FSI inhibition (F) to MSNs introduces a 40% imbalance in firing between D1 and D2 MSNs. B: removing MSN-MSN synapses (M) and replacing those GABAergic synapses with extrinsic input trains (Ext) that keep the overall inhibition similar to control (CNTL) level results in a large increase in firing frequency, but only 18% difference between D1 and D2 MSN firing frequencies. C: reducing the weight (dashed lines) of FSI-MSN synapses to the level of MSN-MSN synapses (8.4 nS to 0.75 nS) produced slight changes (9%) to firing differences between D1 and D2 MSNs. D: removing proximal FSI-MSN synapses and making FSI-MSN synapses as distal as MSN-MSN synapses also did not disrupt balance of firing. E: a delay of 65 ms was added to FSI-MSN synaptic connections to identify the contribution of early FSI firing on disrupting balance in firing. This resulted in a 10% difference in firing between the MSN classes. All percent differences are calculated as difference divided by mean. F: table of mean firing frequencies and %differences between D1 and D2 MSNs for the CNTL condition and for the conditions are represented by the respective panels.
In contrast to FSI removal, replacing the feedback inputs between MSNs produced a smaller change in the balance of firing between D1 and D2 MSNs (18% vs. 40% during FSI block), despite a greater overall increase in firing frequency (Fig. 3B; D1: 19.42 ± 3.99 Hz; D2: 23.29 ± 3.36 Hz). Removing MSN-MSN synapses but not replacing any of those inputs with Poisson trains also produced the same effect; i.e., a smaller change in the balance of firing between the two MSN classes compared with FSI removal (D1: 26.91 ± 3.93 Hz; D2: 23.98 ± 3.78 Hz). GLM reveals that the differences in D1 and D2 MSN firing for the control (fully intact network) and no FSI-MSN and no MSN-MSN groups were significantly different from each other [F(3,19) = 109.24, P < 0.05]. Post hoc tests (Bonferroni correction for paired comparisons) show that difference in firing for FSI-MSN removal > difference for MSN replacement ∼= difference for MSN removal > control (P < 0.01). These results suggest that FSI inhibition is more important for producing balanced firing than is MSN inhibition.
To identify the properties of FSI-MSN synaptic connections that elicit differential responses from D1 and D2 MSNs, simulations were repeated with each property (conductance, proximity, latency) individually changed to match that of MSN-MSN synapses (see Table 2). FSI-MSN synapses with the conductance of MSN-MSN synapses produced a slight imbalance, but only during the beginning of the upstate, with the D2 MSNs firing more than the D1 MSNs (Fig. 3C). A lack of proximal FSI-MSN synapses also produced a slight imbalance in the beginning of the upstate, again with the D2 MSNs firing more than the D1 MSNs (Fig. 3D). Removing early FSI inhibition (adding a latency in FSI firing such that synaptic input from FSI arrives at a similar time as synaptic input from MSNs) did not produce any significant difference in striatal balance during the entire upstate (Fig. 3E). GLM shows that all of these groups are significantly different from the no FSI-MSN group [F(3,19) = 751.56, P < 0.05]. In summary, an isolated modulation of any one of these properties was not sufficient to reproduce the imbalance seen during the FSI block condition.
Synchronization of FSIs by gap junctions is critical for striatal balance.
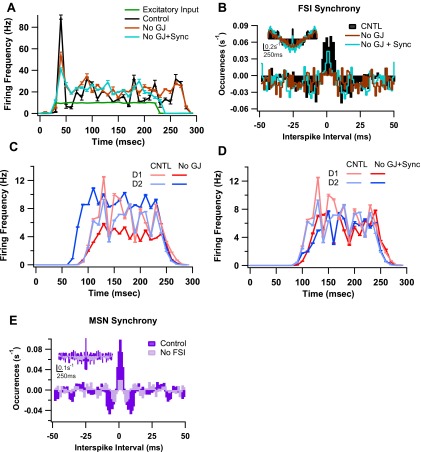
The contribution of FSI-FSI interactions to the balance of firing between D1 and D2 MSNs was investigated next. The FSIs are connected by GABAergic synapses [1 nS; connection probability of 0.58 (Gittis et al. 2010)] and gap junctions [0.5 nS, connection probability of 0.3 (Koós and Tepper 1999)]. Removing the GABAergic synapses did not cause a significant change to overall firing of FSIs (control: 16.89 ± 1.68 Hz; no GABA: 16.45 ± 1.51 Hz; P = 0.703) or to the balance in firing between the two MSN classes (D1: 10.86 ± 2.75 Hz; D2: 9.96 ± 1.812 Hz, P = 0.303). In contrast, removing gap junctions induced differential responses from D1 and D2 MSNs (D1: 6.55 ± 1.61 Hz; D2: 15.02 ± 3.93 Hz; P < 0.01; Fig. 4C), despite producing a nonsignificant change in FSI firing frequency (control: 16.89 ± 1.68 Hz; no gap: 17.58 ± 1.32 Hz; P = 0.161; Fig. 4A). To confirm that the imbalance was not due to the slight increase in FSI firing, simulations were repeated in the absence of gap junctions with either a weaker cortical input (lower frequencies), or synchronous cortical input (see materials and methods) to FSIs. Weaker cortical input restored FSI firing to the level that occurs in the presence of gap junctions, but did not restore the balance between D1 and D2 MSN firing (D1: 5.14 ± 1.51 Hz; D2: 13.82 ± 4.43 Hz). Synchronization of cortical input to FSIs with no gap junctions increased the level of synchronization between FSIs toward the level seen in the intact network (∼30% of control; Fig. 4B). This increase in synchronization was sufficient to restore the balance between D1 and D2 MSN firing (D1: 7.99 ± 1.82 Hz; D2: 7.79 ± 1.55 Hz; Fig. 4D). Removing FSIs leads to a reduction in overall synchrony of MSNs, suggesting that FSIs possibly modulate MSN synchrony in addition to affecting MSN balance (Fig. 4E). GLM reveals that the differences in D1 and D2 MSN firing for the control, no gap junctions, no gap junctions + weak input, and no gap junctions + synchrony groups were significantly different [F(3,19) = 439.33, P < 0.05]. Post hoc tests confirmed that no gap junctions + synchrony did not differ from the control (P = 0.653), and that both of these cases differed from the other two no gap junctions groups (P < 0.01). Collectively, these simulations show that input to MSNs from FSIs, which are weakly synchronized by gap junctions, produces balanced firing between D1 and D2 MSNs.
Fig. 4.
Contributions of FSI-FSI interactions on balance of firing between D1 and D2 MSNs. A: the mean firing frequency of FSIs is minimally affected by GJ inputs from other FSIs. Removal of GJ results in slightly higher firing of the FSIs. Providing synchronous cortical input to FSIs when GJs are removed does not produce significant changes to FSI firing frequencies. B: cross-correlograms of FSIs during the same conditions as in A show that GJ removal decreases synchrony, and synchronous input restores synchrony, although not quite to the same level seen in the intact network. Note that synchrony is calculated between connected FSIs (Hjorth et al. 2009). Cross-correlogram between nonconnected FSIs reveals no synchrony (data not shown). Inset shows cross-correlograms over entire simulation period and depicts slight oscillatory behavior. C: firing frequencies of D1 and D2 MSNs when GJs between FSIs are removed. D2 MSNs fire at a significantly higher frequency compared with D1 MSNs. D: balance in firing is restored when synchronous cortical input is provided to FSIs during GJ block. E: cross-correlograms of MSNs during CNTL and no FSI conditions. In addition to leading to disruption of balance between D1 and D2 MSN firing, removing FSI results in decreased overall synchrony between MSNs. Inset shows cross-correlogram over entire simulation period.
Direct modulation of intrinsic channels is sufficient to disrupt striatal balance.
Given that differences in intrinsic channels are required to produce both the observed F–I curves and balanced firing, we investigated the contribution of individual channel differences between MSN classes on maintaining striatal balance in the intact network. Channel differences were eliminated by changing the maximal conductance of the channels in the D2 MSN to be identical to that of the D1 MSN for four channels: L-type calcium channels (CaL), fast-sodium channels (NaF), inward-rectifying potassium channels (KIR), and slowly inactivating KAs (Table 1). Removing the differences in conductance of any one of these channels, by setting the D2 conductance equal that of the D1 conductance, was sufficient to produce differential firing between D1 and D2 MSNs, with D2 MSNs firing more than D1 MSNs [Fig. 5A; F(4,24) = 210.49, P < 0.05]. The most profound effect was seen when CaL was made the same between D1 and D2 MSNs, possibly due to the bigger difference in conductance of these channels compared with the other channels. To further investigate the role of CaL, simulations were repeated with CaL blocked in both D1 and D2 MSNs. Removal of CaL from both MSN classes did indeed disrupt striatal balance in the striatal network model (D1: 3.7 ± 1.82 Hz; D2: 21.2 ± 4.25 Hz), which is consistent with a recent in vitro study that showed blocking CaL produced imbalanced firing between D1 and D2 MSNs (Flores-Barrera et al. 2011).
Fig. 5.
Effects of modulating channel properties and their interaction with inhibitory input on affecting striatal balance. A: equalizing L-type calcium channels (CaL), fast-sodium channel (NaF), KAs, or KIR produces imbalance between D1 and D2 MSN firing (P < 0.01 for all conditions). B: when CaL or KAs/KIR (data not shown) conductances are equalized between D1 and D2 MSNs, removing FSIs or GJs does not affect the imbalance in firing. When NaF properties are equalized, removing GJs no longer produces the significant difference in firing between the two MSN classes, restoring balance in firing between D1 and D2 MSNs.
The intrinsic channel differences that interact with correlated FSI input in balancing MSN firing were investigated next. Simulations revealed that, when the differences of each of CaL (Fig. 5B), KAs, and KIR were removed in the absence of FSI-MSN synapses or gap junctions, the imbalance was no greater than the effect of removing intrinsic channel differences alone. Curiously, equalizing NaF channels eliminated the difference in firing between D1 and D2 MSNs produced by gap junction removal (Fig. 5B). The difference in firing during this condition (NaF equal + no gap junctions) was significantly different from either no gap junctions or equal NaF alone [F(2,24) = 618.34, P < 0.05]. This suggests that NaF differences might be critical in allowing differential sensitivity of D1 and D2 MSNs to synchronous inhibitory input.
DISCUSSION
Recent studies have evaluated the contributions of differential cortical input (Mallet et al. 2006) and direct channel modulations (Flores-Barrera et al. 2011) on disrupting the balance in firing between D1 and D2 MSNs in the striatum. However, the role of striatal inhibitory circuits in modulating striatal balance is not clear. We constructed a striatal network model of MSNs and FSIs to identify the inhibitory circuits that modulate MSN excitability differently between the two classes. The model was based on a previously published FSI network model (Hjorth et al. 2009) and MSN single-neuron model (Evans et al. 2012). We found that removing FSI inhibition or reducing the correlation between FSI action potentials by blocking gap junctions produced imbalanced firing between D1 and D2 MSNs (Fig. 6). Additionally, intrinsic and synaptic channels of MSNs interact with these inhibitory mechanisms to modulate differences in firing between the two MSN classes.
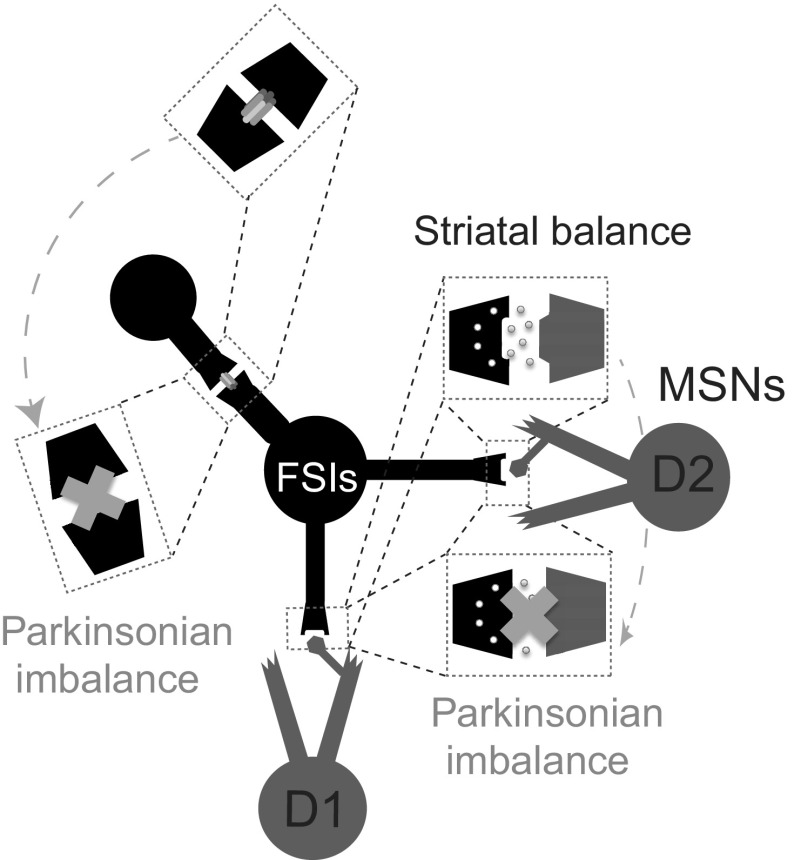
Fig. 6.
Summary of conditions that produce Parkinsonian imbalance and conditions that restore striatal balance. This schematic shows the nature of connections between FSI and MSNs and between MSNs, and the effect of removing these connections. When input from FSIs (black) to D1 (gray) and D2 (gray) MSNs is removed, D2 MSNs fire more than D1 MSNs. When GJs between FSIs are removed, D2 MSNs again fire at higher frequencies compared with D1 MSNs. These two cases have been labeled as Parkinsonian imbalance. In the latter case, balance is restored when the synchronicity of FSIs is increased by alternative means, such as providing highly correlated cortical input to the FSIs. Making NaF the same between D1 and D2 MSNs also restores balance during GJ block. The network output seen in the CNTL network where the connections are intact is labeled as striatal balance. Glutamatergic input to both FSIs and MSNs, along with feedback connections between MSNs, have been omitted from the schematic to highlight the effect of FSI-MSN connections on striatal balance.
FSI inhibition produces striatal balance by reducing D2 MSN activity.
In our striatal network model, feedforward inhibition from FSIs was found to inhibit D2 MSNs more than D1 MSNs. Since D2 MSNs have a higher intrinsic excitability, the increased inhibition by FSIs brings the level of D2 MSN firing down to that of D1 MSNs. The striatal network model by Humphries et al. (2009) that uses Izhikevich neurons suggests that removing FSIs might alternatively lead to a decrease in overall MSN firing. In their network model, they use an FSI-MSN conductance five times stronger than their MSN-MSN conductance. This ratio, lower than that used in our network model, combined with the somatic location of all their MSN synapses, implies that removing FSI synapses eliminates a much smaller fraction of the total inhibitory synaptic current than in our model. Our FSI-MSN-to-MSN-MSN conductance ratio was estimated based on a recent experimental paper that uses transgenic mice (Gittis et al. 2010) that suggests that the FSI-MSN conductance is closer to 8 nS, which is the value used in this paper.
Selective inhibition of FSIs in the striatum recently was shown to produce dyskinesia in mice (Gittis et al. 2011b) and a mixed effect on MSN firing, with 54% of MSNs having increased firing rates and 38% having reduced firing rates. This is consistent with our results that show that the two populations of MSNs have disparate responses to FSI removal. Although that experimental study did not record from identified D1 and D2 MSNs, our simulations predict that the recorded neurons with reduced firing were D1 MSNs, and the neurons with increased firing were D2 MSNs. In that study, block of FSIs produced dyskinesia, and not the hypokinesia typically observed with excess D2 MSN firing (Kravitz et al. 2010), which suggests that the most significant behavioral effect of FSI block may be caused by changes in MSN synchrony, and not an imbalance in firing. Alternatively, dyskinesia may be caused by changes in synchrony interacting with the modest imbalances in firing produced by selective block of FSI activity, as opposed to the strong imbalance and synchronous firing produced by optogenetic stimulation of D2 MSNs. This hypothesis is consistent with our finding, that removing FSI inhibition results in a reduction in overall MSN synchrony and an imbalance in firing between the two MSN classes.
Although inhibition from FSIs and MSNs constitute a critical part of inhibitory input to MSNs, they are not the only sources of inhibition. MSNs receive inhibitory input from other interneurons in the striatum and also from GABAergic projection neurons in the globus pallidus (Mallet et al. 2008, 2012) that innervate both MSNs and interneurons. The contributions of these other inhibitory sources to MSN balance must also be explored to form a complete picture of the contribution of inhibitory input on striatal balance.
Desynchronization of FSIs is sufficient to disrupt striatal balance.
Reducing the correlation between FSIs by removing gap junctions disrupts the balance in firing between the D1 and D2 MSNs. The strength of FSI inhibition is modulated by the presence of gap junctions that reduce the number of FSI action potentials and increase the level of synchronization between the FSIs (Hjorth et al. 2009; Traub et al. 2001). Simulations were performed to confirm that the ability of FSIs to balance firing between D1 and D2 MSNs was not dependent on the level of input correlation (data not shown). Direct measures of FSI correlation in vivo are problematic due to the low numbers of FSIs in the striatum; however, the entrainment of FSIs to the striatal gamma rhythm (Berke 2009; Berke et al. 2004) suggests some degree of correlation among FSIs. The small reduction in FSI correlation due to removal of gap junctions was sufficient to produce striatal imbalance in our network model. That synchronization of FSIs is critical for striatal balance was confirmed when increasing the level of correlation between cortical inputs to FSIs restored the balance. Our finding that a change to correlation between FSIs did not change the mean firing of MSNs in the network is consistent with another computational study that used simplified single-neuron models to study the effects of correlated inputs on striatal function (Yim et al. 2011). This implies that, even though changes to correlation between FSIs might not affect overall firing frequency of MSNs, they can modulate D1 and D2 MSN firing rates differently. This suggests that changes to FSI synchrony can lead to targeted activation of a population of MSNs without changing the overall activity. Gap junctions can thus act as a key modulator of striatal balance because of their specific role in FSI synchronization.
For the FSI-FSI connections in our network, we did not use distance-dependent connections because of the relatively small size of the network. We confirmed that fixed probabilities and distance-dependent probabilities produce similar synchronization levels in our network (data not shown). This is in contrast to the results presented in a recent computational study (Lau et al. 2010) that reports that the synchronous activity of the network is sensitive to the connection type between the neurons (local vs. glob al). We think that this contrast in results might be due to the difference in network architectures between that study and this present study. Their network model consisted of 200 neurons arranged in a one-dimensional lattice compared with our two-dimensional, 49-neuron network.
The imbalance produced by gap junctions was altered in our model by modulating NaF. Lowering the conductance of NaF channels in D2 MSNs (to that of D1 MSNs) increased the firing frequency of D2 MSNs, but it also changed their sensitivity to FSI input. With lowered NaF conductance in D2 MSNs, removing FSI inhibition produces a smaller increase in firing frequency than in the control case. This result in the striatum is similar to that reported recently in the globus pallidus. Edgerton et al. (2010) found that, in the presence of synaptic excitation, the density of NaFs can alter the sensitivity of globus pallidus neurons to synaptic inhibition. The novel aspect of our result is that eliminating the correlation among FSIs restored the balance when NaF is the same in D1 and D2 MSNs. A possible mechanism is that the synchronized FSI inputs, in combination with elevated NaF conductance, may enhance NaF inactivation, leading to lower firing rates. Either lower NaF conductance or desynchronized FSI inputs avoid this NaF inactivation, permitting higher firing of D2 MSNs. This mechanism is analogous to the enhancement in hippocampal FSI firing produced by Kv3.1 (Erisir et al. 1999). Evaluation of the inactivation gate variable of NaF of D2 MSNs was not different between the different conditions (data not shown), possibly because of the increase in the firing frequency of D2 MSNs in the lower NaF condition. The increase in firing in the D2 MSNs is predicted to increase inactivation, which may mask the predicted reduction in inactivation per spike.
Functional implications of dopamine.
Dopamine may modulate the ability of FSIs to control striatal balance. Dopamine depletion has been shown to reduce the immediate-early gene response of PV+ neurons in the striatum (Trevitt et al. 2005). In addition, FSIs are depolarized by dopamine through D1/D5 receptors (Bracci et al. 2002; Centonze et al. 2003), and thus reduced dopamine can result in lower FSI input to both MSN classes. This makes reduction of FSI input a potential mechanism of disrupting striatal balance during dopamine depletion.
The excitability differences between D1 and D2 MSNs in our model are due to modulation to synaptic and intrinsic channels. Changes to intrinsic channels were sufficient to replicate firing frequency differences between the two MSN classes in response to current injection (Fig. 1) measured in identified D1 and D2 MSNs. This implies that the intrinsic excitability of D1 and D2 MSNs in the absence of dopamine should be more similar than in the control condition. This is consistent with recent studies using transgenic mice (Chan et al. 2012; Day et al. 2006), where the current-firing frequency curves of D1 MSNs in the control and dopamine-depleted condition were indistinguishable, whereas that of the D2 MSN had diminished considerably in the dopamine-depleted condition. This reduced firing frequency profile of the D2 MSN during dopamine depletion was much closer to that of the D1 MSN than it was in the control condition (Chan et al. 2012), suggesting that “intrinsic” differences in channel differences might be largely due to basal dopamine in slice preparations.
Modulation of MSN excitability by direct changes to properties of the intrinsic channels can also disrupt balanced striatal firing. In particular, CaL have previously been identified as one of the key targets through which dopamine balances excitability in the striatal network (Flores-Barrera et al. 2011; Surmeier et al. 2007). Additionally, CaL are located near cortical synaptic inputs (Freund et al. 1984; Higley and Sabatini 2010) and may be involved in corticostriatal integration. Flores-Barrera et al. (2011) showed that blocking CaL produces imbalanced firing of D1 and D2 MSNs in response to synchronous input. Here, we show that striatal imbalance is also produced during block of CaL in response to asynchronous, in vivo-like input. Further removal of either FSI inputs or FSI correlation does not enhance the imbalance. This effect is also observed when CaL is increased in the D2 MSNs to match that of D1 MSNs. These results suggest that modulations to FSI inputs and to CaL on MSNs might have similar effects on MSN excitability. However, an alternative explanation for occlusion of the effect of removing FSI inputs is that the difference in firing between D1 and D2 MSNs has reached its maximum in the absence of differential CaL. Thus our simulations suggest that the absence of dopaminergic modulation of CaL may be a mechanism whereby dopamine depletion disrupts striatal balance.
An imbalance in firing between D1 and D2 MSNs is not the only striatal mechanism considered to produce pathological globus pallidus activity. Changes to synchrony among MSNs have also been suggested to occur after loss of dopamine (Burkhardt et al. 2007; Costa et al. 2006). Experimental and computational studies have shown that synchronously firing MSNs dynamically appear after dopamine depletion (Carrillo-Reid et al. 2008; Humphries et al. 2009), with recent large-scale simulations suggesting that the feedback inhibitory connections between MSNs support the formation and switching between synchronous assemblies of MSNs (Ponzi and Wickens 2010). It is not clear whether dopamine also causes a change in FSI correlation, and whether changes to FSI correlation would contribute to the change in MSN synchrony.
A recent in vivo study reported that dopamine depletion leads to doubling of FSI input to D2 MSNs (Gittis et al. 2011a). Simulations done in that study predicted that this remodeling of FSI input increased the level of synchronization between MSNs. Doubling FSI input to D2 MSNs did not affect striatal balance in our network model (data not shown), suggesting that different mechanisms might be involved in producing imbalanced vs. synchronous firing in the striatum. The link between striatal imbalance and increased correlation of MSNs thus should be explored further.
In summary, using a large-scale network model of heterogeneous, biologically realistic striatal neurons, we have demonstrated a critical role for the synchronization of FSIs through gap junctions in maintaining a balance between the direct and indirect pathway neurons of the striatum. We have also shown that the FSI control of this balance is mediated through channels governing intrinsic excitability differences between the D1 and D2 MSNs, specifically the NaFs and CaL.
GRANTS
This work was supported by Office of Naval Research Grant Multidisciplinary University Research Initiative N00014–10-1–0198 and through the joint National Institutes of Health-National Science Foundation Collaborative Research in Computational Neuroscience program through National Institute on Alcohol Abuse and Alcoholism Grant RO1-AA-016022.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D. and K.T.B. conception and design of research; S.D. and R.C.E. performed experiments; S.D. analyzed data; S.D. and K.T.B. interpreted results of experiments; S.D. prepared figures; S.D. drafted manuscript; S.D., R.C.E., and K.T.B. edited and revised manuscript; S.D., R.C.E., and K.T.B. approved final version of manuscript.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62: 707–719, 1994 [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004 [DOI] [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci 30: 848–859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KT, Czubayko U, Plenz D. Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J Neurosci 23: 9123–9132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JM, Beeman D. Constructing realistic neural simulations with GENESIS. Methods Mol Biol 401: 103–125, 2007 [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol 87: 2190–2194, 2002 [DOI] [PubMed] [Google Scholar]
- Burkhardt JM, Constantinidis C, Anstrom KK, Roberts DCS, Woodward DJ. Synchronous oscillations and phase reorganization in the basal ganglia during akinesia induced by high-dose haloperidol. Eur J Neurosci 26: 1912–1924, 2007 [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Tapia D, Hernández-Cruz A, Galarraga E, Drucker-Colin R, Bargas J. Encoding network states by striatal cell assemblies. J Neurophysiol 99: 1435–1450, 2008 [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci 23: 6245–6254, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Peterson JD, Gertler TS, Glajch KE, Quintana RE, Cui Q, Sebel LE, Plotkin JL, Shen W, Heiman M, Heintz N, Greengard P, Surmeier DJ. Strain-specific regulation of striatal phenotype in Drd2-eGFP BAC transgenic mice. J Neurosci 32: 9124–9132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MAL. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52: 359–369, 2006 [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494: 238–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990 [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci 28: 6483–6492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton JR, Hanson JE, Günay C, Jaeger D. Dendritic sodium channels regulate network integration in globus pallidus neurons: a modeling study. J Neurosci 30: 15146–15159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999 [DOI] [PubMed] [Google Scholar]
- Evans RC, Morera-Herreras T, Cui Y, Du K, Sheehan T, Kotaleski JH, Venance L, Blackwell KT. The effects of NMDA subunit composition on calcium influx and spike timing-dependent plasticity in striatal medium spiny neurons. PLoS Comput Biol 8: e1002493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra-Chacón BJ, Bargas J, Tapia D, Galarraga E. Dopaminergic modulation of corticostriatal responses in medium spiny projection neurons from direct and indirect pathways. Front Syst Neurosci 5: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13: 1189–1215, 1984 [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A 99: 12438–12443, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci 28: 10814–10824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Hang GB, LaDow ES, Shoenfeld LR, Atallah BV, Finkbeiner S, Kreitzer AC. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 71: 858–868, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci 31: 15727–15731, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30: 2223–2234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13: 958–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000 [DOI] [PubMed] [Google Scholar]
- Hjorth J, Blackwell KT, Kotaleski JH. Gap junctions between striatal fast-spiking interneurons regulate spiking activity and synchronization as a function of cortical activity. J Neurosci 29: 5276–5286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Wood R, Gurney K. Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit. Neural Netw 22: 1174–1188, 2009 [DOI] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res 536: 1–15, 1990 [DOI] [PubMed] [Google Scholar]
- Kita H. Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience 70: 925–940, 1996 [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci 24: 7916–7922, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999 [DOI] [PubMed] [Google Scholar]
- Kotaleski JH, Plenz D, Blackwell KT. Using potassium currents to solve signal-to-noise problems in inhibitory feedforward networks of the striatum. J Neurophysiol 95: 331–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643–647, 2007 [DOI] [PubMed] [Google Scholar]
- Krüger J, Aiple F. Multimicroelectrode investigation of monkey striate cortex: spike train correlations in the infragranular layers. J Neurophysiol 60: 798–828, 1988 [DOI] [PubMed] [Google Scholar]
- Lau T, Gage GJ, Berke JD, Zochowski M. Local dynamics of gap-junction-coupled interneuron networks. Phys Biol 7: 16015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Partridge JG, Matta JA, Buonanno A, Vicini S. Long-lasting NMDA receptor-mediated EPSCs in mouse striatal medium spiny neurons. J Neurophysiol 98: 2693–2704, 2007 [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 26: 3875–3884, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. Dichotomous organization of the external globus pallidus. Neuron 74: 1075–1086, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci 28: 14245–14258, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Stefani A, Stratta F, Bernardi G. GABA depolarizes neurons in the rat striatum: an in vivo study. Synapse 8: 38–40, 1991 [DOI] [PubMed] [Google Scholar]
- Moyer JT, Wolf JA, Finkel LH. Effects of dopaminergic modulation on the integrative properties of the ventral striatal medium spiny neuron. J Neurophysiol 98: 3731–3748, 2007 [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215, 2000 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz M, Marin C, Alonso F, Zamarbide I, Lanciego JL, Rodriguez-Diaz M. The origin of motor fluctuations in Parkinson's disease: importance of dopaminergic innervation and basal ganglia circuits. Neurology 62: S17–S30, 2004 [DOI] [PubMed] [Google Scholar]
- Oorschot DE, Lin N, Cooper BH, Reynolds JNJ, Sun H, Wickens JR. Local connectivity between rat striatal spiny projection neurons: a three-dimensional ultrastructural study of somal innervation. IBAGS X. New Jersey: 2010, p. 114 [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJJ, Grillner S, Silberberg G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci 30: 3499–3507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci 26: 436–443, 2003 [DOI] [PubMed] [Google Scholar]
- Ponzi A, Wickens J. Sequentially switching cell assemblies in random inhibitory networks of spiking neurons in the striatum. J Neurosci 30: 5894–5911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Saghyan AA, Paré D. NMDA-dependent facilitation of corticostriatal plasticity by the amygdala. Proc Natl Acad Sci U S A 104: 341–346, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci 22: 8158–8169, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature 394: 475–478, 1998 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci 28: 5504–5512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Bargas J, Galarraga E. Dopaminergic modulation of short-term synaptic plasticity at striatal inhibitory synapses. Proc Natl Acad Sci U S A 104: 10258–10263, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res 160: 189–208, 2007 [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci 21: 9478–9486, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevitt JT, Morrow J, Marshall JF. Dopamine manipulation alters immediate-early gene response of striatal parvalbumin interneurons to cortical stimulation. Brain Res 1035: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- Ts'o DY, Gilbert CD. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci 8: 1712–1727, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88: 1263–1269, 2002 [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res 367: 201–213, 1986 [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci 10: 508–519, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16: 2397–2410, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim MY, Aertsen A, Kumar A. Significance of input correlations in striatal function. PLoS Comput Biol 7: e1002254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]