Abstract
In the absence of sensory information, we rely on past experience or memories to guide our actions. Because previous experimental and clinical reports implicate basal ganglia nuclei in the generation of movement in the absence of sensory stimuli, we ask here whether one output nucleus of the basal ganglia, the substantia nigra pars reticulata (nigra), influences the specification of an eye movement in the absence of sensory information to guide the movement. We manipulated the level of activity of neurons in the nigra by introducing electrical stimulation to the nigra at different time intervals while monkeys made saccades to different locations in two conditions: one in which the target location remained visible and a second in which the target location appeared only briefly, requiring information stored in memory to specify the movement. Electrical manipulation of the nigra occurring during the delay period of the task, when information about the target was maintained in memory, altered the direction and the occurrence of subsequent saccades. Stimulation during other intervals of the memory task or during the delay period of the visually guided saccade task had less effect on eye movements. On stimulated trials, and only when the visual stimulus was absent, monkeys occasionally (∼20% of the time) failed to make saccades. When monkeys made saccades in the absence of a visual stimulus, stimulation of the nigra resulted in a rotation of the endpoints ipsilaterally (∼2°) and increased the reaction time of contralaterally directed saccades. When the visual stimulus was present, stimulation of the nigra resulted in no significant rotation and decreased the reaction time of contralaterally directed saccades slightly. Based on these measurements, stimulation during the delay period of the memory-guided saccade task influenced the metrics of saccades much more than did stimulation during the same period of the visually guided saccade task. Because these effects occurred with manipulation of nigral activity well before the initiation of saccades and in trials in which the visual stimulus was absent, we conclude that information from the basal ganglia influences the specification of an action as it is evolving primarily during performance of memory-guided saccades. When visual information is available to guide the specification of the saccade, as occurs during visually guided saccades, basal ganglia information is less influential.
Keywords: inhibition, movement preparation, eye movements, delay period, memory, decision making, vision, action
the substantia nigra pars reticulata (nigra) is one of two output nuclei of the basal ganglia. In the early '80s it was discovered that neurons in the nigra discharged action potentials tonically and ceased their activity around the time that animals moved their eyes or head toward a sensory stimulus (Hikosaka and Wurtz 1983a; Joseph and Boussaoud 1985). Based on these findings, together with the knowledge that nigral neurons contained the neurotransmitter GABA and made synapses with neurons in the superior colliculus responsible for initiating orienting eye, head, and body movements, it was hypothesized that the nigra acted as a gate for the initiation of saccadic eye movements (Appell and Behan 1990; Behan et al. 1987; Bickford and Hall 1992; Chevalier et al. 1981a, 1981b; Chevalier et al. 1984; DiChiara et al. 1979; Hikosaka and Wurtz 1983c, 1989; Karabelas and Moschovakis 1985). Experiments in which the GABA agonist muscimol was injected into the nigra to inactivate neuronal activity transiently resulted in irrepressible saccades in monkeys and orienting head and body movements in cats (Boussaoud and Joseph 1985; Hikosaka and Wurtz 1985). These findings solidified the role of the nigra as a gate for saccades and lent much support to the more general idea that basal ganglia nuclei were critical for the initiation of action. Indeed, the experiments demonstrating the relationship between the nigra and saccades are the textbook illustration of how the basal ganglia control movement initiation through the process of disinhibition (Purves et al. 2012).
We recently introduced electrical stimulation to the nigra while monkeys performed visually guided and memory-guided saccadic eye movements to test whether the nigra gated the initiation of all types of saccades or only certain saccades, such as those guided by memory. We found that when electrical stimulation occurred at the time of saccade initiation, the metrics of the saccades were altered primarily for memory saccades. Electrical stimulation had less of an effect on saccades when a visual target guided them (Basso and Liu 2007). These results demonstrate that the role of the nigra in saccades is context dependent but also raise the interesting question, are basal ganglia manipulations influencing movement or memory? Specifically, would stimulation occurring well before the cue to move alter subsequent eye movements?
Capitalizing on the precise timing afforded by electrical stimulation, in this article we report on experiments in which we introduced electrical stimulation during different intervals of the memory-guided and visually guided saccade tasks. We tested the hypothesis that manipulation of nigral activity occurring before the onset of a cue to move would influence the spatial memory required for an eye movement. We reasoned that if the nigra was involved in the memory process required to specify the saccade, manipulation of nigral activity during the time that the monkey was maintaining the location of the target in memory would alter whether, where, or when the saccade occurred. In contrast, stimulation during the same period while the target remained visible would have little effect on saccades.
We found that when the target appeared only briefly and monkeys had to remember the location of a target, nigral stimulation during the delay period influenced whether, where, and when saccades were made. In contrast, if the target remained visible throughout the delay period, saccades were less affected by the nigral stimulation. Based on our results, we conclude that activity of the basal ganglia influences the memory processing required for the evolving saccadic eye movement.
MATERIALS AND METHODS
Physiological procedures.
For electrophysiological recording of single neurons, electrical stimulation of neuronal elements, and monitoring of eye movements, chambers and eye movement measuring devices were implanted in four rhesus monkeys (Macaca mulatta) using surgical and electrophysiological procedures as described previously (Basso and Liu 2007; Basso and Wurtz 2002; Basso et al. 2005; Liu and Basso 2008). For access to the nigra, the recording chamber was placed stereotaxically at A7–12, M5 and angled lateral-medial 40°. All experimental protocols were approved by the Institutional Animal Care and Use Committee and complied with or exceeded standards set by the Public Health Service policy on the humane care and use of laboratory animals.
In a typical experiment, an electrode was advanced into the brain targeting the nigra using the grid system (Basso and Liu 2007; Basso and Wurtz 2002; Basso et al. 2005; Crist et al. 1988; Handel and Glimcher 1999; Hikosaka and Wurtz 1983d; Liu and Basso 2008). We stimulated in the nigra at locations in which we found clear visual, delay, and/or saccade-related activity, either pauses or increases in neuronal discharge that occurred during the performance of visually guided saccades (Basso and Sommer 2011; Handel and Glimcher 1999; Sato and Hikosaka 2002; Shin and Sommer 2010; Shires et al. 2010). In our experience, the different neuronal response profiles are intermingled throughout the extent of the nigra, and with our 40° angled chamber, this corresponds to a roughly 2 × 2 × 4-mm3 volume of tissue and 2–3 grid holes. Therefore, a precise mapping of neuronal response properties at particular locations to specific stimulation effects is not possible. Electrical stimulation parameters were 300 Hz, pulse width 150–200 μS, and current intensity 40–80 μA. These parameters were selected on the basis of our previous experience with stimulation in the nigra (Basso and Liu 2007; Liu and Basso 2008). A Grass S88k dual-output square-wave pulse generator provided the input driving two PSIU6 photoelectric stimulus isolation units. Each of these units produced one phase of a biphasic pulse with constant current. For safety, these units were optically isolated (Grass Technologies, AstroMed). The pulses in the stimulation trains were current-balanced to minimize tissue damage and generally were anodal leading cathodal pulses. To ensure accurate current intensities, we measured the current before and after stimulation experiments on an oscilloscope using a 10-Ω resistor in series with the stimulating electrode. Train durations, which were determined by the length of the randomized delay period of the task, varied among 200–1,200 ms (see Behavioral procedures).
Behavioral procedures.
For the first set of experiments, we used a real-time experimental data acquisition and visual stimulus generation system (Tempo and VideoSync; Reflective Computing) to create the behavioral paradigms and acquire eye position and single-neuron data as described previously (Li et al. 2006; Li and Basso 2005). Visual stimuli were rear-projected on a tangent screen at a 51-cm distance with the use of a digital light-processing (DLP) projector (LP130, Infocus, Wilsonville, OR; PLC-WXE-45, Sanyo, San Diego, CA) at its native resolution and operating at 60 Hz. The centrally located fixation spot had a luminance of 6.5 cd/m2, and the target spots were white with a luminance of 41.0 cd/m2. The background luminance was 0.6 cd/m2. The visual stimulus presentation was controlled by VideoSync software (Reflective Computing) running on a dedicated personal computer (PC) with a 1,024 × 768 video graphics array (VGA) video controller (Computer Boards).
In the second set of experiments we used the REX and VEX real time experimental system developed and distributed by the Laboratory of Sensorimotor Research, National Eye Institute (Bethesda, MD; Hays et al. 1982) to create the behavioral paradigms and acquire eye position and a single channel of neuronal data or to deliver electrical stimulation through the electrode. Visual stimuli were rear-projected onto a screen at a 51-cm distance from the subject with the use of a short-throw projector (LP130, Infocus; PLC-WXE-45, Sanyo) at its native resolution and operating at 60 Hz. A photocell secured to the screen sent a transistor-transistor logic pulse to the experimental PC, providing an accurate measure of stimulus onset. The fixation spot at the center of the screen had a luminance of 6.5 cd/m2 (mean of 3 measurements). Visual stimuli had luminance values of 41.0 cd/m2 (mean of 3 measurements). The background luminance was 0.6 cd/m2 (mean of 3 measurements). The PC for the visual stimulus display was a slave device to the PC used for experimental control and data acquisition.
Four monkeys performed delayed saccades guided by memory or by vision (Hikosaka and Wurtz 1983c). A fixation spot appeared initially at the center of a screen (Fig. 1, C and D). After a delay (500–1,500 ms), a second spot appeared in the periphery of the visual field. In the memory version of the task, the peripheral, target spot disappeared after 200 ms. In the visual version, this target spot remained illuminated. A second delay period began and lasted for 800–1,200 ms. At the end of the delay, the fixation spot disappeared, cueing the monkeys to initiate a saccade to the location of the visible target in the visual task or the previously visible target in the memory task.
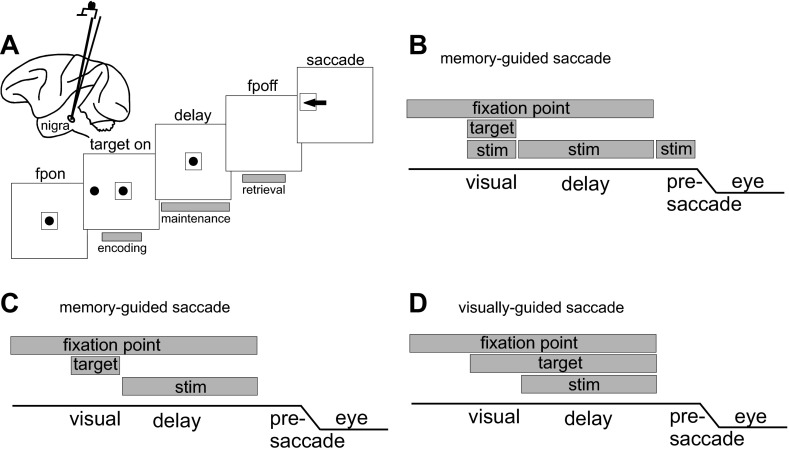
Fig. 1.
Experimental task. A: schematic view of the lateral surface of a monkey brain. Electrical stimulation targeted the substantia nigra pars reticulata (nigra) as shown by the electrode in the brain schematic. The 5 large squares show the spatial arrangement of the memory-guided saccade task. The centrally located black circle indicates the fixation point (fp). The peripherally located black circle shows 1 example target location. The small square around the circles indicates the eye position criterion required for assessment of performance accuracy. The arrow indicates a correct saccade. The gray rectangles below the screen image show the timing of the electrical stimulation that occurred in the first set of experiments in 2 monkeys. B: temporal arrangement of the memory-guided saccade task. The gray rectangle labeled “fixation point” indicates the time and duration of fixation; the gray rectangle labeled “target” shows the time and duration of the visual stimulus, and the gray rectangles labeled “stim” show the time and duration of the electrical stimulation. The line labeled “eye” shows a schematic of the eye position. C: same as in B for the 2nd set of experiments in which visually guided and memory-guided saccades were interleaved with stimulation only during the delay period; this panel shows the memory-guided saccade condition. D: same as in C for the visually guided saccade condition.
The peripheral, target spot appeared at one of eight possible locations throughout the visual field, either contralateral or ipsilateral to the stimulation site and in some cases vertically. The response fields of nigral neurons are generally very large, encompassing one hemifield, so we placed our stimuli at 10–15° amplitude at three locations in either hemifield for the first set of experiments (see Fig. 2) and in the four cardinal and four oblique positions for the second set of experiments (see Fig. 6). We performed two sets of experiments in two different pairs of monkeys. In the first experiment, electrical stimulation occurred during the visual (encoding), delay (maintenance), or presaccade (retrieval) intervals of the task (Fig. 1, A and B, gray bars) in monkeys E and B on randomly interleaved trials. During the visual interval, the stimulation occurred at the same time as the onset of the visual target spot and lasted as long as the stimulus was visible (200 ms). During the delay interval, electrical stimulation depended on the length of the delay period, which was randomized among 800–1,200 ms. As one control for the train duration, we also included a 200-ms-duration train presented at random times during the delay period. During the presaccade phase, electrical stimulation occurred at the same time as the fixation spot removal and lasted until the onset of the saccade (∼200 ms). With the use of an automated algorithm, the stimulator was turned off as soon as the eye moved 0.3°, ensuring no or minimal stimulation during the movement. To ensure further that the effects were not a result of stimulation during the movement, we used a second condition in which a fixed, 100-ms train of electrical stimulation began when the cue to make a saccade appeared. Each of the target locations and each of the stimulation trial types [control (no stimulation), visual, delay, and presaccade] were interleaved randomly to minimize the possibility that knowledge of the trial type influenced the results.
Fig. 2.
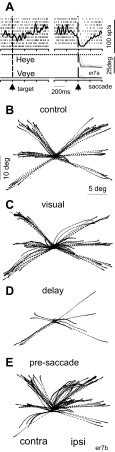
Nigral stimulation during the delay period influences saccades. An example recording and stimulation site show that electrical stimulation of the nigra well before the onset of a saccade influences the occurrence of subsequent saccades. A: raster plot and spike density function (σ = 12 ms) of the neuron recorded at the site. Each tick indicates the time of an action potential; each row of ticks is 1 trial of the delayed saccade task. Left trace is aligned on the onset of the target (vertical dashed line and arrowhead); right trace is aligned on saccade onset (vertical dashed line and arrowhead). The position traces below the rasters are horizontal (H eye) and vertical eye positions (V eye). Downward movement is shown leftward by convention. er7a is a file identifier. B: control (no stimulation) eye position traces plotted from the time the saccade started to 300 ms at the site where the neuron shown in A was recorded. C: same as in B for the condition in which stimulation occurred during the visual (encoding) interval. D: same as in B for the condition in which the stimulation occurred during the delay period (maintenance). E: same as in B for the condition in which the stimulation occurred during the presaccade (retrieval) interval. Contralateral (contra) and ipsilateral (ipsi) hemifields are indicated with respect to the site of stimulation. See Fig. 1 for stimulation intervals relative to the task events. er7b is a file identifier.
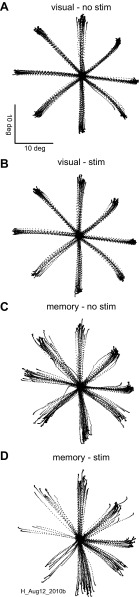
Fig. 6.
Nigral stimulation during the delay period of visually guided and memory- guided saccades influences only memory-guided saccades. A: visually guided saccade trajectories without stimulation (visual no stim) are plotted from the time of the fixation point offset for 300 ms. B: same as in A for the condition in which stimulation occurred (visual stim). C: same as in A for memory-guided saccades (memory no stim). D: same as in B for memory-guided saccades (memory - stim). H_Aug12_2010b is a file identifier.
In the second experiment in monkeys H and L, we randomly interleaved visually guided saccade trials and memory-guided saccade trials along with electrical stimulation only during the delay period of both types of tasks (Fig. 1. C and D). The visually guided saccade task is identical to the memory-guided saccade task except that the target for the saccade remains illuminated until after the saccade is made correctly. By performing this manipulation, we tested directly whether manipulation of basal ganglia neuronal activity influenced the specification of a saccade with or without visual stimuli present. To determine whether the monkeys made saccades accurately, we used an electronic window centered on the target position. If the eye position was located within this window, the trial was scored automatically as correct. Because we expected stimulation to perturb eye movements, we used a very liberal criterion (30–40° square, sometimes extending across hemifields) on stimulated trials. To capture the trials in which monkeys failed to make a saccade, we included these “error” trials. Therefore, the trials used for analysis include both “correct” and “error” trials. We indicate on a case-by-case basis whether correct only or correct and error trials are displayed.
Data analysis.
All data were analyzed using standard parametric, inferential statistics. The data were tested for normality using the Lilliefors test. If this test failed, nonparametric tests (e.g., Wilcoxon signed rank) were used. For determination of the distribution of saccade vectors in the different stimulation conditions, we applied circular statistics. We used the Kuiper test to assess normality (Batschelet 1981). If they passed this test of normality (all data sets in our sample did), we then made comparisons using parametric ANOVA (or F-test) on the endpoints and lengths separately for the different stimulation conditions. If statistical significance was assessed, then where appropriate, paired comparisons were made using the Bonferroni correction by implementing multcompare(). For this we set the alpha level to equal 0.05/(number of comparisons). To assess the differences in variability, we used the F-test [vartest2()] or ANOVA, as indicated in the text, to test the hypothesis that two independent samples come from normal distributions with the same variance against the alternative, that they come from normal distributions with different variances. To assess rotations of vectors, we used circ_kuipertest(), which is the circular analog of the Kolmogorov-Smirnov test. All data and plots were analyzed and created using Matlab (The MathWorks).
RESULTS
We recorded and stimulated from 69 sites in the substantia nigra (nigra) of four monkeys. Eleven sites were in monkey E, 17 were in monkey B, 24 were in monkey H, and 17 were in monkey L. At each site we first recorded neuronal activity to characterize it at that site. We observed response profiles of nigral neurons similar to those previously reported by us and others (Basso and Liu 2007; Basso and Wurtz 2002; Basso et al. 2005; Bayer et al. 2002; Handel and Glimcher 1999, 2000; Hikosaka and Wurtz 1983a, 1983b, 1983c; Liu and Basso 2008). After briefly documenting the local response properties, we introduced electrical stimulation at the site during performance of the memory-guided saccade task or the visually guided saccade task. Because of the small volume of tissue that makes up the visual-oculomotor nigra and the intermingling of response profiles of neurons throughout the nigra, we found no clear relationship between the neuronal responses recorded and the stimulation effects at the same sites. This is likely due to the fact electrical stimulation influences multiple different, intermingled neuronal cell types and that a large population of nigral neurons contributes to the eye movement. Despite this, we found systematic changes in the endpoints of eye movements when stimulation occurred well before the onset of an eye movement and predominantly when the eye movement was made in absence of visual stimuli to guide it. In what follows, we describe the results from the first set of experiments in which we stimulated during different intervals of the memory-guided saccade task only (2 monkeys: monkeys B and E). We then describe the results from experiments in which we stimulated during the delay period of both the memory-guided and visually guided saccade tasks (2 monkeys: monkeys H and L). Figure 1 shows the experimental and behavioral paradigms in the two experiments.
An example experiment from one monkey (monkey E) is shown in Fig. 2. The neuron recorded at this site decreased its activity before and during the saccades made to the hemifield contralateral to the recording site (Fig. 2A). Electrical stimulation at this site during the different epochs of the memory-guided saccade task influenced the subsequent occurrence of saccades. In this example, the most prominent effect occurred with electrical stimulation during the delay interval. This effect was apparent as the monkey making fewer saccades, and when saccades were made, they were often altered (Fig. 2D). When the stimulation occurred during the visual (Fig. 2C) or the presaccade intervals (Fig. 2E), the characteristics of the saccades were different from those made in the no-stimulation trials (cf., Fig. 2, B and E). For example, in some cases, the monkey avoided making saccades into the lower, contralateral hemifield and more frequently made saccades into the upper, ipsilateral hemifield, and these were abnormal as well(Fig. 2E).
To show the influence of stimulation during the different intervals of the memory-guided saccade task on the endpoints of saccades, we plotted the average saccadic endpoint for each target location for each experiment and each stimulation condition in Fig. 3. Each circle shows the mean endpoint for one experiment, and the color of the circles indicates the instructed target position. The size of the circle indicates the frequency of the average endpoints occurring within 5° of the position of the circle. The triangles show the example from Fig. 2. Figure 3 shows that across the sample of 28 stimulation sites in these 2 monkeys, the scatter in the endpoints was most prominent when stimulation occurred during the delay interval (Fig. 3C). The effects appear for endpoints located both ipsilateral and contralateral to the site of stimulation.
Fig. 3.
Stimulation of the nigra during the visual, delay, and presaccade intervals in the memory-guided saccade task. The mean saccadic endpoints for each of the 28 experiments from 2 animals are plotted. Different colors indicate different target positions. The size of the circles indicates the frequency of the endpoints that were within 5° of the target location. Triangles show the data points for the example data shown in Fig. 2. A: endpoints when no stimulation occurred (control). B: endpoints when stimulation occurred during the visual interval. C: endpoints when stimulation occurred during the delay interval. D: endpoints when stimulation occurred during the presaccade interval. Data are taken from correct trials.
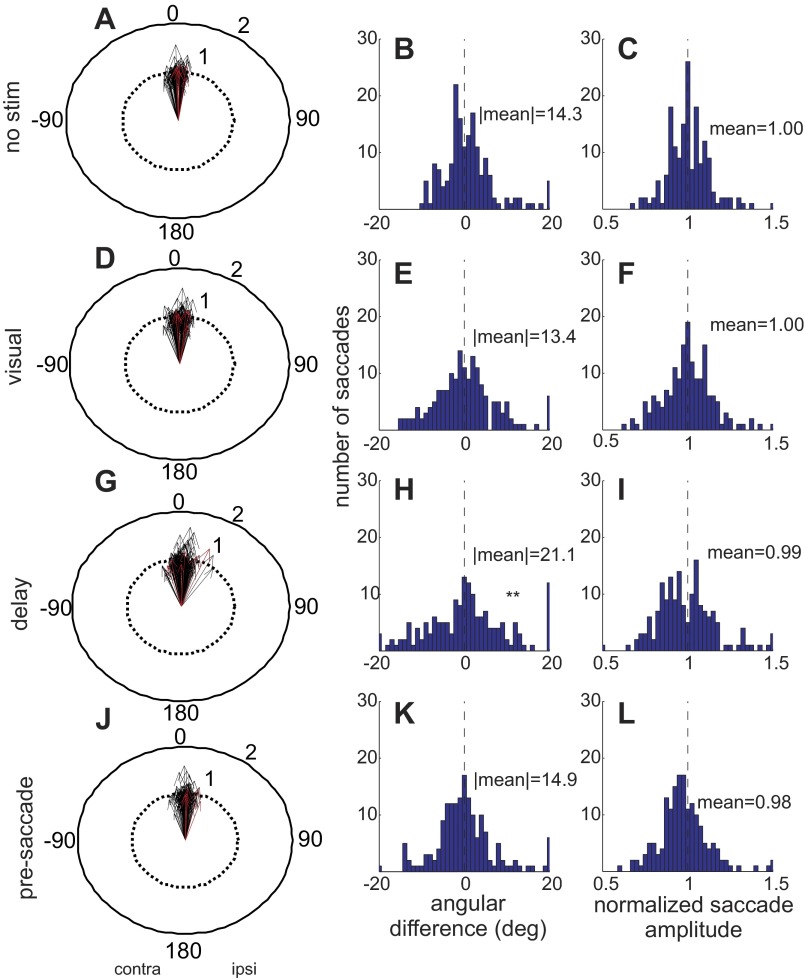
To quantify the changes in saccade endpoints across our sample of stimulation sites, we measured the direction and length of the saccade vectors for all trials in all conditions for each experiment. We calculated an idealized vector as the mean of the saccade endpoints made by an animal within an experimental session for each target position in the no-stimulation condition. Using these idealized vectors, we computed direction and length differences for each saccade made in the stimulation condition of each experiment. For the direction difference, we subtracted each saccadic endpoint measured in stimulation trials from the idealized, no-stimulation endpoint in the equation θstim − θno stim, where θ = x2 + y2. We then reflected the differences so that negative angles indicated contralateral rotations (with respect to the side of stimulation) and positive angles indicated ipsilateral rotations (with respect to the side of stimulation) of the saccade direction. For the amplitude data, we normalized vector lengths similarly using the idealized saccade length in the equation 1 + (ρstim − ρno stim)/ρno stim, where ρ is the length of the vector. We then plotted the differences in polar coordinates for each condition of stimulation (Fig. 4, A, D, G, and J). Vectors pointing to 0° (upward) indicate no change in the direction of the saccade vector from ideal with stimulation. Vectors pointing toward 180° (downward) indicate that the saccade direction was rotated into the opposite hemifield with stimulation of the nigra. A length of 1 in this plot indicates that the amplitude of the saccade was unchanged with nigral stimulation, whereas lengths <1 indicate the saccade was hypometric and lengths >1 indicate the saccade was hypermetric.
Fig. 4.
Stimulation of the nigra during the visual, delay, and presaccade intervals in the memory-guided condition. A, D, G, and J: the normalized vectors of memory-guided saccades are drawn as arrows. Each black arrow is an average of at least 10 saccades. The red arrows show the example result from Fig. 2. The lengths 1 and 2 indicate the length of the saccades relative to the idealized saccade length. The direction of the arrows shows the direction of the saccade relative to the direction of an idealized saccade direction (see text for details). For the plot in A, the saccades that were made on trials without stimulation are normalized to the idealized saccades. B: distribution of the differences in saccade angles between the idealized saccades and the nonstimulated saccades. C: same as in B for saccade lengths. D: same as in A for saccades made while nigral stimulation occurred during the visual interval. E and F: same as in B and C for saccade trials in which stimulation occurred during the visual interval. G: same as in A for saccades made with stimulation during the delay period. H and I: same as in B and C for saccades made with nigral stimulation during the delay period. J: same as in A for saccades made with stimulation during the presaccade interval. K and L: same as in B and C for saccades made with stimulation during the presaccade interval. **P < 0.05, indicating that the distribution was significantly more variable with stimulation than without. All eye movements are from correct trials.
Figure 4 shows the changes in saccades that occurred with stimulation of the nigra from 28 stimulation sites in 2 monkeys. The red arrows indicate the example results shown in Fig. 2. To get a measure of the spread of the saccade directions that occurred with and without stimulation, we took the absolute value of the endpoint differences in the different experimental conditions. For the no-stimulation conditions the spread was 14.3° (Fig. 4B), indicating the inherent variability in the direction of memory-guided saccades.
When stimulation occurred during the visual interval, we measured a 13.4° spread in the distribution of saccade angles (Fig. 4E). When stimulation occurred during the delay interval, the spread of directions increased to 21.1° (Fig. 4H). Stimulation during the presaccade interval, when the stimulation ended before the eye movement started, resulted in a 14.9° spread (Fig. 4K). Comparing these distributions with an ANOVA revealed a significant difference between the four conditions [ANOVA, F(3,111) = 8.34, P < 0.005]. Subsequent multiple comparisons with Bonferroni corrections revealed that the significant difference resulted from the differences between the delay condition and all others (Fig. 4, G and H). None of the other comparisons were significantly different from the no-stimulation condition. Thus electrical stimulation of the nigra during the delay period and not the visual or presaccade intervals altered the variability of saccadic endpoints. In addition to the change in endpoint variability, the mean of the distribution of the endpoints tended to be rotated ipsilaterally when stimulation occurred during the delay interval and not any of the other intervals. Only the mean of the saccade endpoints occurring with delay interval stimulation was significantly different from control, showing an ipsilateral shift of 1.44° from −1.43° to 0.01° (Kuiper test, P = 0.001). The means for the visual interval stimulation and presaccade interval stimulation were −2.78° and −0.85°, respectively, and were not significantly different from control (Kuiper test, P > 0.1 visual; P > 0.1 presaccade). The delay interval stimulation mean was not significantly different from visual interval stimulation (Kuiper test, P > 0.1) but was significantly different from presaccade interval stimulation (Kuiper test, P = 0.005). Given that the stimulation showed no significant bias for particular directions as we also found in our previous work (Basso and Liu 2007), this suggests that the influence of the nigra is widespread. We also quantified changes in amplitude that occurred with stimulation of the nigra. In this case, stimulation during all three intervals had no effect on mean saccade lengths [ANOVA, F(3,671) = 1.04, P = 0.376] or saccade length variability [ANOVA, F(3,111) = 2.17, P = 0.096].
We next quantified the observation shown in Fig. 2D that monkeys sometimes failed to make saccades with stimulation of the nigra. We defined failure as not having made a saccade within 1,000 ms of the fixation point removal (the go cue). We counted the number of saccades made in each trial condition across the 28 stimulation sites. Since all conditions were randomly interleaved, each trial type and each target location occurred with equal probability. Figure 5, A and B, shows the results for the two monkeys. For saccades directed contralaterally relative to the site in the nigra, stimulation during the visual (encoding) interval produced ∼10% reduction in saccade frequency (Fig. 5A). Stimulation during the delay interval (maintenance) reduced the probability of saccade occurrence by ∼20%. Stimulation during the presaccade interval (retrieval) failed to change the probability of saccade occurrence. An ANOVA between the no-stimulation, encoding, maintenance, and retrieval conditions revealed significant differences [ANOVA, F(3,335 = 9.05, P < 0.001], and multiple comparisons revealed that only the maintenance stimulation was significantly different from the no-stimulation condition. For saccades directed ipsilaterally relative to the site of stimulation, there were statistically significant differences between the stimulation conditions, and multiple comparison tests revealed that only stimulation during the delay period (maintenance) showed a significant reduction in saccade probability [Fig. 5B; ANOVA, F(3,335) = 5.28, P = 0.001]. These results show that whether or not a saccade is made is influenced by alterations of the activity of neurons within the nigra during the time that a target for a saccade is maintained in memory.
Fig. 5.
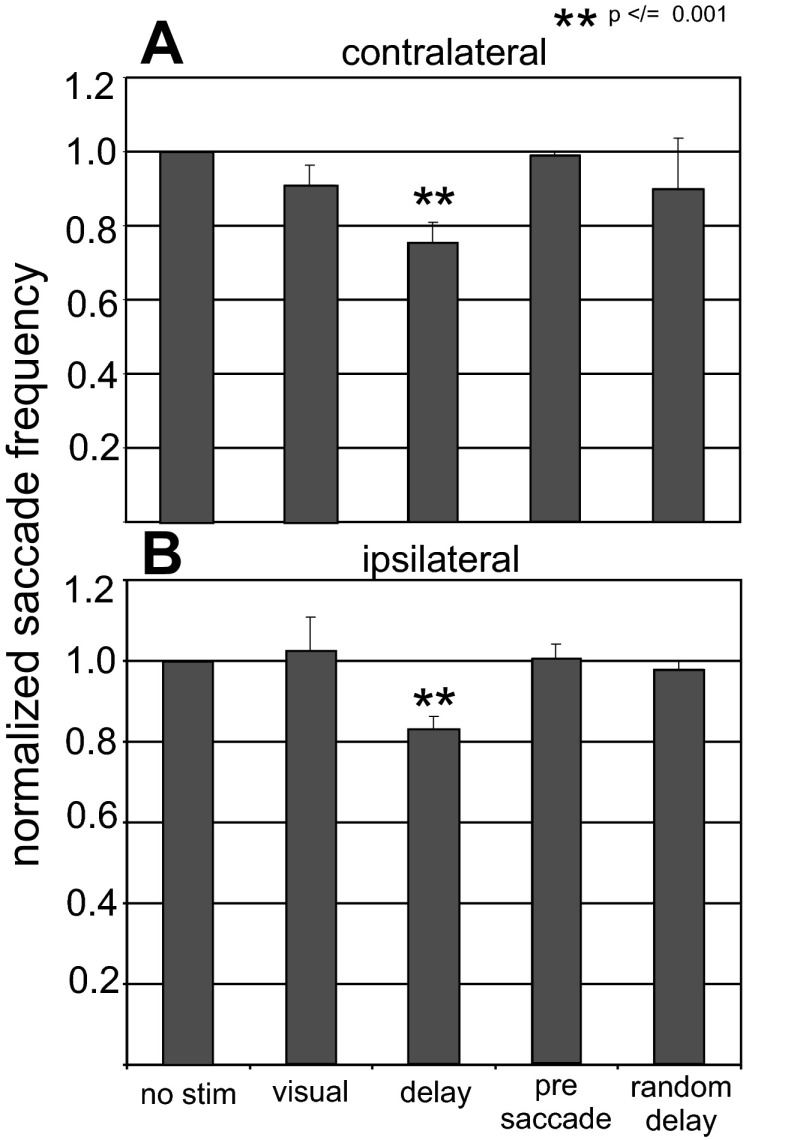
Stimulation of the nigra reduces saccade probability. The number of saccades made with stimulation is normalized to the number made without stimulation, and this ratio is plotted for each of the stimulation conditions from the memory-guided saccade task. Data from the encoding, maintenance, and retrieval conditions come from 2 monkeys and 28 stimulation sites. Data for the random interval condition come from the same 2 monkeys and only 20 of the 28 sites. No stim is the no-stimulation condition, visual is stimulation during the encoding phase, delay is stimulation during the maintenance phase, presaccade is stimulation during the retrieval phase, and random delay is from the condition in which the stimulation occurred for a fixed length of 200 ms at random intervals during the delay period. Contralateral and ipsilateral refer to the direction of the saccades made relative to the hemisphere that was stimulated. **P ≤ 0.001.
Thus far, we have described the results from experiments in which stimulation of the nigra occurred during different intervals of the memory-guided saccade task. This experiment, although insightful, confounds stimulation duration with stimulation interval. One possible interpretation is that the effects seen with delay period stimulation resulted because the length of stimulation was so much longer than the other intervals of stimulation. We performed two control experiments for this. For one, we introduced a brief, 200-ms-duration stimulation at random times during the delay period. We assessed the effects of these short pulses of stimulation on saccades in 21 additional sites in 2 monkeys (monkeys B and E). This manipulation had no significant effect on the variability of saccadic amplitudes or directions, but the 200-ms stimulation did alter saccade probability. The reduction in saccade probability was more prominent in one of our monkeys (∼25% in monkey B vs. ∼3% in monkey E), and overall there was a ∼14% decreased probability of the occurrence of contralateral saccades (Fig. 5). Across both monkeys, an ANOVA comparing the no-stimulation, encoding, maintenance, retrieval, and random delay conditions revealed a significant difference for contralaterally directed saccades [contralateral: ANOVA, F(4,314) = 3.67, P < 0.006; ipsilateral: ANOVA, F(4,314) = 2.24, P = 0.06]. Subsequent multiple comparisons revealed that this main effect was caused by the significant difference between the no-stimulation and maintenance intervals for contralaterally directed saccades. Note that this experiment was performed at only 20 of the 28 stimulation sites, requiring us to eliminate some data for this second analysis. Because the delay period could be as long as 1,200 ms in this task, leaving plenty of time for the monkey to specify and prepare an eye movement, the finding that a brief, 200-ms pulse of stimulation to the nigra influenced saccades occurring much later in time, even occasionally, was remarkable. Nevertheless, the lack of statistical confirmation of this result leaves this experiment inconclusive.
Introducing electrical stimulation during a 200-ms interval at random times during the delay period does not necessarily manipulate the neuronal activity during the portion of the delay period that is critical for remembering the spatial location of the target. This uncertainty may explain the smaller and not statistically significant effects seen in this manipulation compared with those seen when we stimulated during the entire delay period. Therefore, we performed an additional experiment in which we introduced electrical stimulation of the nigra during the entire delay period of the memory-guided saccade task and compared the effects with those measured when the same stimulation occurred during the delay period of the visually guided saccade task. In this way, the timing and duration of the electrical stimulation as well as the instructed saccade and the expected value of the saccade were identical. The only difference was whether or not the monkey had to rely on its memory to guide the saccadic eye movement.
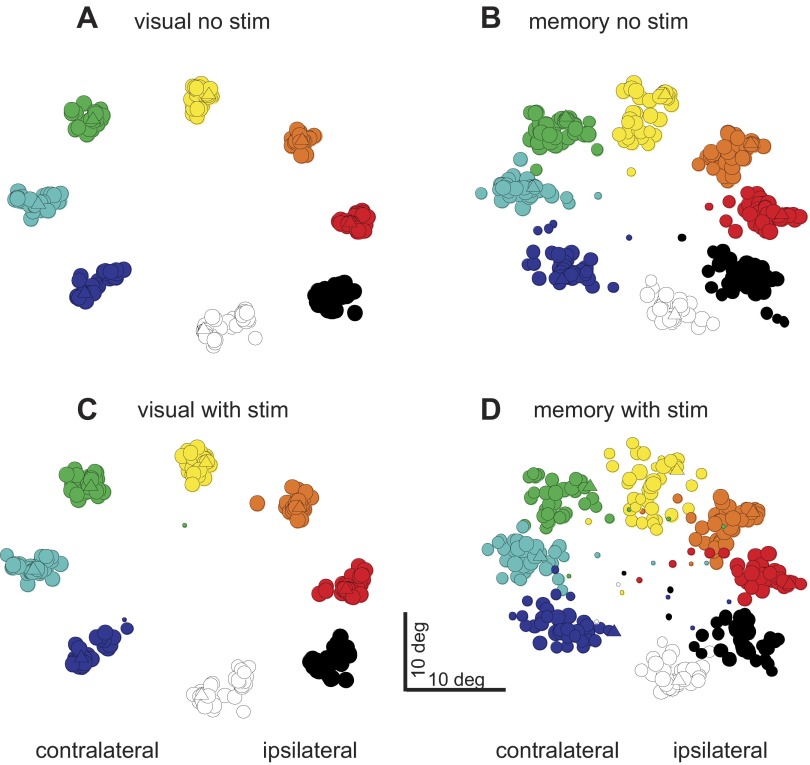
We performed this experiment in 41 sites in 2 additional monkeys (Fig. 1; monkeys L and H). Stimulation during the delay period of memory-guided saccades influenced the directions and amplitudes of saccades as well as the frequency of the occurrence of saccades. We observed this result when the stimulation occurred in the absence of a visual stimulus but not in the presence of a visual stimulus. Figure 6 shows an example of these effects. The stimulation influenced the saccades, but only when it occurred during the delay period of the memory-guided saccade task (Fig. 6D). The same stimulation had little effect if the visual stimulus remained illuminated during the delay (Fig. 6B). To assess these results across our sample of 41 stimulation sites in 2 monkeys, we plotted the endpoints of saccades for correct and error trials for the saccades made in the visually guided and memory-guided tasks with and without stimulation. This plot is the same as that shown in Fig. 3. The triangles show the example data from Fig. 6. Visually guided saccades are quite accurate and precise (Fig. 7A), whereas memory-guided saccades are less so (Fig. 7B). The introduction of electrical stimulation during the delay period of the visually guided saccade task altered the endpoints very little (Fig. 7C), whereas the saccades made in the memory-guided trials with stimulation were often in error (Fig. 7D). The plot shows the frequency with which an endpoint occurred within 5° of a location. The size of the circles indicates the frequency of the endpoint occurrence, and the color indicates the instructed target location. Note that, in some cases, the eye movement was instructed to the upper left target but landed in the upper right (Fig. 7D; green circle in the center of the orange circles and purple circle between the red and black circles). Note that the points in the center of Fig. 7D may also include some “flipped” saccades, but since they are plotted as averages they appear in the middle.
Fig. 7.
Stimulation of the nigra during the delay period of memory-guided saccades alters the variability of saccadic eye movement endpoints. The arrangement is the same as in Fig. 3, although these data are from the 2nd set of experiments and monkeys (2 monkeys, 41 sites). Triangles show the data points from the example data shown in Fig. 6. Data are from correct and error trials. A: visually guided trials without stimulation (visual no stim). B: memory-guided trials without stim (memory no stim). C: visually guided trials with stimulation during the delay period (visual with stim). D: memory-guided trials with stimulation during the delay period (memory with stim). Contralateral and ipsilateral are indicated with respect to the site of stimulation. Note that for some of these trials the eye movement endpoints showed large rotations with stimulation in the memory condition (green circle in the middle of orange circles in D; purple circle next to black and red circles).
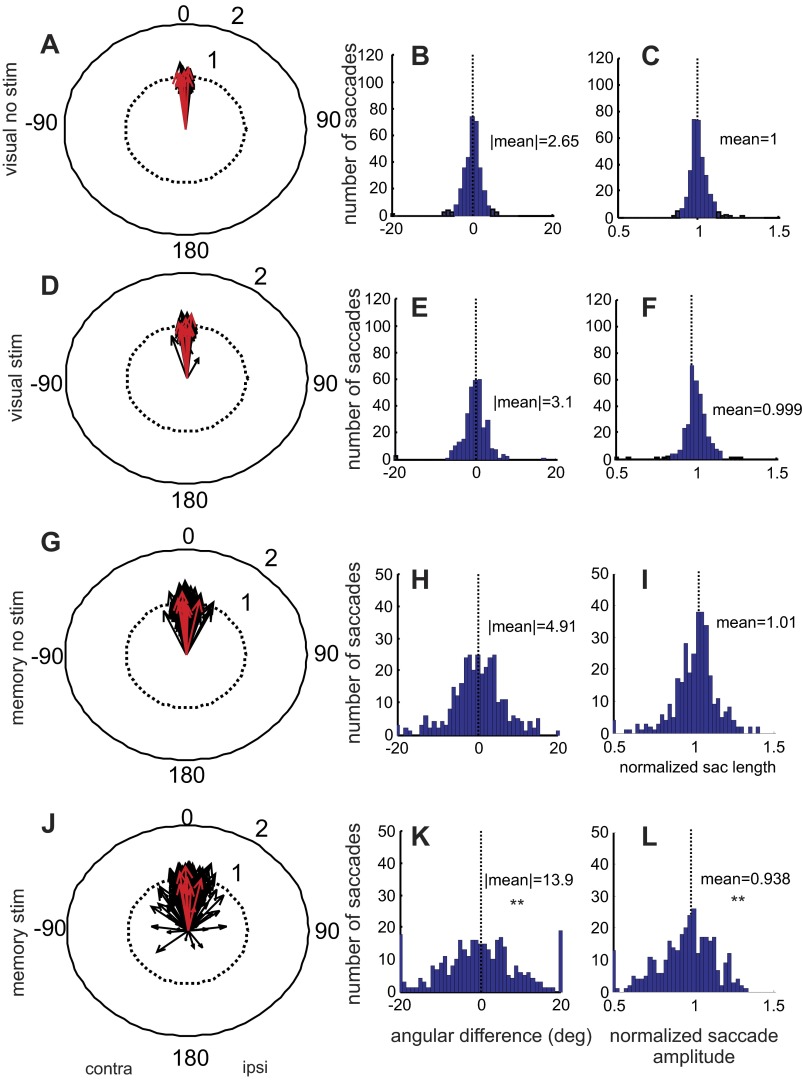
The polar plots shown in Fig. 8 were populated in the same way as those shown in Fig. 4 except the data generated in the second set of experiments were used. The red arrows in Fig. 8 show the example result from Fig. 6. The histograms in Fig. 8 show the pattern of changes in saccades across the sample of 41 stimulation sites in 2 monkeys. To get a measure of the spread of the saccade directions that occurred with and without stimulation, we took the absolute value of the differences in the different experimental conditions as we did for the data shown in Fig. 4. For the no-stimulation visually guided condition, the spread was slight, only 2.65°, consistent with the precision of visually guided eye movements (Fig. 8, A and B). Stimulation of the nigra resulted in a variability measure of 3.1° (Fig. 8, D and E). For memory-guided saccades, the variability in saccade direction even without stimulation was 4.91° (Fig. 8, G and H). Electrical stimulation of the nigra during the delay period of the memory-guided saccade had a large effect on the spread of saccade directions, increasing this to 13.9° (Fig. 8, J and K). We performed an ANOVA on the variability measures across the four conditions and found significant differences [ANOVA, F(3, 163) = 6.69, P = 0.003]. Subsequent multiple comparisons with Bonferroni corrections revealed that the significance stemmed from differences between the memory stimulation condition and all others. There were no differences between the variability of the visual saccades with or without stimulation and the memory saccades without stimulation.
Fig. 8.
Stimulation of the nigra during the delay period alters the variability of saccadic eye movement endpoints and lengths of saccades in the absence of visual information. The arrangement is identical to that of Fig. 4. Data are from experiments in which both the memory-guided and visually guided saccade tasks were performed. Visual no stim indicates visually guided trials without stimulation of the nigra; visual stim indicates trials in which stimulation occurred. Memory no stim indicates trials in which memory-guided saccades were performed and stimulation of the nigra did not occur; memory stim indicates trials in which nigral stimulation occurred. **P < 0.05, indicating that the distribution was significantly more variable with stimulation than without. The red arrows show the example result that appears in Fig. 6.
For saccade lengths, we found that stimulation of the nigra during the delay period shortened the length of the memory-guided saccades by ∼6% compared with the same eye movements without stimulation (cf., Fig. 8, C and L), whereas visually guided saccade length was little affected by stimulation during the delay period (cf., Fig. 8, C and F). The differences in saccade length measured between the no-stimulation and stimulation conditions were statistically significant [ANOVA, F(3,163) = 25.06, P < 0.0001; Fig. 8J], and subsequent multiple comparisons with Bonferroni corrections revealed that the differences occurred only for the memory stimulation condition compared with all others.
When electrical stimulation of the nigra occurred during the delay period, the mean of the distributions of the saccade angles was rotated ipsilaterally compared with the no-stimulation distribution for only the memory-guided saccades. The mean shift for visually guided saccades was from 0.19° to 0.004°, which was not statistically significant (Kuiper test, P = 1.00). For memory-guided saccades, the mean shifted significantly from −2.39° to −0.57°, a +1.86° ipsilateral shift (Kuiper test, P = 0.001). Because the differences in the saccade endpoints with stimulation of the nigra were seen primarily during memory-guided saccades, we conclude that the effects of nigral stimulation during the delay period are greater in the absence of a visual stimulus. Note that if the results depended solely on the length of the stimulus train, we should have observed quantitatively similar effects on visually guided saccades and memory-guided saccades.
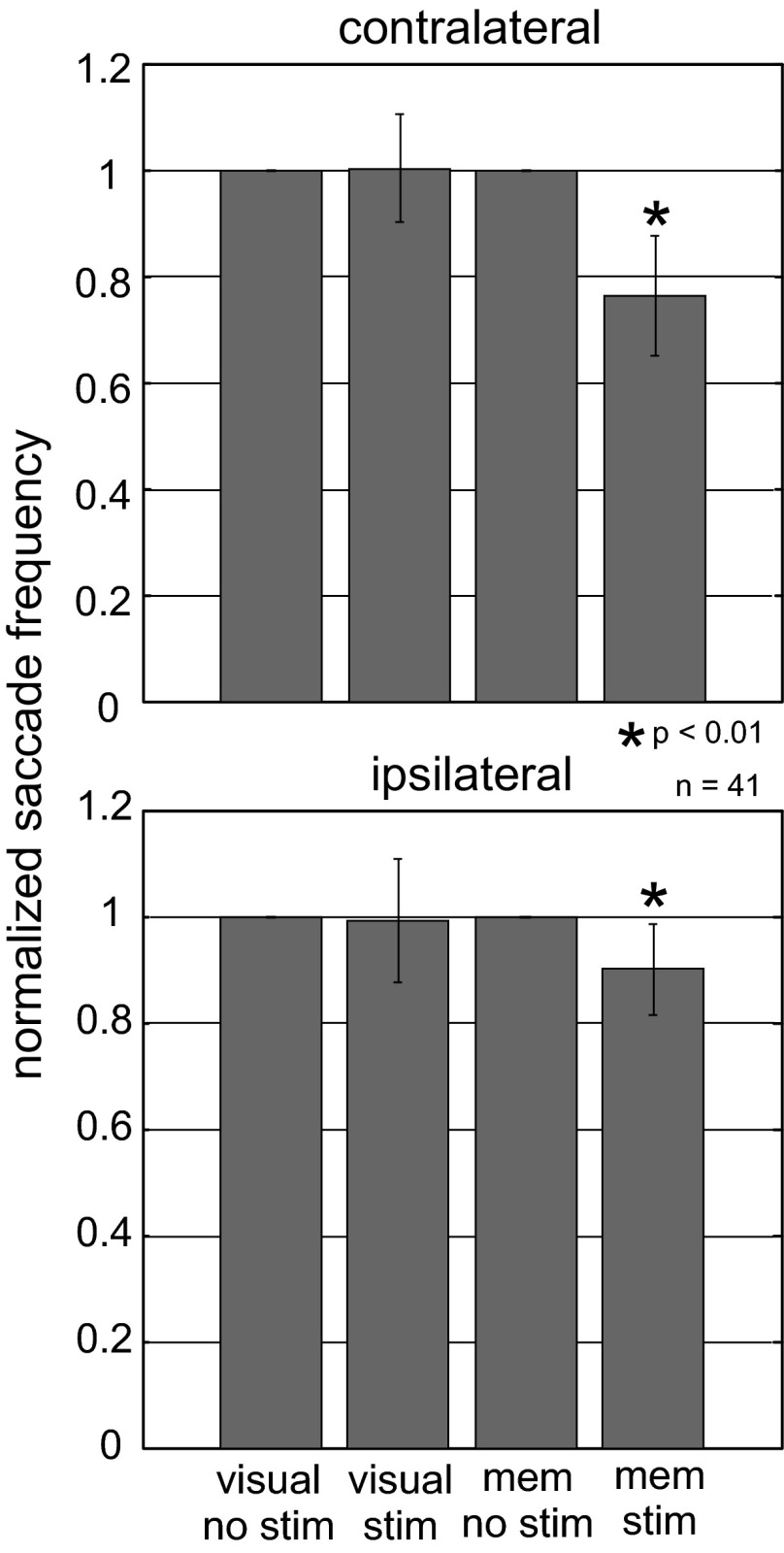
We next assessed whether stimulation of the nigra during memory- or visually guided saccades influenced the probability of making a saccade, as we did in the previous experiments shown in Fig. 5. Indeed, stimulation of the nigra during the delay period altered the ability of monkeys to make saccades in the absence of a visual stimulus but not in the presence of a visual stimulus (Fig. 9). A 23.7% reduction in saccade frequency occurred when the instructed location was contralateral to the side of stimulation. A 9.8% reduction in saccade frequency occurred when the instructed location was ipsilateral to the side of stimulation. The reductions were statistically significant for both sides [contralateral: ANOVA, F(3,163) = 113.08, P < 0.001; ipsilateral: ANOVA, F(3,163) = 4.6, P = 0.004]. Furthermore, the frequency reduction for contralaterally directed saccades was significantly different from the frequency reduction seen for saccades directed ipsilaterally [ANOVA, F(7,320) = 38.54, P < 0.00001].
Fig. 9.
Stimulation of the nigra reduces saccade probability in the absence of visual information. The number of saccades made with stimulation during the delay period was normalized to the number made without stimulation, and this ratio was plotted for the memory-guided saccade task and the visually guided saccade task. Data are from 41 stimulation sites in 2 monkeys. No stim is the no-stimulation condition, stim is the stimulation condition, and mem is the memory condition. Values are means; error bars indicate SE. *P < 0.01, indicating a statistically significant reduction in saccade frequency with stimulation.
Taken together, the results of the experiments presented so far suggest that manipulation of the output of the basal ganglia influences the ability to make saccades well before the onset of the saccades and primarily when sensory information is unavailable. These findings are consistent with the hypothesis that the basal ganglia play a role in maintaining a spatial memory for the upcoming saccade. The effects were somewhat smaller than those seen in our previous work (Basso and Liu 2007), but they were statistically reliable and were always larger than those seen in visually guided saccade conditions. There are a few possible explanations for the small effects seen in the memory condition. One is that our animals were highly over-trained in these tasks, and with these target locations, it is possible that there is little working memory required to perform this task. If this is true, then this begs the question of why there were effects at all. One reason could be that the memory for the learned target locations is very volatile and easily influenced by the stimulation. Another possibility is that the stimulation is influencing a more dynamic process such as the specification of a particular saccade when a visual stimulus is unavailable to drive that process. To test this latter idea, we assessed whether nigral stimulation influenced the time to make a saccade in the presence or the absence of a visual stimulus. We reasoned that influencing the nigra with its input to the superior colliculus (or to the frontal eye fields via the thalamus) might alter the evolving activity in saccadic motor maps as the eye movement specification evolves. If reaction time effects are observed with stimulation of the nigra, it would implicate the nigra in more of the specification process than a memory process per se (Basso and Wurtz 1998; Dorris and Munoz 1998). We predicted that nigral activity alterations would influence saccade evolution predominantly when visual stimuli were absent.
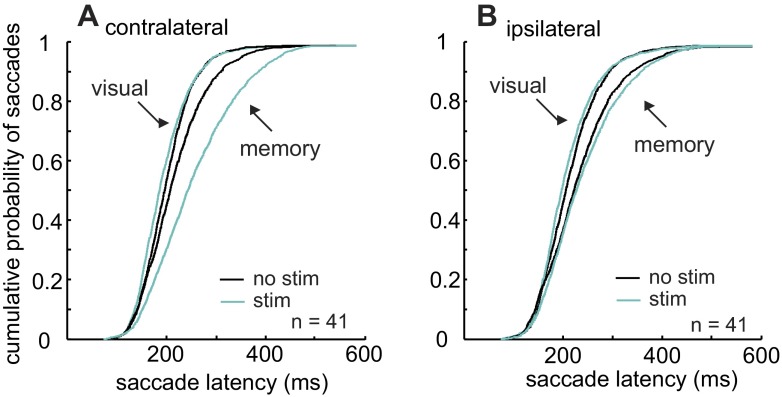
Figure 10 shows the effect of nigral stimulation on the latency of saccades made in the presence or absence of a visual stimulus. Cyan lines show stimulated trials, whereas black lines show nonstimulated trials. Stimulation of the nigra during the time that the choice of a saccade was evolving in the presence of a visual stimulus decreased the latency of saccades in both directions only modestly, but significantly (Kruskal-Wallis, P < 0.001; Bonferroni-corrected multiple comparisons, P < 0.05; Fig. 10, A and B, leftmost cyan lines). The most obvious effect was an increase in the latency of saccades made in the absence of a visual stimulus (Fig. 10, A and B, rightmost cyan lines). The differences between the latencies for contralaterally directed memory-guided saccades in the stimulation and no-stimulation conditions were statistically significant (Kruskal-Wallis, P < 0.001; Bonferroni-corrected multiple comparisons, P < 0.05). Similarly, the differences between the latencies for ipsilaterally directed memory-guided saccades in the stimulation and no-stimulation conditions were statistically significant (Kruskal-Wallis, P < 0.001; Bonferroni-corrected multiple comparisons, P < 0.05). Furthermore, the increase in latency as a result of stimulation on the contralateral side was significantly greater than that on the ipsilateral side (Kolmogorov-Smirnov test comparing stimulation and no-stimulation differences, P < 0.05) . Based on these results, we conclude that alterations in basal ganglia output influence the evolution of a saccade to a greater extent when sensory information is unavailable.
Fig. 10.
Stimulation of the nigra during the delay period increases saccade latency in the absence of visual information. The cumulative probability of a saccade with a particular latency is plotted against time. Cyan functions show the stimulation latencies, and black functions show the no-stimulation latencies. These data are from the correct trials from 41 stimulation sites in 2 monkeys. A: saccades directed to the hemifield contralateral to the hemisphere that was stimulated. B: saccades directed to the hemifield ipsilateral to the hemisphere that was stimulated.
DISCUSSION
We report in this article that stimulation of the nigra, one of two output nuclei of the basal ganglia, influences the occurrence of saccades. Importantly, this influence occurs primarily when the saccade is dependent on information stored in memory. When visual information is available to guide the saccade, the stimulation has little to no effect. Our evidence demonstrates this by showing that manipulation of nigral activity during the delay period (maintenance), but less so during the presentation of a visual stimulus (encoding) or immediately before an eye movement starts (retrieval), results in 1) a reduction in the probability of making saccades directed to the contralateral hemifield, 2) an increase in the latency of saccades directed primarily contralaterally, and 3) changes in the endpoints of saccades with a tendency for them to be rotated toward the ipsilateral hemifield with stimulation of the nigra. The influence of the stimulation on saccades was greater when they were instructed toward targets located in the hemifield contralateral to the side of stimulation, consistent with the known projections of the nigra to the superior colliculus and the thalamus (Anderson and Yoshida 1977, 1980; Deniau et al. 1978; Graybiel 1978; Hikosaka and Wurtz 1983d; Jayaraman et al. 1977; Liu and Basso 2008).
Relationship to previous studies.
The current model of the role of the nigra in saccades is to act as an inhibitory gate on the discharge of superior colliculus neurons and thus the generation of saccades (Hikosaka et al. 2000). The tonic discharge of nigral neurons represents a closed gate, disallowing saccades. In contrast, pausing of the discharge of nigral neurons represents an opening of the gate and a release of inhibition on the superior colliculus, resulting in a saccade. A number of lines of evidence suggest that the nigra has a larger role than just gating saccade initiation. For example, recordings in monkeys suggest that many neurons in the nigra are context dependent, being modulated preferentially during saccades guided by memory and not by vision or reward (Handel and Glimcher 2000; Hikosaka and Wurtz 1983b; but see Bayer et al. 2002). Second, reversible inactivation of the nigra with muscimol results in irrepressible saccades but also severely impairs the ability of monkeys to make memory-guided saccades (Hikosaka and Wurtz 1985). Third, neurons in the nigra are characterized by a variety of response profiles, increases as well as decreases, and the modulations can occur before, during, or after saccades (Basso and Sommer 2011; Handel and Glimcher 1999, 2000). Furthermore, in addition to projecting to the output neurons of the superior colliculus, the nigra also projects to inhibitory interneurons in the superior colliculus (Kaneda et al. 2008). Consistent with this novel anatomic result, electrical stimulation of the nigra speeds up the latency of visually guided saccades (Basso and Liu 2007; current Fig. 10). Taken together, the evidence indicates that a simple gating mechanism does not capture these additional nigral functions. Based on this previous work and our presently reported results, we propose that in addition to its role in gating saccades, the nigra is also involved in events that precede the onset of an eye movement, such as specifying the saccadic eye movement, particularly in conditions when visual stimuli are unavailable to do so.
Relationship to rewarded eye movements.
Recently, emphasis has been placed on the role of the nigra in saccades that are oriented toward stimuli associated with rewards (Handel and Glimcher 2000; Hikosaka et al. 2006). A role for the basal ganglia in saccades informed by reward is consistent with the very large literature showing that the basal ganglia participate in reinforcement learning (e.g., Schultz 2006). It is also consistent with some of the original observations in monkeys that the activity of nigral neurons is not modulated by spontaneous saccades (Hikosaka and Wurtz 1989). Causal evidence that the basal ganglia play a role in reward-based learning for eye movements comes from a recent study in which electrical stimulation of the oculomotor sector of the caudate nucleus, a structure with direct projections to the nigra, can produce a long-lasting facilitation of saccades (Nakamura and Hikosaka 2006). Interestingly, electrical stimulation of the caudate had to occur immediately after a saccade to a fixed direction in order to show this facilitation. This observation is consistent with the caudate providing a reward signal linking the saccade with a particular stimulus location and reward. Similarly, stimulation of the midbrain tegmentum, a structure just posterior to the nigra, results in a facilitation of saccadic adaptation. However, the stimulation must occur after the first saccade ends (Kojima et al. 2007). A similar finding of behavioral facilitation appears in rodents in which learned behaviors are altered with electrical stimulation of dopamine neurons in the compacta portion of the nigra. Here, too, the stimulation occurred after the behavior was produced, consistent with a reward or learning signal (Routtenberg and Holzman 1973).
Our experiment was aimed at assessing the role of the nigra in events that precede the onset of saccades within a single trial and not in the role of feedback information on saccades occurring in subsequent trials. Therefore, the results we report add an important component to our understanding of the role of the basal ganglia in saccades. We show that electrical stimulation targeting the GABA containing neurons of the nigra before a saccade is generated, during the time when a target location is maintained in memory, influences whether a saccade is made on that same trial. Furthermore, and importantly, the effects of nigral stimulation were seen primarily when the sensory stimulus that would specify the saccade was absent and the expected value of the saccade was the same. These data provide compelling evidence that the influence of the nigra on saccades extends beyond reward and may play a role in other cognitive functions such as the maintenance of spatial memories and the evolution of a saccade. Others have examined the role of stimulation in the caudate before saccade onset and its effects on reaction time, for example (Nakamura and Hikosaka 2006; Watanabe and Munoz 2011), with findings similar to those described in this report, but neither of these studies explored the role of stimulation during a memory delay as in the present study. The finding that the effects of stimulation disappear or are statistically undetectable when visual stimuli are available further suggests that the influence of the nigra can be overridden when strong or reliable sensory information is available. Indeed, this interpretation is consistent with our previous work in which we found that electrical stimulation immediately before and during a saccade altered the saccade, but primarily when it was made in the absence of a visual stimulus to guide it (Basso and Liu 2007). The present work extends this finding to show that the influence of the nigra occurs well before the cue to initiate the saccade while the memory of a target location is maintained. Thus, in addition to playing a role in saccade initiation, the nigra also plays a role in remembering a spatial location required for a subsequent movement or in the specification process that leads up to a particular saccade being made.
A role for the nigra in saccade specification.
The present results and those from previous work, taken together, support a role for the nigra in events that precede saccade initiation and occur in the absence of visual information. This result has at least two interpretations. One is that the stimulation interferes with the spatial memory of the target location required to specify the saccade. The second is that the stimulation interferes not with memory, per se, but with the evolution of the choice of whether or not to make a saccade and which one. Of course, for memory-guided saccades, the choice depends on information that is stored in memory. Therefore, these two possibilities are difficult to dissociate. Nevertheless, we believe that the results are best explained by a nigral influence on a dynamic process of specifying a saccade in the absence of sensory information to guide this process. First, in experiments in which multiple possible saccade targets are available, nigral neurons show modulations in activity that correlate with the probability of selecting one of the saccade choices (Basso and Wurtz 2002). Second, the discharge properties of neurons in the nigra are modulated during events that precede saccade initiation, such as the target appearance and the delay period before a cue to move appears, in ways that are reminiscent of modulations seen in brain areas involved in decision making, such as the superior colliculus and the lateral intraparietal area (Hikosaka and Wurtz 1983b, 1983c; Handel and Glimcher 1999; Shires et al. 2010). Third, our findings of electrical stimulation reveal a temporal sensitivity of effects. Stimulation during the visual interval had little effect on saccades. Likewise, stimulation right before the saccade started but not during the saccade had a comparatively small effect on saccades (note: this is different from our previous work in which the stimulation occurred before and during the saccade to great effect; Basso and Lui 2007). In the present experiments, the greatest effects we observed were when the stimulation occurred during the delay period, presumably as the specification of whether and which saccade to make was being formed. The lack of effects seen with the stimulation occurred at random times during the delay period are also consistent with this. In other words, there was still time for the specification to evolve during the delay period when the stimulation train was a brief 200 ms.
Based on our results, we can draw two more conclusions. First, due to the temporal specificity of the effects and the fact that stimulation during the delay period in the target present condition was relatively ineffective, the results are unlikely to be due to spurious correlations with other variables such as current spread into other areas, nonspecific aversive or alerting effects of stimulation, or antidromic activation of other brain areas. Second and more interesting is that stimulation during the delay period had large effects on the endpoints of saccades despite the fact that the monkeys were very over-trained, making the target positions very well known to the monkeys. This final point is further evidence that the manipulation of nigral activity is influencing something dynamic or that the memories for the target positions are much more volatile than we would expect given the exposure to these stimuli the moneys have had.
Taking the results together, we hypothesize that the effects of nigral stimulation are on the dynamic process of specifying whether and where to act when sensory information are unable to influence that process. Furthermore, based on the influence of the stimulation on saccade latency, we propose that the alterations of nigral activity may influence the rise to threshold for a choice, perhaps the slowly increasing activity of superior colliculus neurons or prefrontal cortical neurons (Ratcliff et al. 2003, 2007; Kim and Shadlen 1999; Horwitz et al. 2004; Kim and Basso 2010; Watanabe and Munoz 2011). Alternatively, but not exclusively, the manipulations of nigral activity may also alter the threshold level of a choice process. A similar role for the nigra in decision making has been proposed by recent modeling work (Lo and Wang 2006) and remains to be tested experimentally.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants EY13692 and NS065776, the Esther A. and Joseph Klingenstein Foundation (M. A. Basso), and a Parkinson Disease Foundation Summer Student Fellowship (T. J. Garrison). We acknowledge the support of NIH Grant P51 RR000167 to the Wisconsin National Primate Research Center, NIH Core Grant in Vision Research P30 EY0166665 to the University of Wisconsin-Madison, and NIH Neuroscience Training Grant T32 GM007507 (J. Shires).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M., T.J.G., J.S., and M.A.B. performed experiments; S.M., T.J.G., and M.A.B. analyzed data; S.M. and M.A.B. interpreted results of experiments; S.M. and M.A.B. prepared figures; S.M. and M.A.B. edited and revised manuscript; S.M., T.J.G., J.S., and M.A.B. approved final version of manuscript; M.A.B. conception and design of research; M.A.B. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. James Bisley for providing critical comments on an earlier version of our manuscript.
REFERENCES
- Anderson M, Yoshida M. Axonal branching patterns and location of nigrothalamic and nigrocollicular neurons in the cat. J Neurophysiol 43: 883–895, 1980 [DOI] [PubMed] [Google Scholar]
- Anderson ME, Yoshida M. Electrophysiological evidence for branching nigral projections to the thalamus and the superior colliculus. Brain Res 137: 361–375, 1977 [DOI] [PubMed] [Google Scholar]
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus of the cat. J Comp Neurol 302: 143–158, 1990 [DOI] [PubMed] [Google Scholar]
- Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol 97: 4129–4142, 2007 [DOI] [PubMed] [Google Scholar]
- Basso MA, Sommer MA. Exploring the role of the substantia nigra pars reticulata in eye movements. Neuroscience 198: 205–212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci 22: 1883–1894, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Pokorny JJ, Liu P. Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. Eur J Neurosci 22: 448–464, 2005 [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. London: Academic, 1981 [Google Scholar]
- Bayer HM, Handel A, Glimcher PW. Eye position and memory saccade related responses in substantia nigra pars reticulata. Exp Brain Res 154: 428–441, 2002 [DOI] [PubMed] [Google Scholar]
- Behan M, Lin CS, Hall WC. The nigrotectal projection in the cat: an electron microscope autoradiographic study. Neuroscience 21:529–539, (1987) [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. The nigral projection to predorsal bundle cells in the superior colliculus of the rat. J Comp Neurol 319: 11–33, 1992 [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Joseph JP. Role of the cat substantia nigra pars reticulata in eye and head movements. II. Effects of local pharmacological injections. Exp Brain Res 57: 297–304, 1985 [DOI] [PubMed] [Google Scholar]
- Chevalier G, Thierry AM, Shibazaki T, Feger J. Evidence for a GABAergic inhibitory nigrotectal pathway in the rat. Neurosci Lett 21: 67–70, 1981a [DOI] [PubMed] [Google Scholar]
- Chevalier G, Thierry AM, Shibazaki T, Feger J. Evidence for a GABAergic inhibitory nigrotectal pathway in the rat. Neurosci Lett 21: 67–70, 1981b [DOI] [PubMed] [Google Scholar]
- Chevalier G, Vacher S, Deniau JM. Inhibitory nigral influence on tectospinal neurons, a possible implication of basal ganglia in orienting behavior. Exp Brain Res 53: 320–326, 1984 [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DSG, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988 [DOI] [PubMed] [Google Scholar]
- Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res 32: 409–422, 1978 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res 176: 273–284, 1979 [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18: 7015–7026, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Organization of the nigrotectal connection: an experimental tracer study in the cat. Brain Res 143: 339–348, 1978 [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Contextual modulation of substantia nigra pars reticulata neurons. J Neurophysiol 83: 3042–3048, 2000 [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol 82: 3458–3475, 1999 [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc 2: 1–10, 1982 [Google Scholar]
- Hikosaka O, Wurtz RH. The neurobiology of saccadic eye movements. The basal ganglia. Rev Oculomot Res 3: 257–284, 1989 [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49: 1230–1253, 1983a [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J Neurophysiol 49: 1254–1267, 1983b [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284, 1983c [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301, 1983d [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol 53: 292–308, 1985 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol 95: 567–584, 2006 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol 91: 2281–2296, 2004 [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Batton RR, 3rd, Carpenter MB. Nigrotectal projections in the monkey: an autoradiographic study. Brain Research 135, 147–152, (1977). [DOI] [PubMed] [Google Scholar]
- Joseph JP, Boussaoud D. Role of the cat substantia nigra pars reticulata in eye and head movements. I. Neural activity. Exp Brain Res 57: 286–296, 1985 [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci 28: 11071–11078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabelas AB, Moschovakis AK. Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol 239: 309–329, 1985 [DOI] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci 30: 2340–2355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2: 176–185, 1999 [DOI] [PubMed] [Google Scholar]
- Kojima Y, Yoshida K, Iwamoto Y. Microstimulation of the midbrain tegmentum creates learning signals for saccade adaptation. J Neurosci 27: 3759–3767, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci 25: 11357–11373, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kim B, Basso MA. Transient pauses in delay-period activity of superior colliculus neurons. J Neurophysiol 95: 2252–2264, 2006 [DOI] [PubMed] [Google Scholar]
- Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol 100: 1098–1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9: 956–963, 2006 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Facilitation of saccadic eye movements by postsaccadic electrical stimulation in the primate caudate. J Neurosci 26: 12885–12895, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine G, Fitzpatrick D, Hall WC, LaMantia A, White L. (editors). Neuroscience. Sunderland, MA: Sinauer, 2012 [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol 90: 1392–1407, 2003 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves M. A dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol 97: 1756–1774, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Holzman N. Memory disruption by electrical stimulation of substantia nigra, pars compacta. Science 181: 4094–4096, 1973 [DOI] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57: 87–115, 2006 [DOI] [PubMed] [Google Scholar]
- Shin S, Sommer MA. Activity of neurons in monkey globus pallidus during oculomotor behavior compared with that in substantia nigra pars reticulata. J Neurophysiol 103: 1874–1887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires J, Joshi S, Basso MA. Shedding new light on the role of the basal ganglia-superior colliculus pathway in eye movements. Curr Opin Neurobiol 20: 717–725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Munoz D. Saccade reaction times are influenced by caudate microstimulation following and prior to visual stimulus appearance. J Cogn Neurosci 23: 1794–1807, 2011 [DOI] [PubMed] [Google Scholar]