Abstract
Localization of low-frequency acoustic stimuli is processed in dedicated neural pathways where coincidence-detecting neurons compare the arrival time of sound stimuli at the two ears, or interaural time disparity (ITD). ITDs occur in the submillisecond range, and vertebrates have evolved specialized excitatory and inhibitory circuitry to compute these differences. Glycinergic inhibition is a computationally significant and prominent component of the mammalian ITD pathway. However, evidence for glycinergic transmission is limited in birds, where GABAergic inhibition has been thought to be the dominant or exclusive inhibitory transmitter. Indeed, previous work showed that GABA antagonists completely eliminate inhibition in avian nuclei specialized for processing temporal features of sound, nucleus magnocellularis (NM) and nucleus laminaris (NL). However, more recent work shows that glycine is coexpressed with GABA in synaptic terminals apposed to neurons in both nuclei (Coleman WL, Fischl MJ, Weimann SR, Burger RM. J Neurophysiol 105: 2405–2420, 2011; Kuo SP, Bradley LA, Trussell LO. J Neurosci 29: 9625–9634, 2009). Here we show complementary evidence of functional glycine receptor (GlyR) expression in NM and NL. Additionally, we show that glycinergic input can be evoked under particular stimulus conditions. Stimulation at high but physiologically relevant rates evokes a slowly emerging glycinergic response in NM and NL that builds over the course of the stimulus. Glycinergic response magnitude was stimulus rate dependent, representing 18% and 7% of the total inhibitory current in NM and NL, respectively, at the end of the 50-pulse, 200-Hz stimulus. Finally, we show that the glycinergic component is functionally relevant, as its elimination reduced inhibition of discharges evoked by current injection into NM neurons.
Keywords: glycine receptor, inhibition, nucleus magnocellularis, nucleus laminaris, sound localization
animals use differences in the arrival time of sound at each ear, or interaural time disparities (ITDs), to compute the location of low-frequency sound sources (Rayleigh 1907). In vertebrates, ITDs are computed by binaural coincidence-detecting neurons in the brain stem. Coincidence-detecting neurons reside in nucleus laminaris (NL) in birds (Burger et al. 2011; Carr and Konishi 1990; Parks and Rubel 1975; Pena et al. 1996; Sullivan and Konishi 1986) and in the medial superior olive (MSO) in mammals (Goldberg and Brown 1969; Yin and Chan 1990). To perform sound localization computations, these neurons integrate bilateral excitatory and prominent inhibitory inputs while modulating their firing rate based on submillisecond differences in ITDs.
Inhibitory synaptic transmission is a key feature of sound localization circuitry, contributing to temporal precision over a broad range of sound intensities. In mammals, precisely timed feedforward glycinergic input from the medial nucleus of the trapezoid body (MNTB) modulates the ITD selectivity of MSO neurons (Brand et al. 2002; Pecka et al. 2008). Until recently, investigation of avian systems has focused on slow, depolarizing, GABAergic feedback inhibition from the superior olivary nucleus (SON) to its targets in the brain stem (Hyson et al. 1995; Monsivais et al. 2000; Monsivais and Rubel 2001; Yamada et al. 2013; Yang et al. 1999), while no physiological observations of glycinergic transmission in these nuclei have been documented. Also, previous studies have shown that glycine immunoreactivity is sparse in nucleus magnocellularis (NM) and NL (Code and Rubel 1989), especially compared with GABA immunoreactivity (Carr et al. 1989; Code and Churchill 1991). Furthermore, whole cell recordings in these nuclei have indicated that both spontaneous and evoked inhibitory synaptic currents were completely blocked by application of GABAA receptor (GABAAR) antagonists (Funabiki et al. 1998; Lu and Trussell 2000; Monsivais et al. 2000; Yang et al. 1999). Recent work has revealed glycinergic transmission in nucleus angularis (NA) and SON, components of this circuitry that are not considered to be specialized for temporal processing. Inhibitory input to NA (Kuo et al. 2009) and SON (Coleman et al. 2011) is marked by corelease of GABA and glycine at some synapses. These studies further showed that GABA and glycine colocalize in the terminals that synapse onto NA and SON neurons (Coleman et al. 2011; Kuo et al. 2009). Paradoxically, this staining pattern is also present surrounding neurons in NM and NL, where glycinergic transmission has never been detected electrophysiologically (Kuo el al. 2009). Release of multiple transmitters is known to occur in developing synapses (Awatramani et al. 2005; Gillespie et al. 2005) but has also been demonstrated in mature neurons in the avian (Coleman et al. 2011; Kuo et al. 2009) and mammalian (Lu et al. 2008) auditory brain stem. Recent work in the mammalian cochlear nucleus, where glycine is the dominant inhibitory transmitter, has demonstrated that GABA release emerges when synapses are stimulated at high frequencies (Nerlich et al. 2013). Given these recent findings regarding the presence of multiple inhibitory transmitters in the auditory system, we searched for hallmarks of glycinergic transmission in the NM and NL, two nuclei involved in temporal processing of acoustic signals in the avian brain stem.
Here we report expression of glycine receptors (GlyRs) in all principal nuclei of the mature avian ITD computing circuit, including NM and NL. Physiological recordings in NM and NL show that the expressed GlyRs are functional and generate current in response to exogenous glycine application. Finally, we demonstrate evoked, frequency-dependent synaptic release of glycine in response to long-duration, high-frequency stimulation in NM and NL. Functional experiments suggest that this glycinergic component is sufficiently potent to influence the overall efficacy of inhibition in NM during high-frequency stimulation.
METHODS
All procedures were approved by the Lehigh University Animal Care and Use Committee. In this study, 57 white leghorn chickens (Gallus domesticus; Moyers, Quakertown, PA) aged E17–P5 and of both sexes were used.
Immunohistochemistry.
Immunohistochemical staining for GlyR followed protocols described by Coleman et al. (2011). Briefly, four P5 chickens were deeply anesthetized and transcardially perfused with PBS followed by 4% paraformaldehyde (PFA) in PBS, pH 7.4. Brains were removed and postfixed in 4% PFA overnight at 4°C. Brains were rinsed and blocked and then sectioned at 30 μm (HM650V, Microm). Sections were transferred to a solution for antigen retrieval (10 mM sodium citrate, 0.05% Tween 20; pH 6.0) and maintained at 80°C in a water bath for 30 min. Sections were cooled to 27°C, rinsed, and then blocked in 10% normal goat serum for 1 h. Sections were incubated overnight at 4°C in solution containing 5% normal goat serum, anti-neurofilament (1:200; Millipore, catalog no. AB1987) and anti-GlyR (clone mAb4a, 1:1,000; Synaptic Systems, catalog no. 146011). Sections were rinsed and then incubated for 2 h with secondary antibody conjugated to Alexa Fluor 488 goat anti-mouse and Alexa Fluor 633 goat anti-rabbit to label GlyR and neurofilament, respectively (Invitrogen). Sections were mounted in Vectashield (Vector Laboratories), and confocal images were captured (LSM 510 Meta, Zeiss). Images were processed with Photoshop (Adobe Systems) to match pixel intensity distributions between color channels. No staining was observed when primary antibodies were absent (see Fig. 2E) or when GlyR antibody was preabsorbed with antigen peptide (Coleman et al. 2011).
Fig. 2.
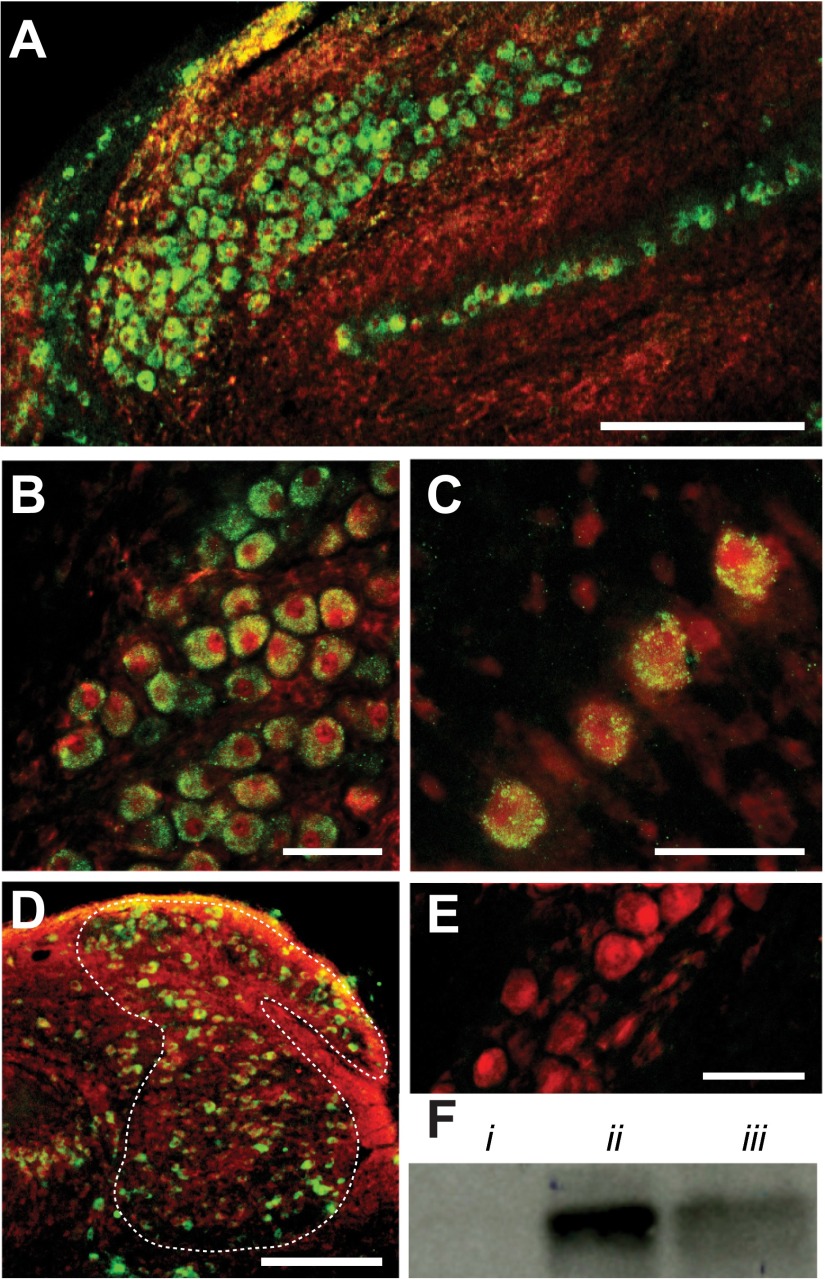
Glycine receptors (GlyRs) are expressed in 4 principal auditory brain stem nuclei. A: low-magnification confocal image of temporal processing nuclei (NM and NL). B: GlyR staining (green) is robust in NM soma colabeled for neurofilament (red). C: NL neurons. D: low-magnification image of NA confirms GlyR expression. E: image of NM neurons shows no GlyR staining when the primary antibody was omitted. F: Western blot analysis using anti-GlyR antibody detects the GlyR α1-subunit (Pfeiffer et al. 1984) in chick brain stem tissue (iii) at a molecular mass (∼48 kDa) comparable to that in gerbil brain stem (positive control, ii). Immunoreactivity of anti-GlyR is absent in chicken lung tissue (negative control, i). Scale bars: 200 μm (A and D) or 50 μm (B, C, and E).
Western blot analysis.
Chicken lung, chicken brain stem, and gerbil brain stem tissue were homogenized in lysis buffer (10 mM Tris·HCl pH 7.4, 0.32 M sucrose, 5 mM EDTA pH 8) supplemented with 0.1 mM PMSF and cOmplete-mini EDTA-free protease inhibitor cocktail (Roche), mixed with an equal volume of 4% SDS, and sonicated. Membrane fractions were collected by centrifugation at 13,000 g (4°C) for 20 min with three washes in supplemented lysis buffer. Membranes were resuspended in modified RIPA buffer (25 mM Tris pH 7.6, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1% SDS) supplemented with 1 mM PMSF for 10 min at room temperature and measured with the DC Protein Assay (Bio-Rad). Twenty micrograms of protein was separated by 10% reducing SDS-PAGE and then electrotransferred onto a 0.2-μm PVDF membrane for Western blot analysis. Antibodies used were mouse anti-GlyR (clone mAb4a, 1:500; Synaptic Systems, catalog no. 146011) and HRP-conjugated goat anti-mouse IgG (1:50,000; Promega). ECL-Plus (GE Healthcare) was used for chemiluminescent detection with Kodak BioMax film.
In vitro brain slice preparation.
For in vitro physiology, 53 white leghorn chickens aged E17–P5 were rapidly decapitated and the brain stem containing auditory nuclei was removed, blocked, and submerged in oxygenated artificial cerebrospinal fluid (ACSF) (in mM: 130 NaCl, 3 KCl, 10 glucose, 1.25 NaH2PO4, 26 NaHCO3, 3 CaCl2, 1 MgCl2) at 22°C. The brain stem was placed rostral surface down on the stage of a vibrating microtome (HM650V, Microm). Coronal sections (150–200 μm) containing the auditory brain stem nuclei were collected, submerged in an incubation chamber of continuously oxygenated ACSF, and incubated at 37°C for ∼1 h. Slices were then maintained at room temperature until used for recording.
Brain stem slices were placed in a custom recording chamber on a retractable chamber shuttle system (Siskiyou Design Instruments), and neurons were visualized with a Nikon FN-1 Physiostation microscope using infrared-differential interference contrast optics. Video images were captured with a CCD camera (Hammamatsu C7500-50) coupled to a video monitor. The recording chamber was continuously perfused with ACSF at a rate of 2–4 ml/min. An inline feedback temperature controller and heated stage were used to maintain chamber temperature at 35 ± 1°C (TC344B, Warner Instruments, Hamden, CT).
In vitro whole cell recordings.
Borosilicate capillary glass pipettes (1B120F-4, WPI) were pulled to a resistance of 4–8 MΩ with a two-stage puller (PC-10, Narishige) and back-filled with internal solution (for voltage clamp; in mM: 105 CsMeSO3, 35 KCl, 5 EGTA, 10 HEPES, 1 MgCl2, 4 ATP-Mg, and 0.3 GTP-Na, pH 7.2 adjusted with KOH). In current-clamp experiments, CsMeSO3 was exchanged for K-gluconate and CsCl for KCl. A liquid junction potential of 10 mV was corrected for in both internal solutions. Five millimolar QX314 was added to the internal solution to prevent antidromic spiking. When the membrane was clamped at −70 mV, these internal solutions yielded depolarizing inhibitory inputs normally observed in the avian brain stem. In voltage clamp, series resistance was compensated at 60–80% (Multiclamp 700B, Molecular Devices). The signal was digitized with a Digidata 1440 data acquisition board and recorded with Clampex software (Molecular Devices).
Inhibitory inputs were pharmacologically isolated in ACSF containing 6,7-dinitroquinoxaline-2,3-dione (DNQX) (40 μM) and d-2-amino-5-phosphonopentanoic acid (AP5) (50 μM) to block AMPA and NMDA receptors. GlyRs were blocked with strychnine (0.5–1 μM). GABAARs were blocked with SR95531 (20 μM). GABAB receptors (GABABRs) were blocked with CGP55845 (2 μM) during high-frequency stimulation protocols. Pharmacological agents were obtained from Tocris except where indicated.
GlyR activation via pressure application of glycine.
Pipettes for pressure application of glycine were pulled to a resistance of ∼1 MΩ and placed within 50 μm of the neuron soma. One hundred-millisecond glycine (Sigma) (0.5–1 mM in ACSF containing DNQX and AP5) puffs were applied with ∼2.5 psi pressure injection (PLI 100A, Warner Instruments).
Synaptic activity in NM and NL.
Inhibitory postsynaptic potentials (IPSPs) or currents (IPSCs) were evoked with 50-μs stimulus pulses with a stimulus isolation unit (Isoflex, A.M.P.I.) through a concentric bipolar electrode (TM53CCINS, WPI) placed to the ventrolateral perimeter of NM or the dorsolateral perimeter of the NL. A schematized view of the recording setup is shown in Fig. 1. Stimulus magnitude (range 10–90 V) was gradually increased until amplitudes stabilized at their maximum. Stimulation protocols ranged from single events to trains of 50 pulses at frequencies from 20 to 200 Hz. The average of 5–10 traces was used for comparison between each condition (depicted in Fig. 4, C and D). For 50-pulse trains, the glycinergic component was analyzed on a pulse-by-pulse basis where the average amplitude at each pulse was compared between the control and GABAAR block conditions. The average residual component remaining during block of both GABAARs and GlyRs was subtracted from each condition before comparisons were made. Differences in the magnitude of the glycinergic component were compared between frequencies by averaging the last five pulses (pulses 46–50) and using Student's t-test (Fig. 5, B and D). Decay time constants, τdecay values, were calculated from standard exponential fits from 10–90% of the peak of IPSCs. The τdecay values were obtained with either single- or double-exponential fits. Goodness of fit was determined by comparing the sum of the squared errors. Double-exponential fits were chosen if the sum of the squared errors was less than half that of the single-exponential fit. A weighted τdecay value was calculated for double-exponential fits with the following equation: weighted τdecay = τ1[A1/(A1 + A2)] + τ2[A2/(A1 + A2)], as in Kuo et al. (2009).
Fig. 1.
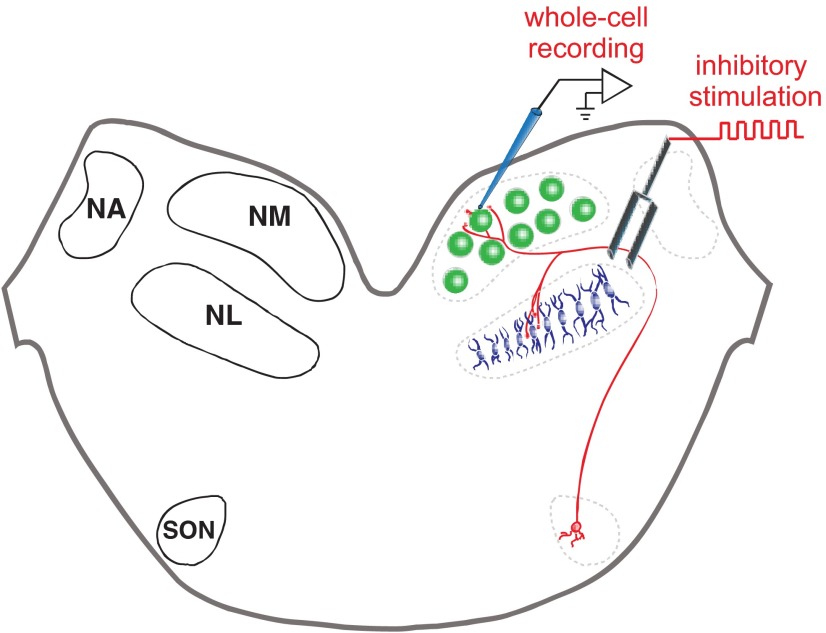
Schematic of a chicken auditory brain stem slice and recording setup. Left: the avian auditory brain stem is composed of 4 main nuclei: two cochlear nuclei, the nucleus magnocellularis (NM) and nucleus angularis (NA); the site of binaural coincidence detection, the nucleus laminaris (NL); and the superior olivary nucleus (SON), which is the source of inhibitory feedback in the circuit. Right: recording setup for whole cell recordings in the NM with the approximate positioning of the stimulating electrode. Stimulator placement was similar during NL recordings.
Fig. 4.
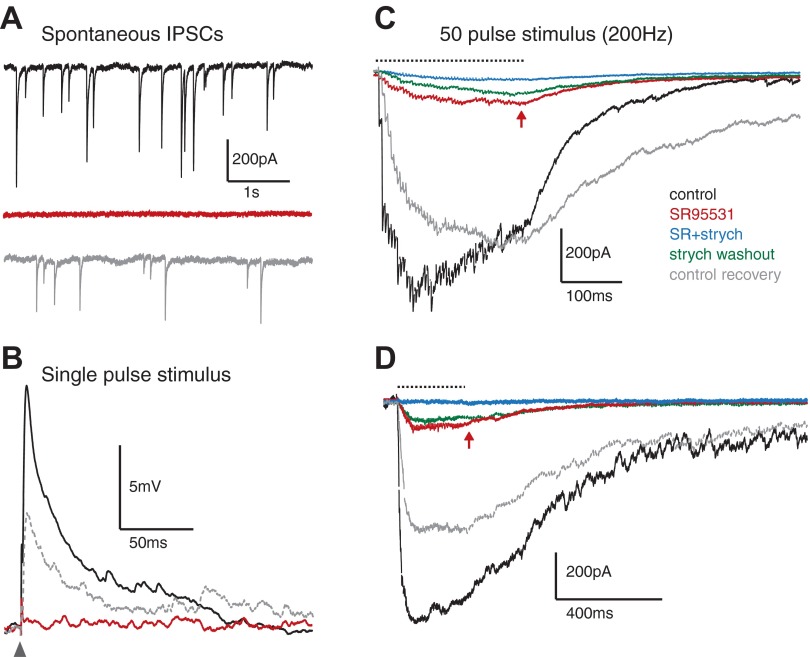
High-frequency stimulation evokes glycinergic synaptic transmission in NM and NL. A: traces from an NM neuron show spontaneous inhibitory postsynaptic currents (IPSCs) that are completely abolished during SR95531 application, suggesting purely GABAergic events (n = 4, population data not shown). B: overlay of averaged inhibitory postsynaptic potentials (IPSPs) evoked during single-pulse stimulation (arrowhead) confirm purely GABAergic events in NM (n = 3). C: averaged IPSC traces evoked with a 200-Hz, 50-pulse stimulus (dashed line) in each pharmacological condition in an NM neuron. GABAAR block reduces the summed IPSC amplitude, leaving a gradually increasing component (arrow) that is eliminated by strychnine application. D: averaged IPSC traces from 200-Hz stimulus train in the NL show similar trend during pharmacological manipulations. Color code in C for pharmacological condition is the same for A–D.
Fig. 5.
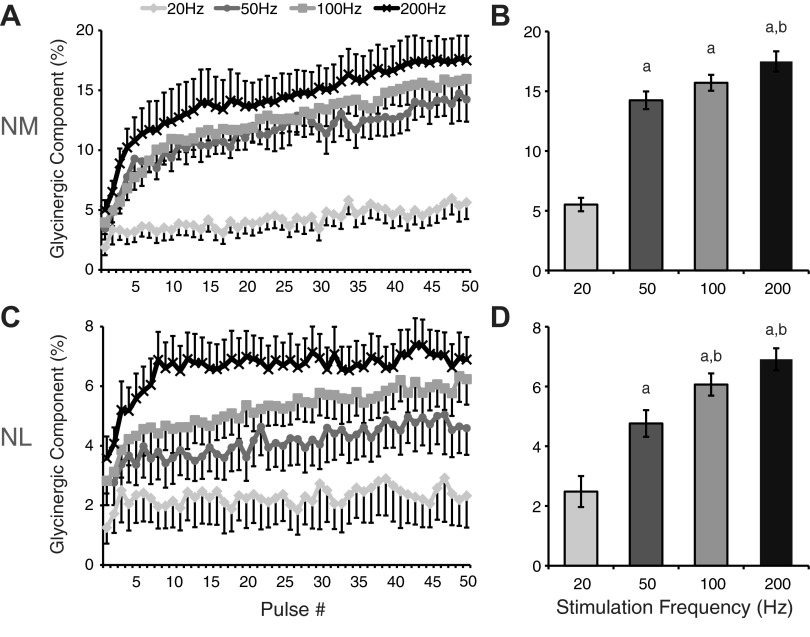
Recruitment of glycinergic transmission is dependent on stimulation frequency. A: population data for NM neurons at each frequency for mean amplitude of the glycinergic IPSC component analyzed pulse by pulse during the high-frequency train stimuli. The glycinergic component was calculated by dividing the IPSC amplitude at pulse n in SR95531 by the IPSC amplitude at pulse n in control. B: population data for the magnitude of the glycinergic component at each frequency tested. Values are average ± SE of the last 5 pulses of the stimulus. C: pulse-by-pulse analysis of glycinergic component in NL at each frequency. D: population data for the average magnitude of the last 5 pulses at each frequency in NL. aSignificantly different from 20 Hz (P < 0.001); bsignificantly different from 50 Hz (P < 0.05).
Functional testing of glycinergic component.
The functional test for glycinergic efficacy consisted of suprathreshold current injection trains (duration 0.5–0.6 ms ranging from 850 to 1,650 pA) at 50 Hz for 50 pulses. The minimum current that evoked 90–100% spiking in the absence of inhibitory input fiber stimulation was used. During the current injection train, synaptic inhibitory input was evoked at 200 Hz for 40 pulses (200 ms) with a bipolar tungsten electrode. Spike probability was calculated and compared between control and strychnine conditions during the 250-ms period initiated at the first pulse of inhibitory stimulation.
RESULTS
GlyR immunohistochemistry in auditory brain stem.
Recent work has confirmed Code and Rubel's (1989) finding of weakly immunopositive glycinergic synaptic terminals apposed to neurons in several avian auditory nuclei, including NM, NL, and SON (Coleman et al. 2011; Kuo et al. 2009). These results are paradoxical, considering that GABAergic input accounted for nearly all of the inhibitory current to NM and NL in previous studies using a variety of stimulus configurations (Funabiki et al. 1998; Lu and Trussell 2000; Monsivais et al. 2000; Yang et al. 1999) and glycinergic transmission has never been physiologically documented in NM and NL. Studies in other systems have shown that postsynaptic responses from multi-transmitter-releasing terminals are determined solely by the complement of receptors expressed by the postsynaptic neuron (Dugué et al. 2005), suggesting that glycine may be released but not received postsynaptically in NM and NL. To resolve whether glycine is a component of synaptic signaling in temporal processing nuclei, we investigated expression of GlyR in NM and NL (Fig. 2). Anti-GlyR immunoreactivity was indeed robust in NM and NL (Fig. 2, A–C), where subcellular staining appeared punctate and was absent from the nucleus. Anti-GlyR-immunopositive neurons in NA (Fig. 2D) and SON support physiological and immunohistochemical evidence of glycinergic synaptic transmission in these nuclei (Coleman et al. 2011; Kuo et al. 2009). Antibody specificity was confirmed by antigen preabsorption prior to tissue staining (Coleman et al. 2011) and Western blot analysis (Fig. 2F), where the GlyR α1-subunit was detected at the predicted molecular mass (∼48 kDa) and at a similar molecular mass observed in gerbil brain stem tissue (Fig. 2Fii), a known GlyR-positive brain region in rodents (Friauf et al. 1997; Korada and Schwartz 1999).
Exogenous glycine application evokes strychnine-sensitive currents in NM and NL.
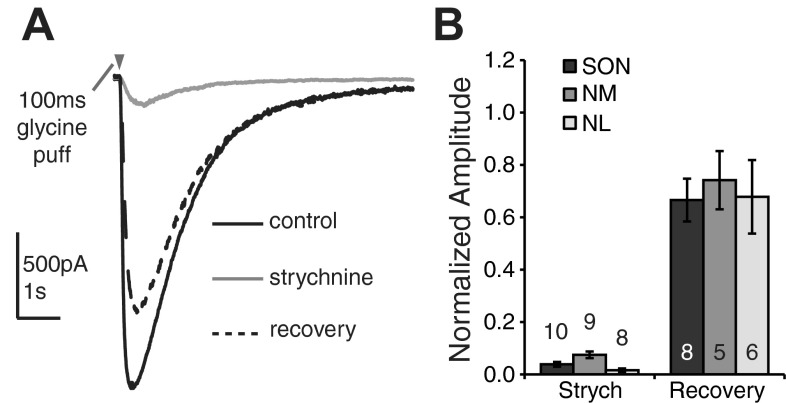
Our immunohistochemical evidence suggests that GlyRs are expressed in NM and NL. Since glycinergic transmission has never been recorded physiologically in these nuclei, we tested whether glycinergic responses could be observed during exogenous glycine application. One hundred-millisecond glycine puffs were applied to neurons in NM, NL, and SON in the presence of glutamate and GABA receptor antagonists. Application of glycine evoked inward current that was almost entirely abolished during bath application of strychnine (0.5–1 μM), a GlyR antagonist. Figure 3A shows the glycine response in control, strychnine, and washout conditions for an E19 NM neuron. Figure 3B depicts the population responses for NM in strychnine and recovery conditions normalized to the control response (strychnine: 0.077 ± 0.065, n = 9; recovery: 0.76 ± 0.49, n = 5; mean ± SD). Similar responses were also observed for all neurons tested in SON (strychnine: 0.039 ± 0.029, n = 10; recovery: 0.67 ± 0.23, n = 8) and NL (strychnine: 0.016 ± 0.019, n = 8; recovery: 0.68 ± 0.34, n = 6) (Fig. 3B). Responses were consistent across the age range (E18–P5). Kuo et al. (2009) demonstrated similar results for NA. Taken together with the immunohistochemical results, these data indicate that functional GlyRs exist in all four brain stem nuclei at ages considered mature for the chick auditory system (Rubel and Fritzsch 2002).
Fig. 3.
Exogenous glycine application evokes strychnine sensitive currents in SON, NM, and NL. A: representative traces from an E19 NM neuron show current responses to a 100-ms puff of glycine (0.5 mM) in control, strychnine, and recovery conditions. B: population data from the 100-ms glycine puff reveals glycinergic currents in all nuclei that are suppressed by GlyR block and recovered after washout. Numbers above (strych) and inside (recovery) bars represent n for each condition.
High-frequency stimulation evokes glycine release in NM and NL.
A number of studies have indicated that inhibitory transmission in the NM and NL is completely blocked by GABAAR antagonists (Funabiki et al. 1998; Lu and Trussell 2000; Monsivais et al. 2000; Yang et al. 1999). Indeed, we too observed no evidence of glycinergic activity in spontaneous events (Fig. 4A) or responses evoked by single-pulse stimuli (Fig. 4B) in voltage (n = 4) or current (n = 3) clamp. However, few of these studies evoked inhibitory synaptic transmission at high stimulation rates approaching those driven in SON by high-intensity acoustic signals. Previous work from our group suggests that SON neurons can reach spike rates exceeding 200 Hz during intense acoustic stimulation (Coleman et al. 2011). Thus we tested whether prolonged high-frequency stimulation could evoke glycine release in the NM and NL. Our protocol consisted of 50-pulse stimulus trains at 200 Hz during whole cell recordings in voltage or current clamp. Figure 4C shows averaged IPSC traces from a representative NM neuron stimulated at 200 Hz in each pharmacological condition in voltage clamp. Control IPSCs were evoked in the presence of DNQX and AP5 to block glutamate receptors and CGP55845 to block GABABRs. These control IPSCs tended to sum over the first several pulses and then plateau or depress for the remainder of the stimulus train (Fig. 4C). The τdecay of the current in this condition was 103 ± 59 ms for the population (n = 12). The GABAAR antagonist SR95531 reduced but did not eliminate the evoked IPSC (Fig. 4C). The remaining IPSC appeared to accumulate over the course of the stimulus (Fig. 4C) and reached ∼18% of the control IPSC by pulse 50 (population data for 200 Hz: 17.5 ± 2.1%, n = 13; Fig. 5, A and B). This component had a τdecay of 168 ± 51 ms (n = 12, P < 0.001 vs. control, paired t-test) and was eliminated with the addition of 0.5 μM strychnine and recovered after washout (Fig. 4C). IPSC amplitude recovered near control levels after SR95531 washout (Fig. 4C).
Neurons in NL responded similarly to those in NM under the same conditions. Figure 4D shows the averaged traces of a representative NL neuron at a 200-Hz stimulus rate in each of the pharmacological conditions tested. Again, GABAAR block reduced, but did not eliminate, the inhibitory currents. For the population of NL neurons the glycinergic component reached ∼7% at 200 Hz (6.9 ± 0.8%, n = 11, measured at pulse 50; Fig. 5, C and D). τdecay of the control and SR95531 conditions were 193 ± 84 ms and 168 ± 66 ms, respectively (n = 12, P < 0.05, paired t-test).
Evoked IPSPs followed a similar response pattern in current clamp in the NM. Again, application of SR95531 resulted in an incomplete suppression of the IPSP, and the residual component was nearly abolished after the addition of strychnine. The glycinergic voltage was greatest at pulse 50 and represented 30% of the control potential at 200-Hz stimulus frequency (30.7 ± 7.9%, n = 4; not depicted). These results suggest that glycine contributes to inhibition in both NM and NL under high but physiologically relevant firing rates.
Magnitude of glycinergic component is frequency dependent.
Our previous results indicated that glycinergic transmission was a component of inhibition during high-frequency stimulation in NM and NL. Next, we varied the stimulus rate to determine the frequency dependence of the glycinergic component. In voltage clamp, we tested the 50-pulse protocol at three additional frequencies: 20, 50, and 100 Hz. These input frequencies are representative of physiologically relevant firing rates that range from spontaneous activity (20 Hz) to maximum rates observed during intense auditory stimulation (200 Hz in SON; Coleman et al. 2011). Figure 5, A and C, shows the glycinergic contribution at each pulse at the four frequencies tested for the population of cells. In the NM we found that the 20-Hz frequency evoked a modest amount of glycinergic signaling in 9 of 11 cells. This glycinergic component represented 5.5 ± 4.2% of the IPSC amplitude compared with control (mean ± SD of the last 5 pulses, n = 11; Fig. 5B). In the NL, 6 of 11 cells showed a small glycinergic component at 20 Hz (2.5 ± 3.9%, n = 11; Fig. 5D). As the stimulation frequency increased, the glycinergic component also increased. In the NM there was a large increase between 20 and 50 Hz and then smaller gains at 100 and 200 Hz (50 Hz, 14.2 ± 4.7%, n = 8; 100 Hz, 15.7 ± 4.5%, n = 9; 200 Hz, 17.5 ± 6.8%, n = 13; Fig. 5, A and B). In the NL, the glycinergic component increased more incrementally and appeared to plateau in the 200 Hz condition (50 Hz, 4.5 ± 3.3%, n = 11; 100 Hz, 6.1 ± 2.9%, n = 12; 200 Hz, 6.9 ± 2.7%, n = 11; Fig. 5, C and D). These results indicate that the recruitment of glycine is frequency dependent and that the threshold for glycinergic transmission is close to 20 Hz for the majority of neurons tested.
GlyR block reduces efficacy of inhibition in NM.
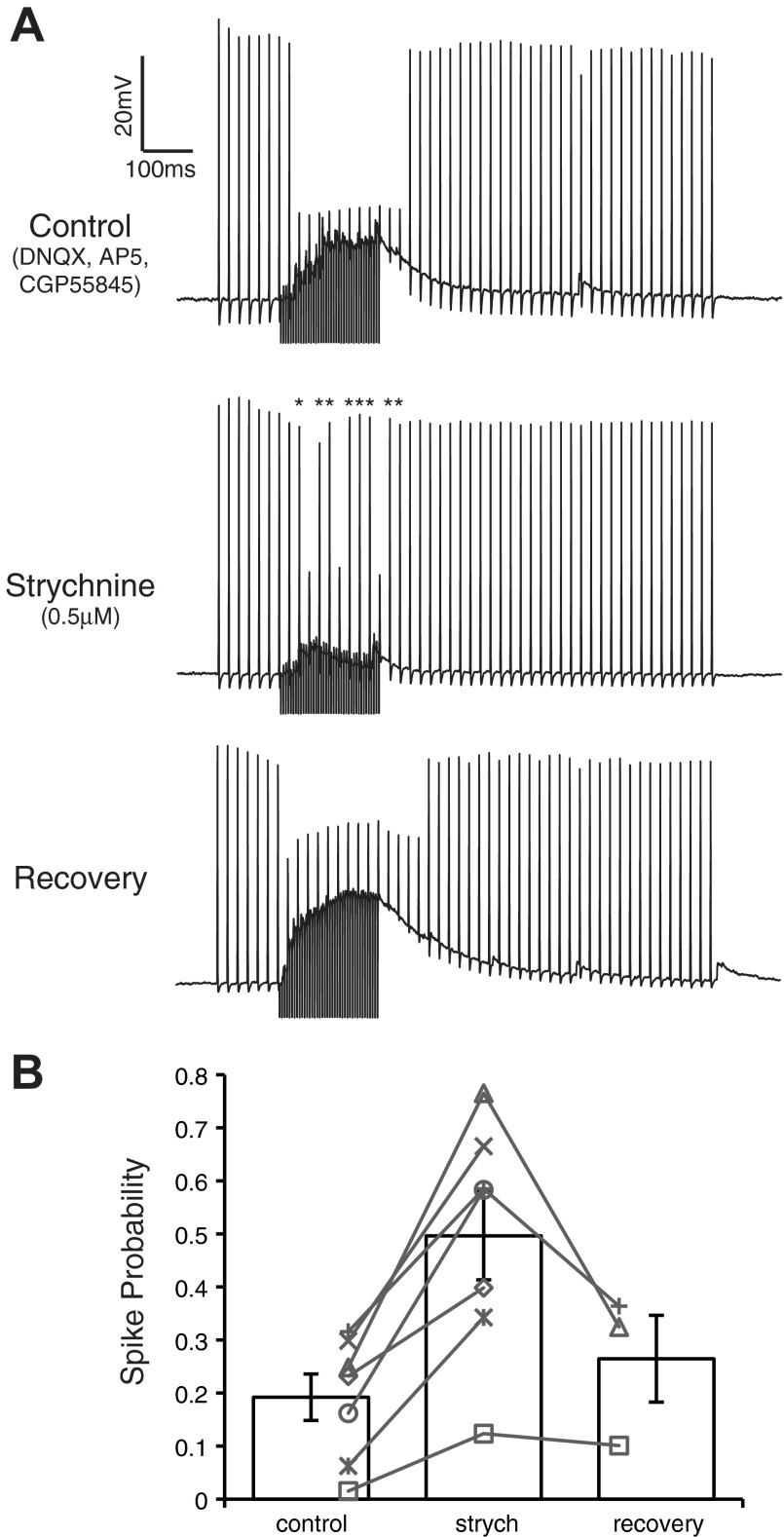
To test the functional efficacy of glycinergic input to NM, we used a protocol similar to that of Monsivais et al. (2000) (see methods) in which 50-Hz suprathreshold current pulse trains were injected into NM neurons to evoke spiking while inhibitory fibers were simultaneously stimulated during a 200-ms window (40 pulses at 200 Hz) (Fig. 6). Representative traces are shown in Fig. 6A for an NM neuron in each condition. Activation of inhibitory inputs in the control condition generally reduced spiking by at least 40% during the 250 ms following the onset of evoked inhibition (see methods). Blocking the glycinergic component of the inhibition with strychnine led to a significant increase in spike probability during inhibitory input activation (control: 0.19 ± 0.12, strychnine 0.50 ± 0.22; mean ± SD, n = 7, P < 0.005, paired t-test; Fig. 6B). After strychnine washout, spike probability returned to near control values (0.27 ± 0.15, n = 3). These results suggest that glycine release evoked under physiologically relevant stimulus conditions contributes to the overall efficacy of the inhibition and modulates the excitability of NM neurons.
Fig. 6.
Blocking GlyRs decreases the ability of evoked IPSPs to suppress action potentials in NM. A: representative current clamp traces from an E19 NM neuron in each pharmacological condition. Application of strychnine resulted in an increase in the firing rate during the 250-ms time window beginning at inhibition onset (asterisks in strychnine condition represent action potentials suppressed in the control). B: population data show a significant increase in spike probability during strychnine application compared with control (n = 7, P < 0.005). Gray lines connect the spike probability at each condition for each neuron tested.
DISCUSSION
Physiological evidence of glycinergic transmission in the sound localization circuitry of birds has only recently been documented (Coleman et al. 2011; Kuo et al. 2009). These studies demonstrated the existence of glycinergic signaling in neurons generally associated with the encoding of sound intensity information, the NA and SON (Takahashi et al. 1984). In the present report, we show for the first time robust expression of GlyRs and evidence of synaptically evoked glycine release in nuclei specialized for temporal processing in the avian auditory system, the NM and NL. We confirm the functionality of the GlyRs and demonstrate that glycinergic transmission is frequency dependent. We also show that the glycinergic transmission is functionally relevant, adding to the efficacy of inhibition to reduce spiking.
GlyR immunohistochemistry and receptor function in sound localization pathway.
Our immunohistochemical results show robust staining for the GlyR in all four of the main nuclei in the avian auditory brain stem. These data are complementary to previous work, which indicated that glycine could be labeled in presynaptic terminals around these nuclei (Code and Rubel 1989; Coleman et al. 2011; Kuo et al. 2009). These studies along with our present physiological evidence of functional GlyRs in NM and NL show that the anatomical pre- and postsynaptic hallmarks of glycinergic transmission observed in these nuclei are indeed indicative of glycinergic signaling in all principal nuclei of this circuitry.
Previous studies suggested that glycinergic transmission accounts for ∼50% of the amplitude of single-pulse evoked IPSCs in the NA and SON (Coleman et al. 2011; Kuo et al. 2009). In NM we confirmed previous results that glycine transmission is not evident from recordings of spontaneous release or release evoked by transient stimuli such as single pulses (Fig. 4, A and B). Rather, our results indicate that glycine transmission was only induced during high-frequency stimulation. One possibility is that the GlyRs may be in an extrasynaptic location that requires spillover for activation. The threshold stimulus for detection of the glycine release was typically between 20- and 50-Hz pulse stimulation. Additionally, the percentage of total inhibitory current attributable to glycine increased over the course of the response. Our protocols simulated high but physiologically relevant input rates, within the range of firing rates observed for SON neurons in response to acoustic stimuli in vivo (Coleman et al. 2011). Given that the spontaneous rates of neurons in this system are similar to the threshold rates for glycine recruitment (>20 Hz, Warchol and Dallos 1990; Coleman et al. 2011; Lippe 1994; Manley et al. 1991; Nishino et al. 2008), it is possible that a low, basal level of glycine is released even in silence in vivo.
Roles for glycine in sound location computation.
Glycinergic transmission in NA and SON results from the corelease of GABA and glycine from inhibitory terminals. One function of glycine was described in vivo for SON neurons, where glycine sharpens phase-locking to acoustic stimuli (Coleman et al. 2011). The role of glycine in NM and NL has not yet been investigated in vivo. Given the results of the present study, we propose that glycinergic transmission would be recruited during intense stimulation, when GABA release may be subject to synaptic depression (Lu et al. 2005 and Fig. 4C). This hypothesis is plausible for several reasons. First, glycine and GABA are trafficked by the same vesicular transport protein, vesicular amino acid transporter (VIAAT, also known as VGAT) (Wojcik et al. 2006). VIAAT has been localized at both GABAergic and glycinergic terminals (Chaudhry et al. 1998; Dumoulin et al. 1999). Since terminals surrounding NM and NL neurons are immunopositive for both GABA and glycine, corelease is a possibility, as similar terminal profiles have been demonstrated to corelease these transmitters in NA and SON (Coleman et al. 2011; Kuo et al. 2009). The finding that glycine is only released during high-frequency stimulation may suggest that glycine is only recruited into vesicles by VIAAT when GABA is depleted in the terminal. Previous studies have demonstrated that transmitter transport into vesicles is concentration dependent and that the relative abundance of GABA or glycine may suppress transport of the complementary transmitter into vesicles (Burger 1991). Additionally, it was recently shown that glycinergic transmission can be suppressed and GABA transmission potentiated via GLYT2 block or increase in GABA synthesis (respectively) in cartwheel cells that corelease GABA and glycine (Apostolides and Trussell 2013). Thus depletion of GABA during intense and prolonged inhibitory stimulation in NM may lead to the recruitment of glycine into vesicles to maintain the inhibitory tone. Our functional test demonstrated that GlyR block does reduce the efficacy of inhibition in the NM. Evidence for a similar frequency-dependent recruitment has recently been documented in the mammalian cochlear nucleus, where GABAergic transmission is observed during high-frequency stimulation of primarily glycinergic inputs to bushy cells (Nerlich et al. 2013). These results indicate a similar scenario for inhibitory neurotransmission in mammals and birds, where one mode of transmission dominates the activity in each system but, interestingly, the complementary mode plays a role in maintaining the circuit.
Kinetic features of inhibitory transmission.
Corelease of inhibitory transmitters has been shown to affect the decay kinetics of IPSCs. For example, in the mammalian MNTB, co-application of GABA with glycine acts to speed up the kinetics of IPSCs (Lu et al. 2008). The kinetics of inhibition in this circuit are diverse and dependent on the specific nucleus. For IPSCs evoked with single stimuli, decay kinetics in the NM were observed to be approximately threefold slower than those recorded in NA, NL, and SON (Coleman et al. 2011; Kuo et al. 2009). In the NA and SON, where glycine was a prominent component of these IPSCs, the isolated glycinergic component had faster kinetics. While kinetic analysis was not a focus of this study, we did analyze IPSC decay kinetics. For high-frequency stimuli (50 pulses at 200 Hz), we observed that the decay in NM was about twofold faster than for NL in the control condition (NM: 103 ± 59 ms, NL: 193 ± 84 ms; P < 0.005, t-test). This is the opposite of what was observed in the single-pulse stimulus responses, where decay was faster in the NL. Additionally, we found that blocking GABAARs with SR95531 had different effects on the kinetics in each nucleus. Compared with the control condition, decay kinetics in the NM were slower with SR95531, whereas in the NL they were faster. The kinetics of the glycinergic component remaining after SR95531 application were remarkably similar for both nuclei (NM: 168 ± 51 ms, NL: 168 ± 66 ms). There are a number of possible mechanisms that could contribute to these rather complex differences in kinetics including variations in extrasynaptic receptors, rates of spillover or cross-reactivity of receptors (reviewed in Muller et al. 2008). There was also a considerable amount of asynchronous release in both nuclei that prolonged the decay times (>100 ms) as was well described previously in NM (Lu and Trussell 2000).
Source(s) of glycine.
The source of glycinergic terminals in NM and NL is unknown, but one possibility is the SON. The somas of some SON neurons stain positively for glycine and GABA, suggesting that high levels of both GABA and glycine are present in populations of these cells (Coleman et al. 2011). These neurons would seem to be the most parsimonious source of glycine, but it is puzzling that spontaneous glycine release occurs in NA and SON but not NM and NL, as these nuclei share SON collateral inputs (Burger et al. 2005). However, it is possible that different subsets of SON fibers project to subsets of target nuclei or that collateral terminals have different release characteristics. Whether the GABA/glycine-positive SON neurons are the population projecting to NM and NL is unresolved and requires further investigation. Alternatively, several other studies have described a small population of neurons located between the NM and NL that are immunopositive for markers of GABA and glycine (Carr et al. 1989; Kuo et al. 2009; Müller 1987; von Bartheld et al. 1989). Yamada et al. (2013) recently showed that these neurons receive excitatory input from NM and provide a stimulus-locked inhibitory input to low-frequency NL neurons. It is unknown whether these neurons also have collateral postsynaptic targets in the NM, and thus could explain the NM data presented in this report.
Summary.
This report shows that functional GlyRs are expressed in four principal nuclei of the avian auditory brain stem and that endogenous glycine is released in NM and NL by high-frequency stimulation. Importantly, glycine transmission was only recruited during long, repetitive stimulus trains albeit within the range of in vivo SON firing frequencies. This glycinergic transmission contributes to the efficacy of the overall inhibition by suppressing spikes evoked by current injections into NM neurons. These findings indicate that glycinergic inhibition is more ubiquitous in the avian brain stem than previously understood, and that models of ITD processing circuitry in avians must incorporate glycinergic components of inhibition for intense or long-duration stimuli.
GRANTS
This work is funded by grants from the Deafness Research Foundation and the National Institute on Deafness and Other Communication Disorders (R01 DC-008989).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.F., S.R.W., and R.M.B. conception and design of research; M.J.F., S.R.W., and M.G.K. performed experiments; M.J.F., S.R.W., and M.G.K. analyzed data; M.J.F., S.R.W., M.G.K., and R.M.B. interpreted results of experiments; M.J.F., S.R.W., and R.M.B. prepared figures; M.J.F. and R.M.B. drafted manuscript; M.J.F., S.R.W., M.G.K., and R.M.B. edited and revised manuscript; M.J.F., S.R.W., M.G.K., and R.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jana Nerlich for her insights on protocol development, Stefan Oline for assistance with data collection, and Drs. Julie Haas, Yong Lu, and William Coleman for comments on earlier versions of the manuscript.
REFERENCES
- Apostolides PF, Trussell LO. Rapid, activity-independent turnover of vesicular transmitter content at a mixed glycine/GABA synapse. J Neurosci 33: 4768–4781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Turecek R, Trussell LO. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol 93: 819–828, 2005 [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature 417: 543–547, 2002 [DOI] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron 7: 287–293, 1991 [DOI] [PubMed] [Google Scholar]
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol 481: 6–18, 2005 [DOI] [PubMed] [Google Scholar]
- Burger RM, Fukui I, Ohmori H, Rubel EW. Inhibition in the balance: binaurally coupled inhibitory feedback in sound localization circuitry. J Neurophysiol 106: 4–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Fujita I, Konishi M. Distribution of GABAergic neurons and terminals in the auditory system of the barn owl. J Comp Neurol 286: 190–207, 1989 [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18: 9733–9750, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code RA, Churchill L. GABAA receptors in auditory brainstem nuclei of the chick during development and after cochlea removal. Hear Res 54: 281–295, 1991 [DOI] [PubMed] [Google Scholar]
- Code RA, Rubel EW. Glycine-immunoreactivity in the auditory brain stem of the chick. Hear Res 40: 167–172, 1989 [DOI] [PubMed] [Google Scholar]
- Coleman WL, Fischl MJ, Weimann SR, Burger RM. GABAergic and glycinergic inhibition modulate monaural auditory response properties in the avian superior olivary nucleus. J Neurophysiol 105: 2405–2420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci 25: 6490–6498, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Lévi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci 112: 811–823, 1999 [DOI] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol 385: 117–134, 1997 [DOI] [PubMed] [Google Scholar]
- Funabiki K, Koyano K, Ohmori H. The role of GABAergic inputs for coincidence detection in the neurones of nucleus laminaris of the chick. J Physiol 508: 851–869, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci 8: 332–338, 2005 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969 [DOI] [PubMed] [Google Scholar]
- Hyson RL, Reyes AD, Rubel EW. A depolarizing inhibitory response to GABA in brainstem auditory neurons of the chick. Brain Res 677: 117–126, 1995 [DOI] [PubMed] [Google Scholar]
- Korada S, Schwartz IR. Development of GABA, glycine, and their receptors in the auditory brainstem of gerbil: a light and electron microscopic study. J Comp Neurol 409: 664–681, 1999 [DOI] [PubMed] [Google Scholar]
- Kuo SP, Bradley LA, Trussell LO. Heterogeneous kinetics and pharmacology of synaptic inhibition in the chick auditory brainstem. J Neurosci 29: 9625–9634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci 14: 1486–1495, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Rubio ME, Trussell LO. Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron 57: 524–535, 2008 [DOI] [PubMed] [Google Scholar]
- Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron 26: 683–694, 2000 [DOI] [PubMed] [Google Scholar]
- Lu Y, Burger RM, Rubel EW. GABAB receptor activation modulates GABAA receptor-mediated inhibition in chicken nucleus magnocellularis neurons. J Neurophysiol 93: 1429–1438, 2005 [DOI] [PubMed] [Google Scholar]
- Manley GA, Kaiser A, Brix J, Gleich O. Activity patterns of primary auditory-nerve fibres in chickens: development of fundamental properties. Hear Res 57: 1–15, 1991 [DOI] [PubMed] [Google Scholar]
- Monsivais P, Rubel EW. Accommodation enhances depolarizing inhibition in central neurons. J Neurosci 21: 7823–7830, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais P, Yang L, Rubel EW. GABAergic inhibition in nucleus magnocellularis: implications for phase locking in the avian auditory brainstem. J Neurosci 20: 2954–2963, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CM. gamma-Aminobutyric acid immunoreactivity in brain-stem auditory nuclei of the chicken. Neurosci Lett 77: 272–276, 1987 [DOI] [PubMed] [Google Scholar]
- Muller E, Le-Corronc H, Legendre P. Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front Mol Neurosci 1: 3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlich J, Künzel T, Keine C, Korenic A, Rübsamen R, Milenkovic I. Synergistic action of GABA and glycine provides powerful inhibition to spherical bushy cells in the cochlear nucleus of gerbil. 36th Annual Midwinter Meeting for the Association for Research in Otolarygology Abstract 640, 2013 [Google Scholar]
- Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci 28: 7153–7164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of brain stem auditory nuclei of the chicken: organization of projections from n. magnocellularis to n. laminaris. J Comp Neurol 164: 435–448, 1975 [DOI] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci 28: 6914–6925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16: 7046–7054, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Simler R, Grenningloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81: 7224–7227, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Philos Mag 13: 214–232, 1907 [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25: 51–101, 2002 [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Neural map of interaural phase difference in the owl's brainstem. Proc Natl Acad Sci USA 83: 8400–8404, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Moiseff A, Konishi M. Time and intensity cues are processed independently in the auditory system of the owl. J Neurosci 4: 1781–1786, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Code RA, Rubel EW. GABAergic neurons in brainstem auditory nuclei of the chick: distribution, morphology, and connectivity. J Comp Neurol 287: 470–483, 1989 [DOI] [PubMed] [Google Scholar]
- Warchol ME, Dallos P. Neural coding in the chick cochlear nucleus. J Comp Physiol A 166: 721–734, 1990 [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 50: 575–587, 2006 [DOI] [PubMed] [Google Scholar]
- Yamada R, Okuda H, Kuba H, Nishino E, Ishii TM, Ohmori H. The cooperation of sustained and phasic inhibitions increases the contrast of ITD-tuning in low-frequency neurons of the chick nucleus laminaris. J Neurosci 33: 3927–3938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Monsivais P, Rubel EW. The superior olivary nucleus and its influence on nucleus laminaris: a source of inhibitory feedback for coincidence detection in the avian auditory brainstem. J Neurosci 19: 2313–2325, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990 [DOI] [PubMed] [Google Scholar]