Abstract
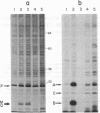
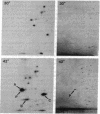
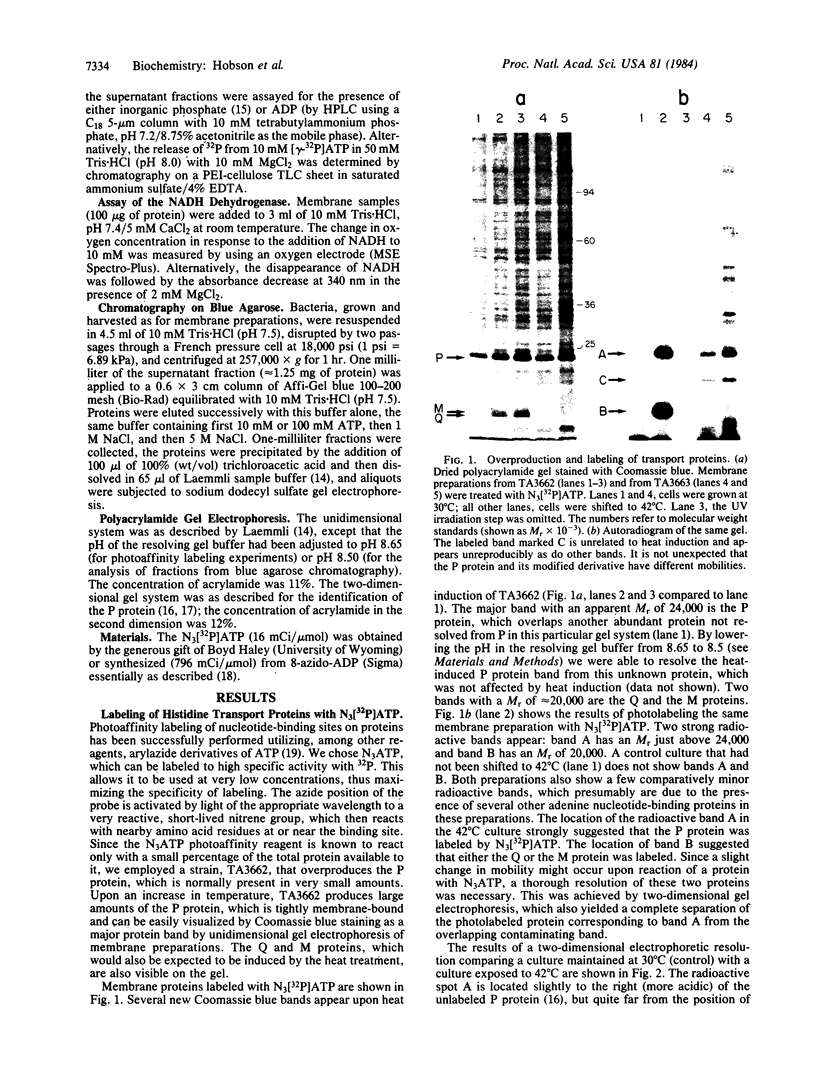
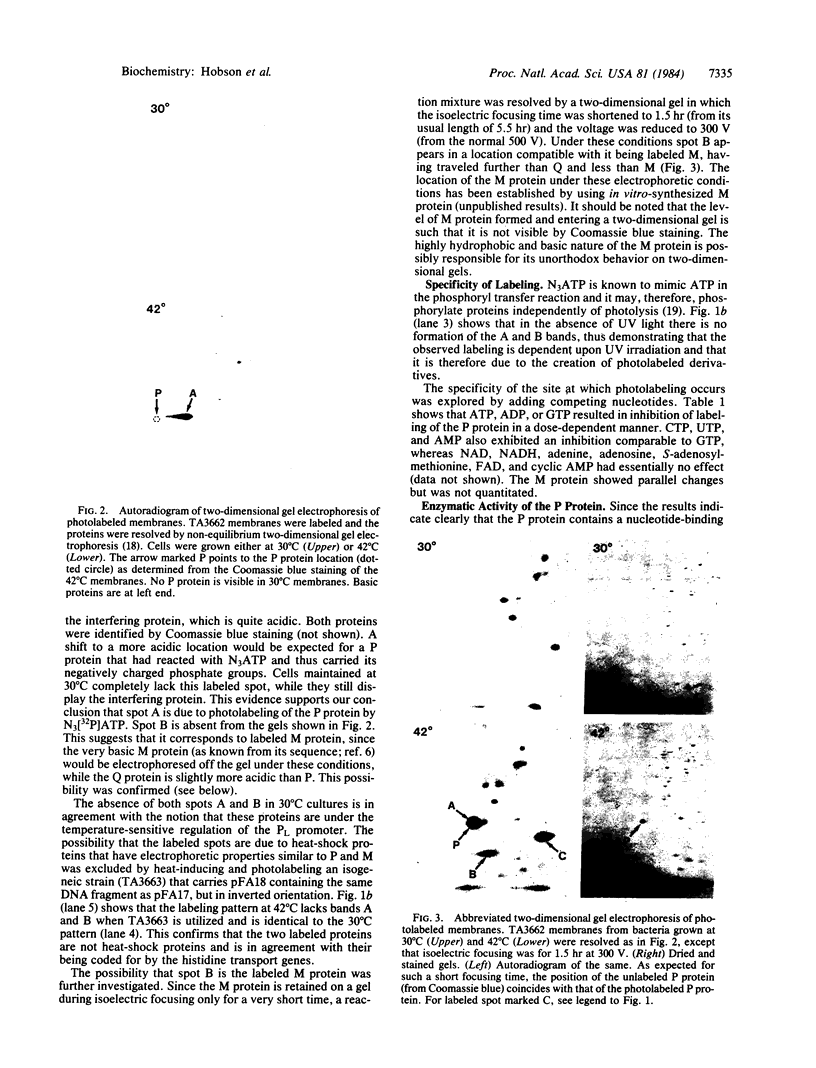
Two components of the histidine permease in Salmonella typhimurium, the membrane-bound P and M proteins, react with the photoaffinity labeling reagent 8-azido-ATP in isolated membranes. The extent of labeling is decreased by the addition of ATP and somewhat less by addition of GTP, CTP, UTP, and ADP. Cyclic AMP, NAD, FAD, and S-adenosylmethionine have little effect. We propose that one or both of these proteins have a site capable of binding an adenine nucleotide and that, therefore, they may be involved in the energy-coupling step in active transport.
Full text
PDF
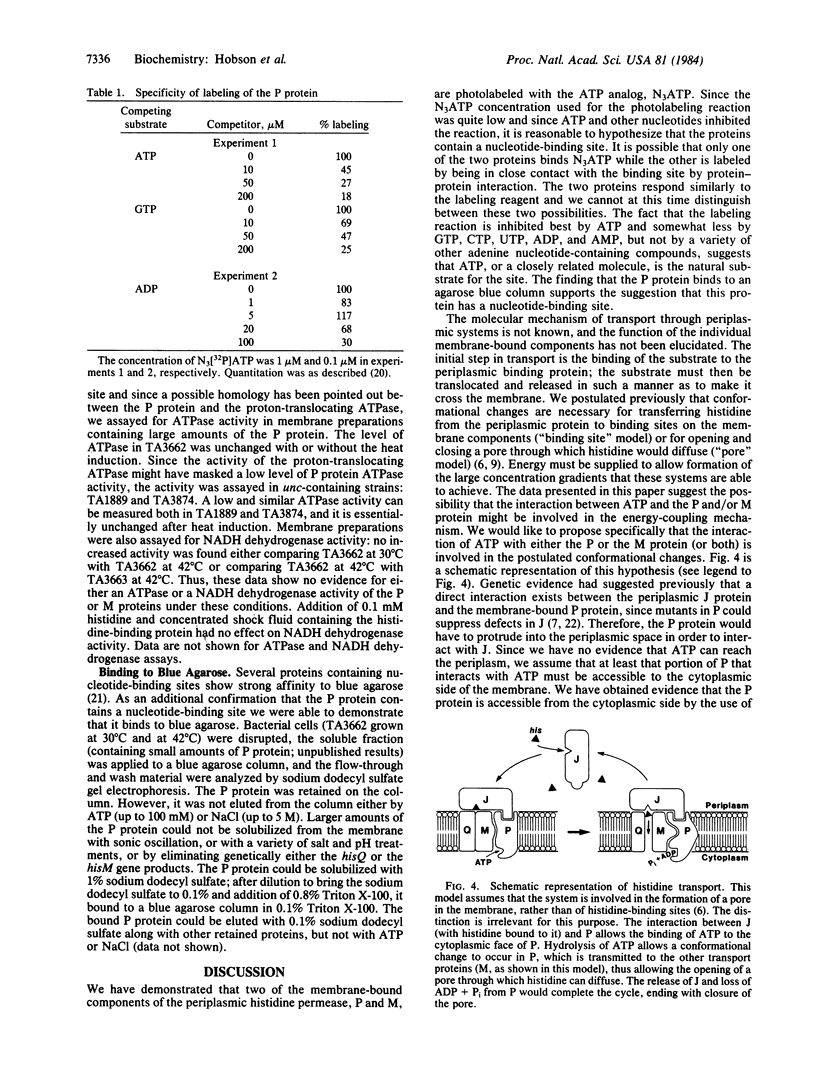

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Identification of a membrane protein as a histidine transport component in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5447–5451. doi: 10.1073/pnas.75.11.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G., Nikaido K. Phosphate-containing proteins of Salmonella typhimurium and Escherichia coli. Analysis by a new two-dimensional gel system. Eur J Biochem. 1981 Apr;115(3):525–531. [PubMed] [Google Scholar]
- Garavito R. M., Rosenbusch J. P. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol. 1980 Jul;86(1):327–329. doi: 10.1083/jcb.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Higgins C. F., Hofnung M., Ferro-Luzzi Ames G., Nikaido H. Extensive homology between membrane-associated components of histidine and maltose transport systems of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):9915–9918. [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- Khatoon S., Atherton R., Al-Jumaily W., Haley B. E. Use of nucleotide photoaffinity probes to study hormone action. Biol Reprod. 1983 Feb;28(1):61–73. doi: 10.1095/biolreprod28.1.61. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The histidine-binding protein J, a histidine transport component, has two different functional sites. J Biol Chem. 1974 Nov 10;249(21):6976–6983. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]