Abstract
Cerebral palsy (CP) results from a perinatal brain injury that often results in sensory impairments and greater errors in motor performance. Although these impairments have been well catalogued, the relationship between sensory processing networks and errors in motor performance has not been well explored. Children with CP and typically developing age-matched controls participated in this investigation. We used high-density magnetoencephalography to measure event-related oscillatory changes in the somatosensory cortices following tactile stimulation to the bottom of the foot. In addition, we quantified the amount of variability or errors in the isometric ankle joint torques as these children attempted to match a target. Our results showed that neural populations in the somatosensory cortices of children with CP were desynchronized by the tactile stimulus, whereas those of typically developing children were clearly synchronized. Such desynchronization suggests that children with CP were unable to fully integrate the external stimulus into ongoing sensorimotor computations. Our results also indicated that children with CP had a greater amount of errors in their motor output when they attempted to match the target force, and this amount of error was negatively correlated with the degree of synchronization present in the somatosensory cortices. These results are the first to show that the motor performance errors of children with CP are linked with neural synchronization within the somatosensory cortices.
Keywords: magnetoencephalography, motor control, variability, error correction, sensory, tactile
it is well recognized that children with cerebral palsy (CP) often have sensory deficits that limit their proprioception, stereognosis, and tactile discrimination (Auld et al. 2012; Clayton et al. 2003; Cooper et al. 1995; Sanger and Kukke 2007; Wingert et al. 2009). Diffusion tensor imaging (DTI) studies have suggested that these deficits are related to the extent of structural damage along the thalamocortical tracts (Hoon et al. 2009; Trivedi et al. 2008, 2010). A few previous studies that used DTI have provided some insight on this symptomatic relationship, by showing that a greater amount of damage present in the thalamocortical tracts is related to reduced muscular strength and a more severe Gross Motor Function Classification System level in children with CP (Hoon et al. 2009; Trivedi et al. 2010). Because CP can involve damage to both the sensory and motor tracts, it is unclear whether these sensory deficits generally contribute to the motor impairments seen in these children, or if they are more independent symptoms that share a common cause (i.e., perinatal brain damage). Further understanding of how the deficient somatosensory processing in children with CP interacts with the motor system is paramount for understanding the movement impairments seen in these children.
Prior experimental work has established that the brain maintains and updates an internal model that is used to predict the ideal muscular synergies to achieve a motor goal (Kording et al. 2004; Shadmere 2004; Wolpert 2007). Outcomes from these experiments have shown that an internal model of the motor system is used to formulate a motor plan based on the available sensory feedback, and this plan is transformed into a motor command that contains the predicted muscle synergies for achieving the goal state (Fig. 1). During the execution of the motor command, the internal sensory feedback that occurs is compared with the expected sensory feedback of the internal model, and any mismatch between the expected and returned sensory feedback is used to make online error corrections in the movement trajectory (Kording et al. 2004; Shadmere 2004; Wolpert 2007). The notion that the motor impairments seen in children with CP may be partly attributable to deficient processing by the sensorimotor cortices, and related error checking networks, is a novel concept that has only recently been explored (Kulak et al. 2005, 2006; Kurz et al. 2012; Kurz and Wilson 2011; Riquelme and Montoya 2010; Teflioudi et al. 2011). Intuitively, improper transmission of sensory feedback would limit the child's ability to make initial adjustments in the motor plan, and to execute online error corrections to the motor command.
Fig. 1.
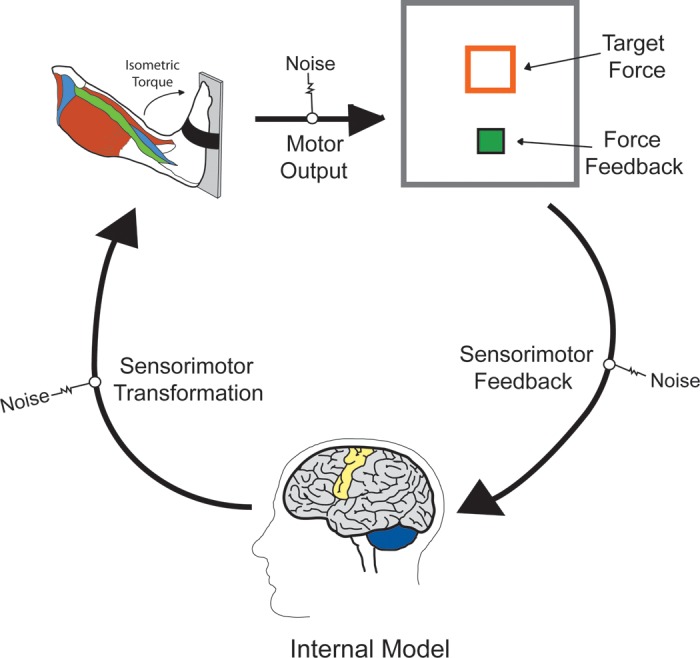
Conceptual scientific model of the sensorimotor transformation, execution, and sensory feedback stages that are involved in completing a goal-oriented isometric motor task with the ankle musculature. These stages are based on an internal model that is used to predict the muscular synergies that will best match the desired motor outcome. Sensory information is used during the formulation of the motor plan and online corrections in the evolving motor pattern.
The impression that the movement abnormalities seen in children with CP may be a product of deviant somatosensory processing has gained favorable attention over the last few years. Several studies have used magnetoencephalography (MEG) and electroencephalography (EEG) to examine neural oscillatory activity in the somatosensory cortices following sensory stimulation. The overwhelming consensus from these investigations has been that somatosensory evoked potentials/fields for the hand, foot, and lips are diminished, and in some cases they are latent in children with CP (Kulak et al. 2005, 2006; Kurz et al., 2012; Kurz and Wilson 2011; Riquelme and Montoya, 2010; Teflioudi et al. 2011). Results from a functional magnetic resonance imaging (fMRI) study have provided further support for these outcomes by showing that the somatosensory BOLD response is diminished when sensory stimulation is provided to the hand (Wingert et al., 2010). Moreover, a recent EEG study has extended these findings by revealing that aberrant event-related potentials are strongly correlated with the two-point tactile discrimination deficits often seen in children with CP (Maitre et al. 2012). Altogether these experimental results indicate that the somatosensory cortices of children with CP may not adequately process sensory stimulation. Intuitively, abnormally suppressed somatosensory processes should affect the motor performance in children with CP; however, this link has not been established.
Our prior investigation showed that children with CP have a greater amount of errors when they attempt to generate an isometric lower-extremity muscular force that matches a target (Arpin et al. 2013). Based on our scientific model presented in Fig. 1, these errors may arise during the sensorimotor transformation, the motor output, and/or the sensory feedback stages. In this investigation, we hypothesized that these errors are strongly associated with the contribution of the somatosensory cortices at the sensorimotor transformation and sensory feedback stages. To this end, the purpose of our investigation was to evaluate the relationship between oscillatory activity of the somatosensory cortices and the amount of error seen in ankle joint motor performance of children with CP. Our primary hypothesis was that greater errors in motor performance would be correlated with diminished oscillatory activity in neuronal groups of the somatosensory cortices.
MATERIALS AND METHODS
Participants.
Eleven children with a diagnosis of either spastic diplegic or hemiplegic CP (age = 14.5 ± 0.7 yr; height = 1.61 m; weight = 52.6 kg) and a Gross Motor Function Classification System score between I and III participated in this investigation. Children classified as level I could walk with minimal limitations, children classified as level II had limitations walking long distances and wore orthotics, and the child classified as level III required a walker to assist in ambulation (Palisano et al. 1997). Further details on the children with CP who participated in the experiment are shown in Table 1. Children with known large, occupying lesions and/or volume loss were not included in our investigation. An additional 11 age- and gender-matched typically developing (TD) children (age = 14.1 ± 0.7 yr; height = 1.64 m; weight = 56.8 kg) served as a control group. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Informed consent was acquired from the parents, and the children assented to participate in the experiment.
Table 1.
Demographics of the participating children with cerebral palsy
| Participant No. | Age, yr | Gender | GMFCS | Type of CP |
|---|---|---|---|---|
| 1 | 13 | Female | III | Spastic diplegia |
| 2 | 17 | Male | I | Hemiplegia |
| 3 | 17 | Male | I | Spastic diplegia |
| 4 | 13 | Male | I | Spastic diplegia |
| 5 | 17 | Male | I | Hemiplegia |
| 6 | 18 | Male | II | Spastic diplegia |
| 7 | 12 | Male | II | Hemiplegia |
| 8 | 16 | Male | II | Spastic diplegia |
| 9 | 14 | Male | II | Spastic diplegia |
| 10 | 13 | Male | III | Spastic diplegia |
| 11 | 10 | Female | I | Spastic diplegia |
GMFCS, Gross Motor Function Classification System level; CP, cerebral palsy.
Motor experimental methods.
On a separate day from the MEG experiment, an isokinetic dynamometer (Biodex, Shirley, NY) was used to measure the steady-state isometric torques generated by the ankle plantar flexors for the participating children. The largest torque generated from two maximum isometric plantar-flexion contractions was used to establish the child's maximum voluntary torque (MVT). The child then performed two submaximal steady-state isometric contractions at 20% of their MVT. We selected this percentage because our prior experimental work has shown that, compared with TD children, children with CP have greater errors in maintaining this torque level with their ankle (Arpin et al. 2013). The target and the torque exerted were displayed as boxes on a large monitor that was positioned ∼1 meter away from the subject at eye level. The box that represented the exerted torque moved vertically as the child applied greater force to the apparatus. The child was instructed to apply a torque such that the box was within the target force box, and to hold the force as accurately as possible for 30 s (Fig. 1). The maximum on the visual feedback (vertical) scale was two times the target value. The child was given ample time to practice achieving the target torque before the two actual trials were recorded. These two trials were then averaged together for all data measures. The voltage output from the torque motor was read by custom LabVIEW (National Instrument) software and sampled at 1 kHz by a 14-bit National Instruments analog-to-digital converter. The coefficient of variation [CV = (SD of torque/mean torque) × 100] was used to assess the amount of variability or error present in the middle 15 s of the steady-state torque.
MEG experimental paradigm.
Throughout the somatosensory experiment, the children were seated in a custom-made nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array. The children were instructed to close their eyes, and a unilateral tactile stimulation was applied to the bottom of the foot at the first metatarsal using a small airbladder. The same foot used for the motor experiment was used for the MEG experiment. For each child, >135 trials were collected using an interstimulus interval that varied randomly between 2,900 and 3,300 ms.
MEG data acquisition.
All recordings were conducted in a one-layer magnetically shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio-video feeds from inside the shielded room. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system (Helsinki, Finland) with 306 MEG sensors, including 204 planar gradiometers and 102 magnetometers. With the use of the MaxFilter software (Elekta), each MEG dataset was individually corrected for head motion during task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu et al. 2005).
MEG coregistration and structural MRI processing.
Four coils were affixed to the head of the subject and were used for continuous head localization during the experiment. Before the experiment, the location of these coils, three fiducial points, and the scalp surface were digitized to determine their three-dimensional position (Fastrak 3SF0002; Polhemus Navigator Sciences, Colchester, VT). Once the child was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Because coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant's MEG data was coregistered with structural T1-weighted MRI data before source space analyses. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into the Talairach coordinate system (Talairach and Tournoux 1988) using BrainVoyager QX version 2.2 (Brain Innovations).
MEG preprocessing.
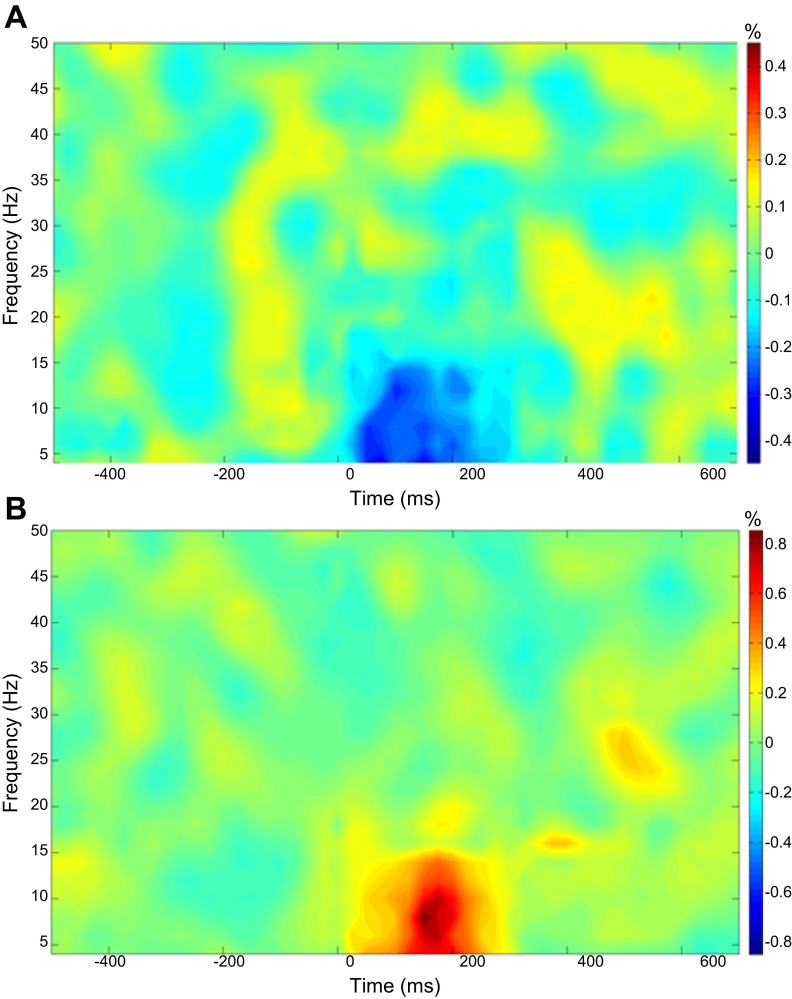
Artifact rejection was based on a fixed threshold method, supplemented with visual inspection. The data analysis epochs were a total duration of 1.2 s (−0.5 to +0.7 s), with the onset of the mechanical stimulation defined as time 0.0 s and the baseline defined as −0.5 to 0.0 s. Artifact-free epochs for each sensor were transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms) and averaged over the respective trials. The power of each time-frequency bin was normalized by dividing it by the amount of power present in the respective frequency's baseline period. This normalization procedure allowed for the visual inspection of power changes that were present in sensor space, and these spectrograms were inspected for maximal responses in the frontoparietal region (since our experiment focused on somatosensory stimulation). The MEG sensor with the maximum response (increase or decrease) in the frontoparietal region was then chosen, and this sensor was averaged across the group to derive the precise time-frequency band of interest. Based on this procedure, we identified a 4- to 14-Hz frequency range from 25 to 275 ms (see Fig. 2).
Fig. 2.
Average time-frequency spectra in controls and children with cerebral palsy (CP) during somatosensory stimulation. Time (in ms) is denoted on the x-axis, with 0 ms defined as stimulation onset. Frequency (in Hz) is shown on the y-axis. The average patterns of event-related spectral changes during the foot stimulation task, expressed as % difference from baseline (−500 to 0 ms), are shown in the children with CP (A) and the control group (B). In each case, the frontoparietal magnetoencephalography sensor with the greatest response amplitude was chosen for each participant, and these were averaged across the person's respective group. As shown, the children with CP (A) exhibited a decrease in the 4- to 14-Hz band during the 25- to 275-ms time window, whereas the healthy children showed a strong increase in this same time-frequency window (4–14 Hz, 25–275 ms). Note that the color scale for each plot is shown to the right, and that the scale for controls is two times that of the children with CP.
MEG source imaging.
A minimum variance vector beamforming algorithm was employed to calculate the source power across the entire brain volume (Gross et al. 2001). The single images were derived from the cross spectral densities of all combinations of MEG sensors within the time-frequency ranges of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per subject using a separately averaged prestimulus noise period of equal duration and bandwidth (Hillebrand et al. 2005; van Veen et al. 1997). Thus, the normalized power per voxel was computed for a 4- to 14-Hz frequency band over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Each subject's functional images, which were coregistered to anatomical images before beamforming, were transformed into a standardized space (Talairach and Tournoux 1988) using the transform previously applied to the structural MRI volume. Subsequently, the entire volume was resampled to 1.0 × 1.0 × 1.0 mm voxels. This method follows that of our other recent studies and has been further described elsewhere (Heinrichs-Graham et al. 2013; Wilson et al. 2010, 2011). MEG preprocessing and imaging used the BESA software (BESA version 5.3.2), and MEG-MRI coregistration used the BrainVoyager QX (version 2.2) software.
Statistical analysis.
Group effects were examined using a random-effects analysis for the time-frequency component of interest, and one-sample t-tests were conducted to determine the activation patterns present in each group. The statistical parametric maps were initially thresholded, and a cluster-based correction method (i.e., 40 contiguous voxels) was applied to the suprathreshold voxels to reduce the risk of false positive results. Thus, we imaged these responses using beamforming and statistically evaluated the resulting three-dimensional maps of functional brain activity using a mass univariate approach based on the general linear model. A t-test was used to determine if there were differences in the CV of the isometric ankle joint torque generated by the children with CP and the TD children. In addition, we performed Pearson product-moment and Spearman rho correlations using each participant's amplitude value in the peak voxel of the group-level statistical parametric map and the CV of the isometric ankle torque.
RESULTS
Children with CP exhibited 4–14 Hz desynchronization in the medial wall of the contralateral postcentral gyrus [P < 0.05, cluster-corrected; peak Talairach coordinates (x,y,z): −7,−23,67; Fig. 3A], whereas TD children had strong synchronization of neuronal discharges in this same brain area (P < 0.05, cluster-corrected; peak Talairach coordinates: −16,−41,58; Fig. 3A). This pattern of responses gave rise to a significant group effect (P < 0.05, cluster-corrected) in this same region of the medial postcentral gyrus (peak Talairach coordinates: −10,−29,64; Fig. 3B). These results indicate that the responsiveness of primary somatosensory cortices to the external afferent feedback was weaker and aberrant in the children with CP.
Fig. 3.
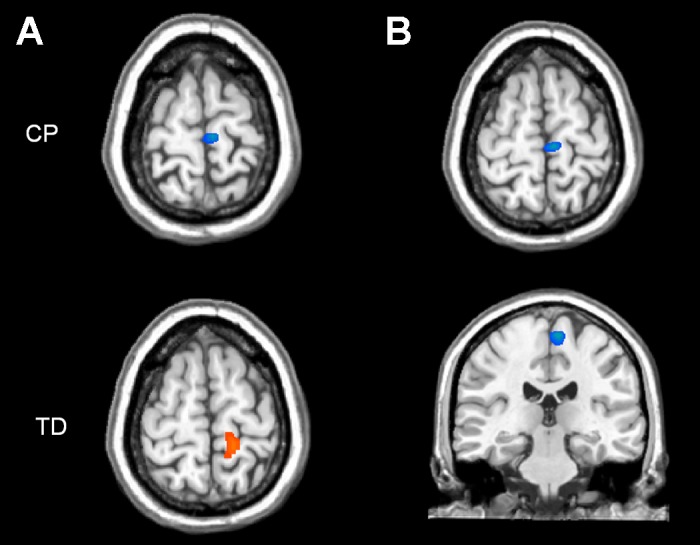
A: statistical parametric maps (SPMs) of the 4- to 14-Hz response from 25- to 275-ms poststimulation for the typically developing (TD) children (bottom) and children with CP (top). Both maps have been thresholded at P < 0.05, cluster-corrected. As can be discerned, both the children with CP and the TD children had activation in the medial wall of the contralateral postcentral gyrus. In addition, it is apparent the children with CP had a desynchronized response (blue) to the external stimulus, whereas the TD children had a synchronized response (orange). B: 2-dimensional SPMs of the group effect for the 4- to 14-Hz response (25–275 ms). Images have been thresholded at P < 0.05, cluster-corrected. As shown, the group difference was in the same region of the medial postcentral gyrus, and was weaker and aberrant in the children with CP. The images are displayed following the radiological convention (right = left).
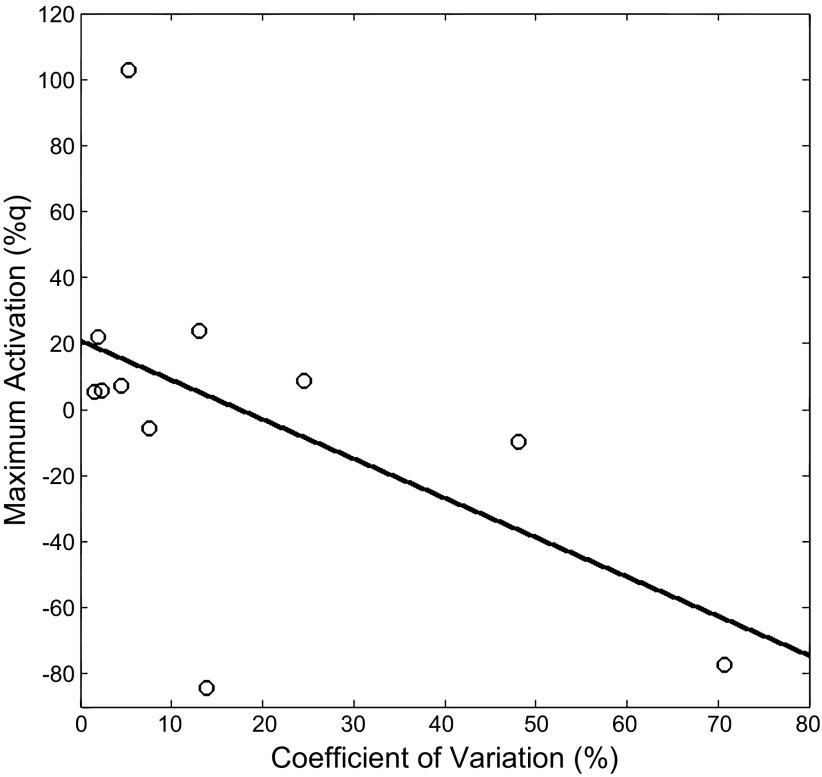
The children with CP had weaker MVT compared with the TD children (CP = 0.36 ± 0.07 nm/kg; TD = 0.98 ± 0.14 nm/kg; P = 0.0001). In addition we found that the children with CP had a larger CV while attempting to sustain the 20% MVT steady-state torque values (CP = 17.5 ± 7%; TD = 2.7 ± 0.5%; P = 0.01), implying that they had greater errors in their ability to adjust the force output to remain at the target value. The Pearson product moment calculated using the data from all of the children showed that there was a negative correlation between the CV and the amount of activity in the somatosensory cortices (r = −0.50; P = 0.01), indicating that greater errors in matching the target force were related to less synchronization in the somatosensory cortices. Further interrogation of the individual groups revealed that there was a negative correlation between the CV and the amount of activity in the somatosensory cortices (r = −0.53; P = 0.04) in the children with CP (Fig. 4), but not for the TD children (r = 0.03; P = 0.46).
Fig. 4.
Scatter graph of the relationship between the coefficient of variation of the isometric torque and the peak activation in the somatosensory cortices of the children with CP. The graph shows that a larger amount of variability or errors in the steady-state isometric torques was related to a decreased activity in the somatosensory cortices to the tactile stimulation.
The Spearman rho calculated using the data from all of the children indicated that the rank order correlation between the CV and the amount of activity in the somatosensory cortices was marginally significant (rho = −0.32; P = 0.08). Similar results were found when we evaluated the separate rank order correlations for the children with CP (rho = −0.42; P = 0.1), but not the TD children (rho = −0.15; P = 0.34). This pattern of results is consistent with those obtained using the Pearson product-moment correlations, and we expect the marginal findings simply reflect the small sample size used in this investigation.
To further explore the results, we divided the data from the children with CP into spastic diplegic (n = 8) and hemiplegic (n = 3) presentation groups. Qualitatively, it was apparent that the amplitude value in the peak voxel of the group-level statistical parametric maps was generally weaker in the children who had a spastic diplegic presentation. In addition, the children with a spastic diplegic presentation also had a larger CV while attempting to sustain the 20% MVT steady-state plantarflexion torques (spastic diplegic mean CV = 22.7%; hemiplegic mean CV = 3.8%). These exploratory results give the qualitative impression that children with a spastic diplegic presentation may be more likely to have uncharacteristic activity of the somatosensory cortices and greater errors in their motor performance.
DISCUSSION
Our results indicated that neural populations representing the foot in the somatosensory cortices abnormally desynchronized in response to tactile stimulation in children with CP. In TD children, these same neural populations exhibited increased synchronization in response to the tactile stimulation. These results provide new support to the notion that the somatosensory cortices in children with CP have an atypical response to external stimuli (Kulak et al. 2005, 2006; Kurz et al. 2012; Kurz and Wilson 2011; Maitre et al. 2012; Teflioudi et al. 2011). Based on our scientific model shown in Fig. 1, it is likely that the inability of the somatosensory cortices to properly respond to sensory feedback would have a downstream effect on the sensorimotor transformation and error checking stages. The impact on the sensorimotor transformation stage is especially salient since a large portion of the corticospinal tract originates in the postcentral gyrus (Lemon and Griffiths 2005).
The children with CP had greater errors in their ability to match and sustain the target force with their ankle. This result concurs with prior experiments that have shown that children with CP have larger errors when attempting to control the force production by the muscles of the hands or the lower-extremity joints (Arpin et al. 2013; Bandholm et al. 2009; Valvanno and Newell 1998). Interestingly, there was a negative correlation between the amount of error in motor performance and the amount of synchronization present in the somatosensory cortices in the 4- to 14-Hz frequency band. This indicates that greater errors occurred in the motor output when there was less synchronization in neural populations of the somatosensory cortices, which provide feedback to the motor system. This finding provides further support for the notion that the motor performance errors seen in children with CP are partly related to the responsiveness of the sensorimotor cortices to afferent feedback.
Variability is a prominent feature of the motor output of both TD children and children with CP. In part, some of these variations arise from subtle adjustments in the motor command to reduce errors in motor performance, whereas others arise from noise that is present at all levels of the nervous system (Churchland et al. 2006; Faisal et al. 2008). Noise at the sensory level can be problematic if it is equal to or greater than the incoming sensory information (Faisal et al. 1998). When this occurs, it may be difficult for the somatosensory cortices to discern if there has been a change in afferent feedback. Prior studies have shown that the variability seen in the motor output can be largely attributed to sensory estimation errors (Osborne et al. 2005). Because it is well established that children with CP often have sensory deficits, we suspect that they may have a heightened amount of noise present during the transmission of sensory information (Auld et al. 2012; Clayton et al. 2003; Cooper et al. 1995; Sanger and Kukke 2007; Wingert et al. 2009). Potentially, the noise-induced errors may hamper the ability of the somatosensory cortices to discern changes in the afferent feedback. We suspect that this may partly explain the uncharacteristic desynchronized response to external stimulation in this investigation.
Numerous transcranial magnetic stimulation and fMRI investigations have shown that the sensorimotor cortices of children with CP often dynamically rewire themselves throughout development. For example, in children with more severe hemiplegic presentations, the ipsilateral homolog cortices often assume the role of the damaged contralateral cortices that would normally be involved in the control of movement (Carr et al. 1993; Holmstron et al. 2010; Staudt et al. 2002; Thickbroom et al. 2001; Vandermeeren et al. 2003b). A recent fMRI study has also reported that bilateral activation of the sensorimotor cortices can occur when children with spastic diplegic and quadraplegic presentations perform a finger-to-thumb opposition task (Lee et al. 2013). Altogether, these data clearly support the notion that the sensorimotor cortices of children with CP may attempt to compensate by recruiting a larger network of brain regions for controlling movement and responding to sensory feedback. The children included in our investigation did not display such compensations in the somatosensory cortices when the foot was stimulated. We suspect that the degree and location of perinatal damage and/or behavioral experience may instigate the neuroplastic changes that encompass a larger network of brain regions. However, whether this potential compensation represents an optimization of the available cortical networks, or deviant plasticity that further degrades the child's motor performance, remains unknown.
A considerable amount of scientific evidence from animal models has shown that the topographical organization and activation of the somatosensory cortex is use dependent (Jenkins et al. 1990; Merzenich et al. 1983). In addition, a DTI study has shown that the structural connectivity of the white matter fiber tracts can be improved in children with CP after an intensive therapeutic protocol (Trivedi et al. 2008). These results suggest that the somatosensory deficits noted in children with CP may be somewhat reversible. We suspect that employing an intensive sensory-training program (touch, proprioception, vibration, and stereognosis) with children with CP may help to restore the somatosensory cortical response to the afferent feedback, and promote beneficial structural improvements in the corticospinal and thalamocortical tracts (cf. Riquelme et al. 2013). Potentially, improvements in the synchronization of the somatosensory cortices to the afferent feedback would reduce the errors that are present in the motor performance of children with CP. However, further studies are warranted to test these hypotheses.
GRANTS
Funding for this project was provided by the Hattie B. Munroe Foundation, a research support fund grant from the Nebraska Health System and the University of Nebraska Medical Center, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (1R21-HD-077532-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.K. and T.W.W. conception and design of research; M.J.K., E.H.-G., and D.J.A. performed experiments; M.J.K., E.H.-G., D.J.A., K.M.B., and T.W.W. analyzed data; M.J.K. and T.W.W. interpreted results of experiments; M.J.K. and T.W.W. prepared figures; M.J.K. and T.W.W. drafted manuscript; M.J.K. and T.W.W. edited and revised manuscript; M.J.K., E.H.-G., D.J.A., K.M.B., and T.W.W. approved final version of manuscript.
REFERENCES
- Arpin DJ, Stuberg W, Stergiou N, Kurz MJ. Motor control of the lower extremity musculature in children with cerebral palsy. Res Dev Disabil 34: 1134–1143, 2013 [DOI] [PubMed] [Google Scholar]
- Auld ML, Boyd RN, Moseley GL, Ware RS, Jonston LM. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehab 93: 696–702, 2012 [DOI] [PubMed] [Google Scholar]
- Bandholm T, Rose MH, Slok R, Sonne-Holm S, Jensen B. Ankle torque steadiness is related to muscular activation variability and coactivation in children with cerebral palsy. Muscle & Nerve 40: 402–410, 2009 [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 116: 1223–1247, 1993 [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron 52: 1085–1096, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Fleming JM, Copley J. Behavioral responses to tactile stimuli in children with cerebral palsy. Phys Occup Therapy Peds 23: 43–62, 2003 [PubMed] [Google Scholar]
- Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. J Child Neuro1 10: 300–309, 1995 [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Nat Acad Sci USA 98: 694–699, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb Cortex In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25: 199–211, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, Forssberg H, Eliasson AC. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol 52: 145–152, 2010 [DOI] [PubMed] [Google Scholar]
- Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell M, Levey E, Mori S, Johnston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51: 697–704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophys 63: 82–104, 1990 [DOI] [PubMed] [Google Scholar]
- Kording K, Ku S, Wolpert DM. Bayesian integration in force estimation. J Neurophsiol 92: 3161–3165, 2004 [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and visual evoked potentials in children with cerebral palsy: correlations and discrepancies with MRI findings and clinical picture. Ped Rehab 9: 201–209, 2006 [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and MRI findings in children with cerebral palsy: correlations and discrepancies with clinical practice. J Ped Neurol 3: 77–78, 2005 [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther 36: 16–172, 2012 [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Let 490: 1–5, 2011 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lee DR, Shin YK, Lee NG, Han BS. Comparative neuroimaging in children with cerebral palsy using fMRI and a novel EEG-based brain mapping during a motor task-a preliminary investigation. NeuroRehab 32: 279–285, 2013 [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the cortiospinal system in different species: organizational differences for motor specialization. Muscle & Nerve 32: 261–279, 2005 [DOI] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP, Key APF. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol 27: 1276–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8: 33–55, 1983 [DOI] [PubMed] [Google Scholar]
- Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39: 214–223, 1997 [DOI] [PubMed] [Google Scholar]
- Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurol 121: 1314–1320, 2010 [DOI] [PubMed] [Google Scholar]
- Riquelme I, Zamorano A, Montoya P. Reduction of pain sensitivity after somatosensory therapy in adults with cerebral palsy. Fronters Hum Neurosci 7: 1–7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Kukke SN. Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol 22: 289–293, 2007 [DOI] [PubMed] [Google Scholar]
- Shadmer R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Human Mov Sci 23: 543–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz Krageloh-Mann IJ. Two types of ipsilateral reorganization in congenital hemiparesis a TMS and fMRI study. Brain 125: 2222–2237, 2002 [DOI] [PubMed] [Google Scholar]
- Talairach G, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme, 1998 [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS). IEE Trans Signal Proc 53: 3359–3372, 2005 [Google Scholar]
- Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E, Tsikoulas I. Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatr Neurol 44: 177–182, 2011 [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol 49: 320–327, 2001 [PubMed] [Google Scholar]
- Trivedi R, Gupta RK, Shah V, Tripathi M, Rathore RKS, Kumar M, Pandey CM, Narayana PA. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Pediatr Neurol 39: 341–349, 2008 [DOI] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RKS, Gupta RK. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiol 52: 759–765, 2010 [DOI] [PubMed] [Google Scholar]
- Valvano J, Newell KM. Practice of a precision isometric grip-force task by children with spastic cerebral palsy. Dev Med Child Neurol 40: 464–473, 1998 [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Bastings E, Fadiga L, Olivier E. Long-latency motor evoked potentials in congenital hemiplegia. Clin Neurophys 114: 1808–1818, 2003 [DOI] [PubMed] [Google Scholar]
- van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880, 1997 [DOI] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 73: 75–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol 36: 596–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton J, Sinclair RJ, Brunstom JE, Damiano DL. Joint-position sense and kinesthesia in cerebral palsy. Arch Phys Med Rehabil 90: 447–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Sinclair RJ, Dixit S, Damiano DL, Burton H. Somatosensory-evoked cortical activity in spastic diplegic cerebral palsy. Hum Brain Map 31: 1772–17785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM. Probabilistic models in human sensorimotor control. Hum Mov Sci 26: 511–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]