Abstract
Individuals learn new skills at different rates. Given the involvement of corticostriatal pathways in some types of learning, variations in dopaminergic transmission may contribute to these individual differences. Genetic polymorphisms of the catechol-O-methyltransferase (COMT) enzyme and dopamine receptor D2 (DRD2) genes partially determine cortical and striatal dopamine availability, respectively. Individuals who are homozygous for the COMT methionine (met) allele show reduced cortical COMT enzymatic activity, resulting in increased dopamine levels in the prefrontal cortex as opposed to individuals who are carriers of the valine (val) allele. DRD2 G-allele homozygotes benefit from a higher striatal dopamine level compared with T-allele carriers. We hypothesized that individuals who are homozygous for COMT met and DRD2 G alleles would show higher rates of motor learning. Seventy-two young healthy females (20 ± 1.9 yr) performed a sensorimotor adaptation task and a motor sequence learning task. A nonparametric mixed model ANOVA revealed that the COMT val-val group demonstrated poorer performance in the sequence learning task compared with the met-met group and showed a learning deficit in the visuomotor adaptation task compared with both met-met and val-met groups. The DRD2 TT group showed poorer performance in the sequence learning task compared with the GT group, but there was no difference between DRD2 genotype groups in adaptation rate. Although these results did not entirely come out as one might predict based on the known contribution of corticostriatal pathways to motor sequence learning, they support the role of genetic polymorphisms of COMT val158met (rs4680) and DRD2 G>T (rs 1076560) in explaining individual differences in motor performance and motor learning, dependent on task type.
Keywords: individual differences, COMT, DRD2, sequence learning, visuomotor adaptation
practice of a motor task results in improved performance. However, there is variation in the rate of improvement across individuals. Recent work has demonstrated that investigating the sources of these individual variations, as opposed to treating them as “noise,” can aide in identification of the underlying neurocognitive mechanisms of behavior (Bo and Seidler 2009; Frank et al. 2009; Anguera et al. 2010; Kanai and Rees 2011).
Several associations have been identified between genetic polymorphisms and interindividual performance variation, in factors ranging from neuroanatomical phenotypes such as cortical size or integrity of gray/white matter (Rimol et al. 2010) to the function and availability of various neurochemicals (Erickson et al. 2008). In the current study, we used two motor learning paradigms (sequence learning and visuomotor adaptation) to investigate, first, whether genetic polymorphisms of two dopaminergic genotypes (COMT val158met and DRD2 G>T) serve as an index of individual differences in motor learning, and second, to what extent these effects depend on the type of task being learned.
Motor sequence learning is defined as acquisition of a series of new skills (e.g., learning how to play guitar or drive a car) through practice (Doyon 2008), which ultimately leads to automated performance with high accuracy and fast execution time. Sequence learning has been shown to rely on corticostriatal pathways (Doyon et al. 1997, 2003; Doyon and Benali 2005; Rieckmann et al. 2010) and is associated with visuospatial working memory capacity (Bo and Seidler 2009).
Visuomotor adaptation involves adapting motor output to account for distortion of visual representations of movement (for example, adjusting to mirror representations of objects and their distances while driving a car) (Krakauer 2009). Sensorimotor adaptation has been linked to cerebellar and parietal regions (Clower et al. 1996; Imamizu et al. 2003). There is some controversy over whether this type of learning also relies on corticostriatal pathways; we have reported bilateral basal ganglia activation during the early stages of this type of learning (Seidler et al. 2006) whereas others have not (Krakauer et al. 2004). Moreover, several studies have documented that patients with Parkinson's disease exhibit normal visuomotor adaptation (Muslimović et al. 2007; Bédard and Sanes 2009) but others have reported impairments (Doyon et al. 1997; Contreras-Vidal and Buch 2003; Shin and Ivry 2003; Paquet et al. 2008; Leow et al. 2013). Thus the extent to which adaptation relies on corticostriatal pathways remains an open question.
Markers of individual differences, particularly, the alleles of genes involved in dopaminergic metabolism, may help to clarify the underlying mechanisms of these two types of learning. Recent findings of genotype associations with dopamine availability in the prefrontal cortex and corticostriatal circuits highlight the role of a single nucleotide polymorphism of the catechole-O-methyltranspherase (COMT) gene at codon 158/108 (Krämer et al. 2007; Frank et al. 2009). The substitution of a valine (val) with methionine (met) allele at this codon (G to A) results in reduced COMT enzymatic activity, which leads to less dopamine degradation and higher prefrontal dopamine availability (Chen et al. 2004). COMT met homozygotes show comparatively better performance in working memory tasks (Malhotra et al. 2002; Foltynie et al. 2004; Dumontheil et al. 2011). It is unknown whether individuals homozygous for the met allele exhibit faster motor learning. However, the positive association between working memory efficacy and the increased rate of both sequence learning and visuomotor adaptation (Bo and Seidler 2009; Anguera et al. 2010) raises the possibility that met homozygotes will learn these two tasks more quickly and accurately than val-met or val-val individuals.
In addition to COMT val158met, the DRD2 G>T polymorphism influences dopamine availability by regulating the expression of striatal dopamine receptors. D2 receptor activity in the striatum has been associated with motor control, coordination, and error avoidance (Xu et al. 2007; Doll et al. 2011). The T allele of the DRD2 genotype (rs 1076560) is associated with reduced D2 expression and consequently with declines in cognitive processing and motor performance (Bertolino et al. 2009). Individuals who are carriers for the DRD2 T allele show a greater area of activated brain regions and reduced levels of performance in working memory tasks, indicating less efficient neural processing (Zhang et al. 2007). Furthermore, we have recently shown that DRD2 genotype is predictive of the effect that l-DOPA has on motor sequence learning in patients with Parkinson's disease (Kwak et al. 2013).
Here we evaluated the proposed role of alleles for genes involved in dopaminergic transmission (COMT val158met and DRD2 G>T) as an index of individual differences in motor sequence learning and visuomotor adaptation. We hypothesized that individuals homozygous for high-performance-associated alleles (COMT-met and DRD2-G) would demonstrate faster rates of motor learning and adaptation. We also determined genotype effects on working memory performance and its relation to rate of learning.
MATERIALS AND METHODS
Participants
We recruited 72 young (20 ± 1.9 yr) healthy females of Caucasian/European descents. We limited the gender and ethnicity of our recruitment because these factors are thought to interact with genotype effects for our genes of interest (Duara et al. 1996; Farrer et al. 1997). With the adjusted α = 0.01 and maintaining the power of 0.08, we were able to detect a moderate effect size of d = 0.45 for the current sample size. Participants were excluded for any major medical issues or neurological impairment. Individuals participated in two test sessions scheduled on consecutive days. The University of Michigan Institutional Review Board approved the study, and all participants gave their written informed consent before starting study procedures. Two subjects were excluded due to missing data.
Genotyping
Saliva samples were obtained from all participants with an Oragene DNA self collection kit. Following the same protocol that we have used previously (Kwak et al. 2013), genotype analysis was performed using a polymerase chain reaction. The single nucleotide polymorphisms (SNPs) for COMT (rs4680) and DRD2 (rs1076560) were determined. The number of subjects in each genotype group was as follows for COMT (met-met = 20, val-met = 35, and val-val = 11) and DRD2 (GG = 45, GT = 22, and TT = 3; Table 1). The COMT genotype could not be determined for four subjects due to DNA contamination. The allelic genotype distributions were consistent with the literature and the typical ratio in the population (according to Hardy-Weinberg equilibrium) (Lindenberger et al. 2008; Bertolino et al. 2009).
Table 1.
Allelic distribution of COMT val158met and DRD2 G>T across 70 healthy young females
| Genotype/Allele | Frequency, % | Expected Frequency, % |
|---|---|---|
| COMT | ||
| met-met | 20 (30.3) | 28 |
| val-met | 35 (53) | 47 |
| val-val | 11 (16.6) | 25 |
| DRD2 | ||
| GG | 45 (64.2) | 75 |
| GT | 22 (31.4) | 22 |
| TT | 3 (4.2) | 3 |
The catechol-O-methyltransferase (COMT) genotype could not be determined for 4 subjects. The allelic distribution of each genotype is in Hardy-Weinberg equilibrium for the European-derived population. DRD2, dopamine receptor D2.
We also defined an independent factor based on the number of alleles associated with high performance that each subject carries. Based on the number of COMT-met and DRD2-G alleles, individuals can be carriers of zero, one, two, three, or four high-performance alleles (Table 2). As shown in Table 2, all participants had at least one high-performance allele.
Table 2.
Frequency of the number of high performance alleles across 68 healthy young females
| Number of High Performance Alleles | Frequency, % |
|---|---|
| 1 | 8 (11.4) |
| 2 | 15 (21.4) |
| 3 | 30 (42.9) |
| 4 | 15 (21.4) |
The COMT genotype could not be determined for 4 subjects.
Although previous researchers have proposed different loci of the effect for each genotype, most recent studies have emphasized differences caused by the presence of high- or low-performance alleles. The effect of a single genotype on measured variation in phenotype can be examined as using several models. In a “dominant” model, individuals having either one copy or two copies of a specific allele can be classified as a single group. For example, if the “val” allele of COMT were modeled as a “dominant” effect, individuals with either one copy (heterozygous, val-met) or two copies (homozygous, val-val) are assigned to one group and compared with the group of homozygote met-met individuals. In an alternative model, three separate groups are assigned: 1) homozygous val-val, 2) homozygous met-met, and 3) heterozygous val-met, where each of the three groups may result in a different phenotype outcome. If the three phenotype groups differ and show a directional influence of the number of alleles held by an individual (e.g., the average phenotype value of val-met group is between the val-val and met-met groups), then the model can be described as “additive.” Here we applied the “additive” model since our preliminary analysis showed a direct effect of each genotype group which would be overlooked if we used the “dominant” model.
Sensorimotor Tasks
Motor sequence learning.
Visual stimuli were presented in a random (“R”) or sequential (“S”) pattern on the computer screen, and participants were instructed to make responses to them by pressing the corresponding button on a bimanual key-press device in front of them as quickly and accurately as possible (Bo and Seidler 2009; Kwak et al. 2010). The sequences consisted of eight repeating elements (e.g., 2, 1, 4, 2, 3, 1, 3, 4). Throughout the experiment, participants learned the repeating pattern and became able to predict the next element and, consequently, responded faster. The same structure (i.e., no runs of 1, 2, 3, 4, no trills 1, 2, 1, 2, and no repeats of the same element in a row) existed in the random blocks; however, elements did not repeat in a specific pattern. There were 96 trials within each of 11 blocks appearing in the following order: R1-R2-S3-S4-R5-S6-S7-R8-S9-S10-R11. The first block (R1) was to acquaint participants with the task and was not included in the analyses. However, it was depicted in the figures to reflect the familiarization process of the task, showing the reaction time alterations in the transition from block 1 to block 2. Each block started with the appearance of a message on the screen to explicitly inform the participant that the following block would be either “R” or “S.” When participants made an error, the same trial repeated again. Blocks were divided into three different stages of learning: early, middle, and late; the first block was considered as baseline performance and it was not included in the analysis. Across the sequence and random pairs of blocks, we categorized the last sequence blocks and the subsequent random blocks into three pairs: S4&R5, S7&R8, and S10&R11 representing three learning phases, early, middle and late, respectively. In each pair, we identified the learning extent by subtracting the mean reaction time of the random block, from the mean reaction time in the previous sequence block. The dependent variables for this task were the participant's mean reaction time, the extent of learning in each phase, and the mean number of errors that each participant made across blocks (accuracy factor).
Visuomotor adaptation.
In the visuomotor adaptation task, participants manually controlled the movement of a cursor on a computer screen with a Logitech Extreme 3D joystick. The movement starting point was the center of the screen, and the target was a red circle that randomly appeared for 4 s either above, below, to the right, or the left of the central location. Data were sampled at 250 Hz, and the intertrial interval was ∼5 s. Participants were instructed to move the cursor with the joystick to the target on the computer screen and wait until it disappeared before returning the cursor back to the central start location. There were 14 blocks, with each including 24 trials. After the first two blocks with veridical visual feedback, a 30° clockwise rotation was applied to the cursor feedback. Participants were not advised of the rotation, and they needed to adjust their movement to the required direction and move the cursor to the correct spot. The angle between the line made by the participant's movement at the time of peak velocity and the line connecting the starting point to the target was defined as the direction error (DE). The rate of adjustment was calculated based on the size of DE and the exponential decay across adaptation trials. The last two blocks were the washout period when the visual feedback was returned to normal (Anguera et al. 2009, 2011).
Working Memory Assessments
Visual array change.
We modified Luck and Vogel's visuospatial working memory task (1997). In our design, there were 150 trials (5 sets of 30 trials). Each trial consisted of two arrays of colored squares displayed on the computer screen: a sample array and a test array. The first array (sample array) was presented for 250 ms, followed by a blank screen and, immediately, the same array of squares appeared in the exact same positions on the screen, except that one of them was encircled (test array). Participants had to indicate whether the encircled square in the test array had the same color as the one that had been presented just before in the sample array. The array size (number of squares in each array) was randomly changed between 2, 4, 6, 8, or 10. Participants were instructed to press the “A” key if the encircled square had the same color, and the “L” key if it appeared in a different color. Sets were divided evenly between each correct response (i.e., 15 A, 15 L), and the response duration was subject dependent. We calculated the visual array change (VAC) score based on the equation introduced by Vogel and Machizawa (2004), where K (working memory capacity) = S (size of array) × [H (hit rate) − F (false alarm rate)].
Card rotation test.
The Card Rotation test is designed to measure an individual's spatial processing ability. In this test participants compared an original figure with eight different representations of that same figure to decide whether each was a rotated version or had been flipped-over (mirror image) with respect to the original figure. There were 2 test pages, each including 10 rows of different figures (total number of 80) and participants responded to them by marking the figures as “same” (rotated version) or “different” (flipped-over version). Participants were given a maximum of 3 min to finish each page, and their score was calculated separately for each page as the number of correct responses minus the number of incorrect responses. The final score was averaged across the two pages.
Data Analysis
To account for the effect of small sample sizes in some of the genotype cells and the nonnormality of group distribution, we conducted a nonparametric mixed model ANOVA (using a bias corrected accelerated bootstrap) for both the sequence learning task and visuomotor adaptation tasks. The genotype effect on reaction time in motor sequence learning was determined by using genotype as a between subject factor (fixed factor) and blocks as within subject factor (repeated factor). The same method was applied for assessing genotype effects on accuracy. The result of these analyses indicated whether there is a main effect of genotype, block, or a genotype by block interaction. We controlled for the effect of multiple comparisons for the five different phenotypes by adjusting the P value to P ≤ 0.01 for the omnibus F-tests.
In addition, for the sequence learning task we categorized the last sequence blocks and their subsequent random blocks into three pairs: S4&R5, S7&R8, and S10&R11, representing three learning phases: early, middle, and late, respectively. Using each pair as a within subject factor and genotype as a between subject factor, we were able to identify whether there is a main genotype effect on the extent of learning in each phase. The post hoc Mann-Whitney U-test was conducted based on the difference of average reaction times (RT) in each phase as follows for early = mean RTR5 − mean RTS4, middle = mean RTR8 − meanRTS7, and late = mean RTR11 − meanRTS10. It was also used as a follow up analysis on the average reaction time and average number of errors across all blocks.
We also applied a nonparametric linear regression to find whether there is a correlation between learning extent in each phase and the number of high-performance alleles that each subject carries.
The same nonparametric mixed model ANOVA was used for analyzing the visuomotor adaptation task, using genotype as a between subject factor (fixed factor) and block as a within subject factor (repeated factor). These analyses determined whether there is a main effect of genotype or block on the size of direction error. Also, whether there is a genotype by block interaction reflecting the genotype effect on the rate of motor adaptation. A nonparametric linear regression was applied to find whether there is a correlation between the rate of adjustment and number of high-performance alleles as well.
The genotype effect on working memory capacity was assessed using a nonparametric one-way ANOVA, taking the VAC and Card Rotation scores as dependent variables, and COMT and DRD2 genotypes as between subject factors. The VAC score and Card Rotation score were used as measures of working memory capacity. Additionally, a nonparametric linear regression was conducted to find a correlation between the working memory capacity and number of high-performance alleles.
RESULTS
Motor Sequence Learning
Reaction time_ COMT.
We found a main effect of block (F10 = 25, P = 0.0001), and a main effect of COMT genotype (F2 = 16.3, P = 0.0001), but no block by COMT interaction on reaction time in the sequence learning task (F20 = 0.30, P = 0.99). A post hoc Mann-Whitney U-test was conducted on the average reaction time across blocks and showed that the main effect of COMT is driven by a significantly higher reaction time of the val-val group compared with the met-met group (U = 67, P = 0.03). Also, the main effect of block is driven by higher reaction time in random blocks compared with sequence blocks (U = 27827.5, P = 0.0001; Fig. 1A).
Fig. 1.
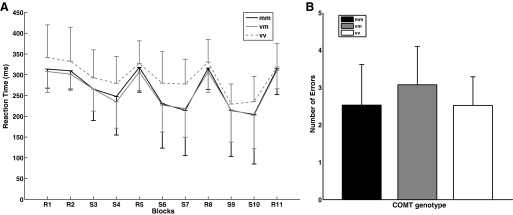
A: sequence learning task. Reaction time across random (R) and sequence (S) blocks for catechol-O-methyltransferase (COMT) val158met (vm) genotype. The first (R) block is the familiarization block. The val-val (vv) group exhibited higher reaction time (P = 0.03) compared with the met-met (mm) group in the middle learning phase (S7-R8). This difference was not significant in the post hoc test on the reaction time differences across S7-R8. B: sequence learning task. Average number of errors for COMT val158met genotype. There was no significant difference between the number of errors that each genotype group made.
Learning extent_ COMT.
An exploratory analysis was conducted to compare the performance in the last sequence block with the subsequent random block in each phase of learning (i.e., blocks 4 and 5 in early, blocks 7 and 8 in middle, and blocks 10 and 11 in late learning phase). A nonparametric group by blockS4-R5 mixed model ANOVA showed a main effect of block (F1 = 29.56, P = 0.0001), no main effect of COMT (F2 = 3.01, P = 0.052), and no COMT by block interaction (F2 = 0.29, P = 0.74). For the middle learning phase, a nonparametric group by blockS7-R8 mixed model ANOVA revealed a main effect of block (F1 = 44.22, P = 0.0001), and a main effect of COMT (F2 = 4.23, P = 0.01), but no COMT by block interaction (F2 = 0.56, P = 0.56). Lastly, a nonparametric group by blockS10-R11 mixed model ANOVA revealed a main effect of block (F1 = 57.8, P = 0.0001), but no main effect of COMT (F2 = 1.54, P = 0.21), nor a COMT by block interaction (F2 = 0.24, P = 0.78). The post hoc Mann-Whitney U-test for the average reaction time in each learning phase did not show any significant difference between COMT genotype groups.
In sum, all participants learned the sequence equally well, regardless of COMT genotype. The met-met group exhibited shorter reaction times than the val-val group, but this effect was not sequence-specific (Fig. 1A).
Accuracy_ COMT.
A nonparametric group by block mixed model ANOVA for the number of errors per block revealed a main effect of block (F10 = 7.22, P = 0.0001), and a main effect of COMT (F2 = 4.44, P = 0.01), but no COMT by block interaction (F20 = 0.44, P = 0.98). A post hoc Mann-Whitney U-test for the average number of errors across blocks revealed no significant difference between the numbers of errors made by each COMT genotype group, making it difficult to disentangle the source of the COMT main effect (Fig. 1B).
Reaction time_DRD2.
The nonparametric group by block mixed model ANOVA revealed a main effect of block (F10 = 6.44, P = 0.0001), and a main effect of DRD2 genotype (F2 = 6.83, P = 0.001), but no DRD2 by block interaction (F20 = 0.29, P = 0.99) on reaction time in the motor sequence learning task (Fig. 2A). The post hoc Mann-Whitney U-test on average reaction time across blocks showed only a trend for higher reaction time of the TT group compared with the GT group (U = 12, P = 0.07). No significant difference was found between GG and TT, or GG and GT group reaction times. Also, the main effect of block is driven by the higher reaction time in random blocks compared with sequence blocks (U = 27827.5, P = 0.0001).
Fig. 2.
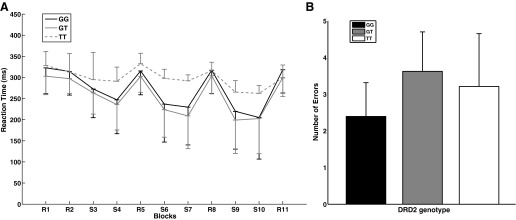
A: sequence Learning task. Reaction time across random and sequence blocks for DRD2 G>T genotype. The first (R) block is the familiarization block. The TT group exhibited a trend for over all higher reaction time (P = 0.07) compared with the GT group. There was no group by block interaction in any of the 3 learning phases. B: sequence learning task. Average number of errors for dopamine receptor D2 (DRD2) genotype. The GG group showed a trend for making fewer errors than the GT group (P = 0.06).
Learning extent_ DRD2.
Similar to COMT genotype described above, an exploratory analysis was conducted to compare the performance in the last sequence block with the subsequent random block in each phase of learning (i.e., blocks 4 and 5 in early, blocks 7 and 8 in middle, and blocks 10 and 11 in late learning phase). A nonparametric group by blockS4-R5 mixed model ANOVA revealed a main effect of block (F1 = 9.83, P = 0.002), but no main effect of DRD2 (F2 = 1.40, P = 0.24), nor a DRD2 by block interaction (F2 = 0.12, P = 0.88). A group by blockS7-R8 showed a main effect of block (F1 = 10.95, P = 0.001), but no main effect of DRD2 (F2 = 1.62, P = 0.20), nor a DRD2 by block interaction (F2 = 0.69, P = 0.50). A group by blockS10-R11 revealed a main effect of block (F1 = 13.58, P = 0.0001), but no main effect of DRD2 (F2 = 0.55, P = 0.57), nor a DRD2 by block interaction (F2 = 0.82, P = 0.44; Fig. 2A).
Accuracy_ DRD2.
A nonparametric group by block mixed model ANOVA for the number of errors per block showed a main effect of block (F10 = 3.86, P = 0.0001), and a main effect of DRD2 (F2 = 21.08, P = 0.0001), but no DRD2 by block interaction (F20 = 0.51, P = 0.96). The post hoc Mann-Whitney U-test for the average number of errors across blocks showed a trend for a larger number of errors made by the GT group compared with the GG group (U = 358, P = 0.06; Fig. 2B).
In sum, all individuals learned the sequence equally well regardless of DRD2 genotype. TT genotype was associated with longer reaction times whereas the GT genotype was associated with more errors, but these effects were marginally significant, and not sequence-specific.
Sequence learning_ high-performance alleles.
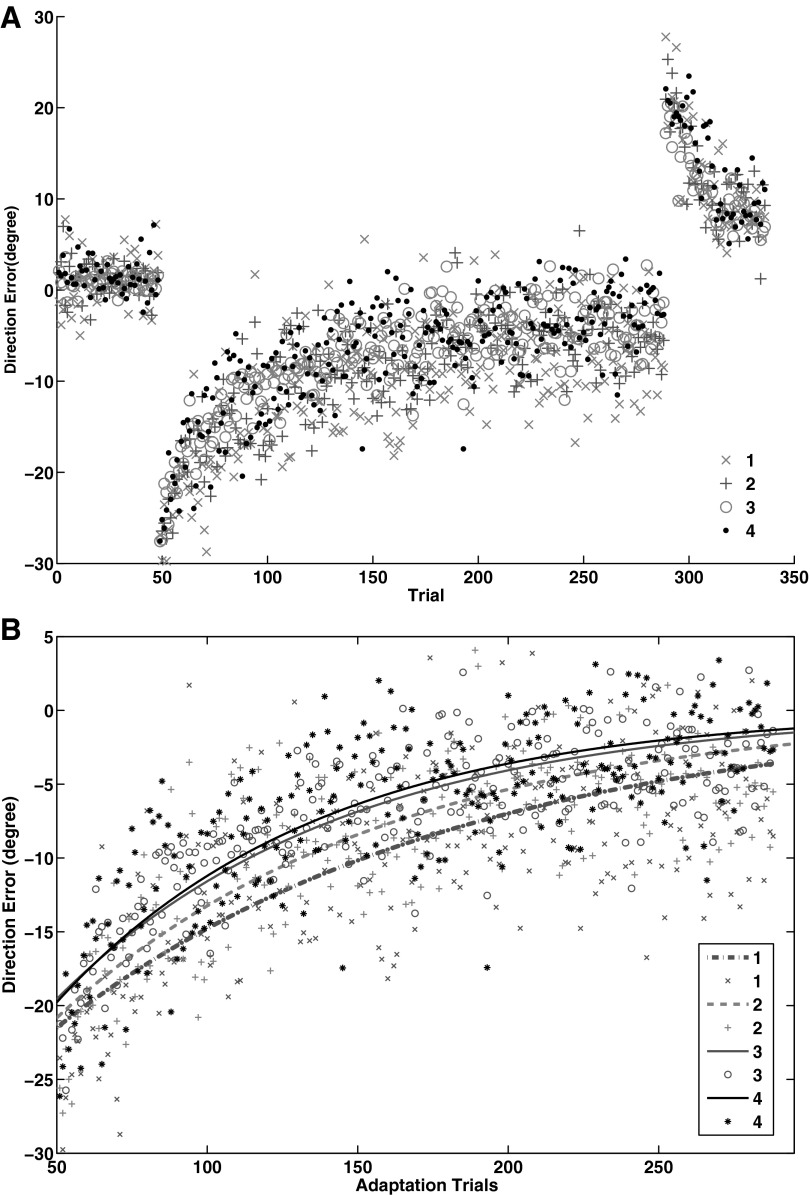
No significant correlation was found between the number of postulated “high-performance alleles” (NHPA) and the learning extent in each learning phase using a nonparametric linear regression (early: R = 0.15, F1,66 = 1.65, P = 0.20; middle: R = 0.17, F1,66 = 2.12, P = 0.15; late: R = 0.19, F1,66 = 2.62, P = 0.11). Also, there was no correlation between NHPA and average reaction time across blocks (R = 0.16, F1,66 = 1.83, P = 0.18). However, a nonparametric mixed model ANOVA for the effect of NHPA on sequence learning showed a main effect of block (F10 = 22.63, P = 0.0001), and a main effect of NHPA (F3 = 5.70, P = 0.001), but no block by NHPA interaction (F30 = 0.30, P = 1; Fig. 3A). The post hoc Mann-Whitney U-test for average reaction time across blocks showed that the main effect of NHPA is driven by a significantly longer reaction time for the carriers of one high-performance allele compared with carriers of four high-performance alleles (U = 27, P = 0.03). Also, there was no significant correlation between NHPA and accuracy level (R = 0.18, F1 = 2.19, P = 0.14). However, a nonparametric mixed model ANOVA for the effect of NHPA on errors across blocks showed that there was a main effect of NHPA (F3 = 8.07, P = 0.0001), a main effect of block (F10 = 8.9, P = 0.0001), but no NHPA by block interaction (F30 = 0.47, P = 0.99). The post hoc Mann-Whitney U-test showed that the main effect of NHPA on the number of errors across blocks is driven by the significantly higher number of errors made by carriers of one high-performance allele compared with carriers of four (U = 5721, P = 0.005), three (U = 10569, P = 0.0001), and two (U = 5876, P = 0.01) high-performance alleles (Fig. 3B).
Fig. 3.
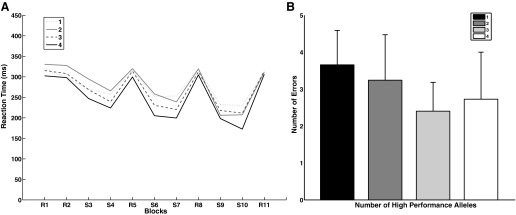
A: effect of the number of postulated “high-performance allele” (NHPA) factor on reaction time in sequence learning task. Carriers of 1 high-performance allele showed higher reaction time across blocks compared with the 4 high-performance allele carriers (P = 0.03). B: effect of NHPA factor on number of errors across blocks in sequence learning task. Carriers of 1 high-performance allele made higher number of errors compared with carriers of 4 (P = 0.005), 3 (P = 0.0001), and 2 (P = 0.01) high-performance alleles.
Overall, these findings demonstrate that COMT and DRD2 genotypes, when combined, are associated with motor performance (i.e., reaction time and errors) but not learning in the sequence task.
Visuomotor Adaptation
Pretest phase_ COMT.
A nonparametric group by block mixed model ANOVA for the first two blocks of the visuomotor adaptation task showed no main effect of block (F1 = 0.12, P = 0.72), or COMT (F2 = 2.46, P = 0.08), nor a COMT by block interaction (F2 = 0.12, P = 0.88) (Fig. 4A).
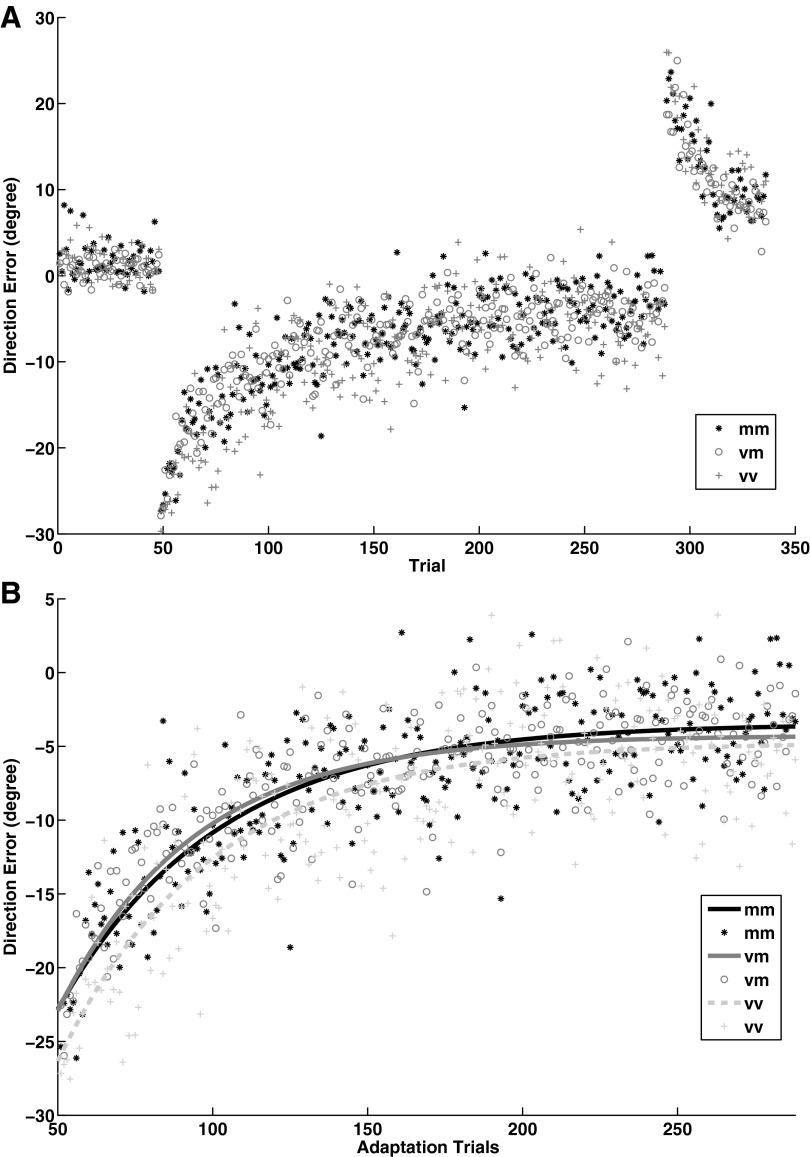
Fig. 4.
A and B: visuomotor adaptation task. A: direction error across pretest (blocks 1–2), adaptation (blocks 3–12) and washout period (blocks 13–14) for COMT val158met. The COMT val-val group exhibited greater direction error across the adaptation trials than both met-met (P = 0.001) and val-met (P = 0.002) groups. B: rate of exponential decay across adaptation trials showed no significant difference between COMT genotype groups.
Pretest phase_ DRD2.
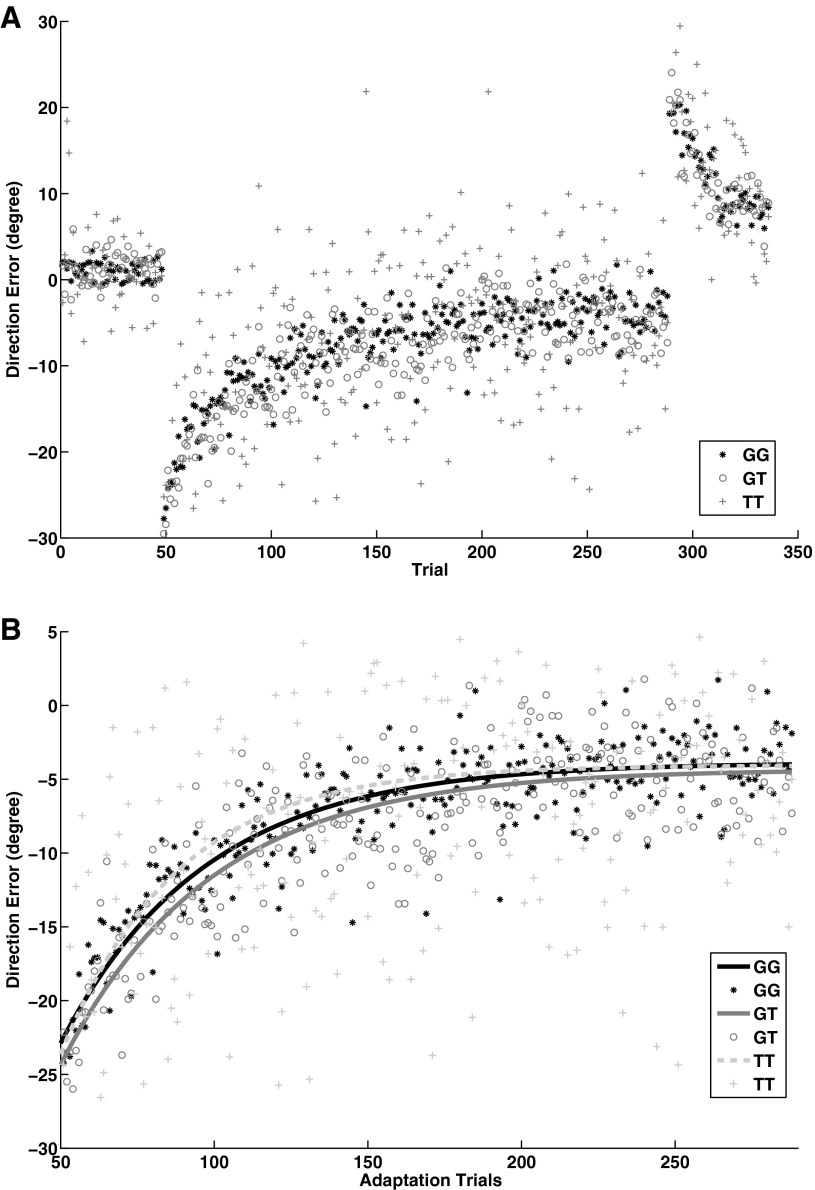
A nonparametric group by block mixed model ANOVA showed no main effect of block (F1 = 0.33, P = 0.56), or DRD2 (F2 = 0.27, P = 0.75), nor a DRD2 by block interaction (F2 = 1.16, P = 0.31; Fig. 5A).
Fig. 5.
A and B: visuomotor adaptation task. A: direction error (DE) across pretest (blocks 1–2), adaptation (blocks 3–12) and washout period (blocks 13–14) for DRD2 G>T. There was no significant main effect of DRD2 genotype on the size of direction error. B: rate of exponential decay across adaptation trials showed no significant difference between DRD2 genotype groups.
These findings support that all participants, regardless of their genotype group, had equal pretest performance.
Adaptation phase _COMT.
A nonparametric group by block mixed model ANOVA showed a main effect of block (F9 = 107.07, P = 0.0001), and a main effect of COMT (F2 = 28.10, P = 0.0001), but no COMT by block interaction (F18 = 1.03, P = 0.42) on direction error during the adaptation phase. The post hoc Mann-Whitney U-test on average DE across blocks revealed that the val-val group demonstrated a larger DE compared with both met-met (U = 31, P = 0.001) and val-met (U = 70, P = 0.002) groups. These findings demonstrate reduced performance in the adaptation phase for the val-val group that stayed constant throughout the adaptation period (Fig. 4A). The rate of exponential decay was not significantly different between COMT genotype groups (Fig. 4B).
Adaptation phase _DRD2.
A nonparametric group by block mixed model ANOVA showed a main effect of block (F9 = 37.64, P = 0.0001), and a main effect of DRD2 (F2 = 7.04, P = 0.001), but no DRD2 by block interaction on direction error during the adaptation blocks (F18 = 0.62, P = 0.88). The post hoc Mann-Whitney U-test on average DE across blocks showed no significant difference between the sizes of DE (Fig. 5A) and the decay constants (Fig. 5B) in each DRD2 genotype groups, making it difficult to disentangle the genotype main effect.
Finding a main effect of block on DE across adaptation blocks supports that performance improved across blocks for all subjects. The level of improvement was affected by genotype, as supported by genotype main effects on the adaptation blocks but not the pretest blocks.
Readaptation phase (washout)_COMT.
A nonparametric group by block mixed model ANOVA for the last two blocks (blocks 13 and 14) revealed a main effect of block (F1 = 101.83, P = 0.0001), but no main effect of COMT (F2 = 2.46, P = 0.08), nor a COMT by block interaction (F2 = 0.73, P = 0.48; Fig. 4A).
Readaptation phase (washout)_ DRD2.
A nonparametric group by block mixed model ANOVA showed a main effect of block (F1 = 40.67, P = 0.0001), but no main effect of DRD2 (F2 = 0.27, P = 0.76), nor a DRD2 by block interaction (F2 = 0.46, P = 0.62; Fig. 5A).
The main effect of block found for both genetic loci in the washout period indicates that aftereffects were reduced with practice for all subjects, and the degree of this effect was not associated with genotype.
Visuomotor adaptation_ high-performance alleles.
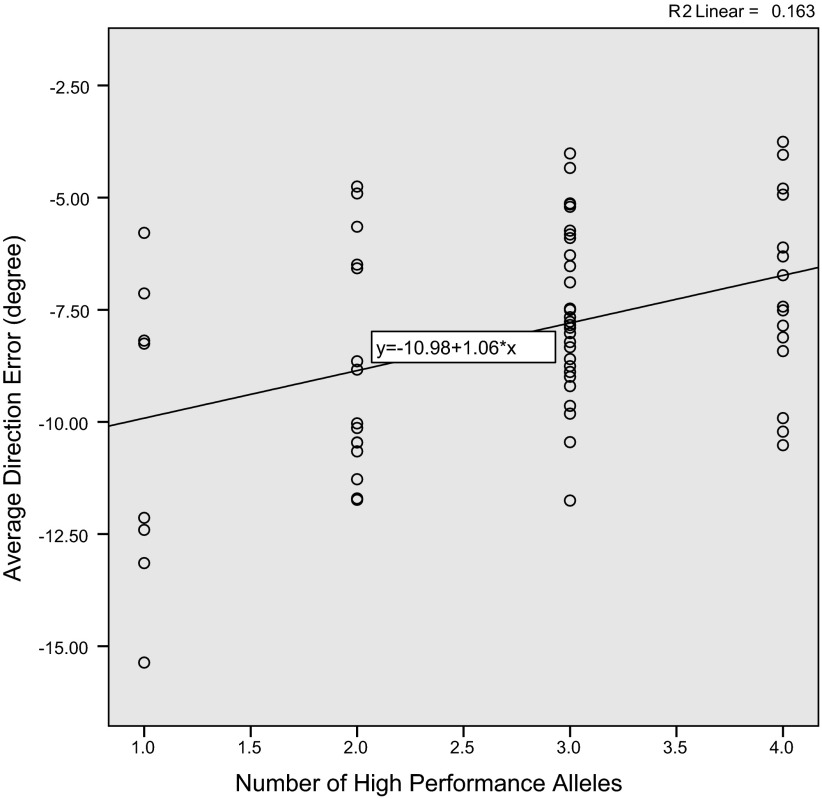
A significant correlation between the additive effect of high-performance alleles and average DE in the adaptation phase was found using a nonparametric linear regression (R = 0.40, F1,65 = 12.62, P = 0.001; Fig. 6). In contrast, no correlation was found in the baseline or washout period. Further, a nonparametric mixed model ANOVA for the effect of NHPA on visuomotor adaptation showed a main effect of block (F9 = 102,26, P = 0.0001), and a main effect of NHPA (F3 = 17.55, P = 0.0001), but no NHPA by block interaction (F27 = 0.61, P = 0.94; Fig. 7A). The post hoc Mann-Whitney U-test on average DE across adaptation blocks revealed that carriers of one high-performance allele showed poorer adaptation (larger DE) compared with carriers of four (U = 27, P = 0.03) and three (U = 62, P = 0.04) high-performance alleles. Also, carriers of two high-performance alleles showed a trend for poorer adaptation compared with carriers of four high-performance alleles (U = 66, P = 0.054). There was no significant difference between the sizes of decay constants corresponding to NHPA (Fig. 7B).
Fig. 6.
There was a significant correlation between the adaptation rate and number of high-performance alleles (P = 0.001).
Fig. 7.
A and B: additive effect of number of high-performance alleles on the adaptation rate. A: carriers of 1 high-performance allele showed poorer adaptation (larger DE) compared with carriers of 4 (P = 0.03) and 3 (P = 0.04) high-performance alleles. Also, carriers of 2 high-performance alleles showed a trend for poorer adaptation compared with carriers of 4 high-performance alleles (P = 0.054). B: there was no significant difference among the carriers of 1, 2, 3, or 4 high-performance alleles in the rate of exponential decay across adaptation trials, or the size of decay constant.
In sum, these findings reveal that COMT and DRD2 genotypes are associated with differences in visuomotor adaptation.
Visuospatial Working Memory Capacity
Working memory tests_ COMT.
A nonparametric one-way ANOVA revealed no main effect of COMT on VAC score (F2,63 = 0.04, P = 0.95) or Card Rotation score (F2,63 = 0.70, P = 0.49).
Working memory tests_ DRD2.
A nonparametric one-way ANOVA revealed no main effect of DRD2 on VAC score (F2,65 = 0.25, P = 0.77) or Card Rotation score (F2,65 = 0.59, P = 0.55).
Working memory tests_ high-performance alleles.
A nonparametric linear regression for the correlation between the working memory capacity and NHPA revealed no significant correlation for VAC score (R = 0.06, F1,64 = 0.27, P = 0.60) or Card Rotation score (R = 0.002, F1,65 = 0.0001, P = 0.99). Also, a nonparametric one-way ANOVA showed no main effect of NHPA on VAC score (F3,62 = 0.36, P = 0.78) and Card Rotation score (F3,63 = 0.18, P = 0.90).
In summary, we found no effects of COMT and DRD2 genotypes on working memory, suggesting that genotype associations with motor learning are not driven by reliance on working memory.
The summary of the results for sequence learning, visuomotor adaptation, and working memory tasks is shown in Table 3, representing the main effects of COMT and DRD2 genotypes and NHPA factor in each task separately.
Table 3.
Summary of the results
| Phenotype/Genotype | COMT val158met | DRD2 G>T | NHPA |
|---|---|---|---|
| Reaction time | F2 = 16.3, P = 0.0001 | F2 = 6.83, P = 0.001 | F3 = 5.70, P = 0.001 |
| Accuracy | F2 = 4.44, P = 0.01 | F2 = 21.08, P = 0.0001 | F3 = 8.07, P = 0.0001 |
| Learning extent (early, middle, late) | F2 = 4.23, P = 0.01 (middle) | — | — |
| Direction error | F2 = 28.10, P = 0.0001 | F2 = 7.04, P = 0.001 | F3 = 17.55, P = 0.0001 |
| Working memory capacity | — | — | — |
The main effects of COMT val158met and DRD2 G>T genotypes and number of postulated “high-performance alleles” (NHPA) factor on the selected motor and cognitive phenotypes. The results of post hoc Mann Whitney U-tests are not shown in this table.
DISCUSSION
In the current study we hypothesized that COMT val158met (re4680) and DRD2 G>T (rs 1076560) genetic polymorphisms would be associated with interindividual variability in motor learning. We also hypothesized that visuospatial working memory capacity would contribute to this genotype effect in both motor sequence learning and visuomotor adaptation tasks. We found that performance, but not sequence-specific learning in the sequence learning task, is associated with common variations in both the COMT and DRD2 genes. We also found evidence supporting a COMT genotype association with the size of direction errors in the visuomotor adaptation task, indicating a poorer adaptation of the val-val group. We found no difference between DRD2 genotype groups in performance of the visuomotor adaptation task. Moreover, our results do not support the contribution of visuospatial working memory capacity to any of these genotype effects in the sequence learning and visuomotor adaptation tasks.
Our findings on genotype-phenotype associations may bring up the question of whether there is any potential compensation for genetic differences in a transmitter system. There are several factors that need to be taken into account when evaluating any correlations in a complex system. Here, we only looked at dopaminergic signaling and its influence on motor learning and adaptation. However, a more comprehensive study is needed to explore possible compensations of other neuronal systems, neurotransmitters, and neurochemicals that have impacts on learning and memory. For instance, carriers of COMT val and DRD2 T alleles (associated with lower dopamine availability) could potentially benefit from a compensatory mechanism through cholinergic system or estrogen signaling (Patterson et al. 2011; Huang et al. 1999). Moreover, an individual's accumulated lifetime experiences and long-term training of specific motor and cognitive tasks could also overcome genotype effects (McHughen et al. 2011).
Sequence Learning
In general, we found a main effect of genotype on the average reaction time in the sequence learning task, which suggests that motor control may be affected by an individual's genotype. We found no interaction between the individuals' genotype group and performance in different blocks (where the block's structure serves as a marker of learning that occurs only in sequence blocks) and therefore, we propose that this genotype effect may be restricted to performance benefits and not sequence learning.
In line with some previous studies (Nagel et al. 2008; Frank et al. 2009; Dumontheil et al. 2011), the effect of COMT genotype with better performance (faster response with no death of accuracy) is associated with met-met homozygosity. Although, in our study, the association of met-met homozygotes with fewer errors across blocks was not significant, the direction of the effect indicates that reaction time benefits are unlikely to reflect a speed-accuracy trade-off.
For the DRD2 genotype, the TT group's small sample size (3 subjects) provides low statistical power for interpretation of additive effects. Our results showed impaired performance of those with the TT genotype on overall reaction time but no significant genotype association with the extent of learning in any learning phase. The results suggest that the G-allele carriers (heterozygotes and homozygotes) may demonstrate an overall performance benefit (and not a learning benefit) compared with the TT homozygote group. The DRD2 T allele has been linked to decreased expression of striatal D2 receptors, which results in less efficient cognitive and motor processing (Bertolino et al. 2010). Neuroimaging studies have shown greater fMRI BOLD responses in the striatum, premotor and motor cortex of T-allele carriers performing a simple motor task, which suggests reduced neural efficiency (Zhang et al. 2007; Fazio et al. 2011). These studies compared the homozygote GG group with the heterozygote GT group (due to the lack of subjects who are homozygous for the T allele) and suggest poorer performance for the GT group. In line with those findings, we found that the GG group made fewer errors across blocks than the GT group.
Our results for the sequence learning task demonstrate that both COMT and DRD2 alleles may underlie motor performance but not motor learning.
Visuomotor Adaptation
Sensorimotor adaptation varied only with COMT genotype and not with DRD2. We found that the COMT val-val homozygote group exhibited greater direction error (poorer adaptation) across the adaptation trials than both met-met and val-met groups, indicating a learning deficit for the val-val group. Finding no genotype effects in pretest performance, while finding a significant genotype effect in the adaptation phase, supports an association between COMT genotype and sensorimotor adapation Doyon and Benali (2005) reviewed the neural correlates of motor sequence learning and visuomotor adaptation. They proposed that there are overlapping neural substrates of sequence learning and sensorimotor adaptation during early learning, including basal ganglia, and cerebellum and prefrontal, premotor, and parietal cortexes, with activation shifting to reduced and more specialized regions later in learning. Early learning engagement of the striatum and prefrontal cortex in sensorimotor adaptation (Seidler et al. 2006; Anguera et al. 2007) fits well with the COMT allelic effects that we observed. There are several explanations for why we did not observe DRD2 effects on adaptation. This may be because the DRD2 genotype is thought to influence subcortically mediated behaviors, while COMT has been more frequently linked to prefrontally mediated tasks (Wiener et al. 2011). Several studies have reported prefrontal cortical involvement during sensorimotor adaptation (Anguera et al. 2010, 2011), whereas very few report striatal activation with adaptation (Seidler et al. 2006). Sensorimotor adaptation is more frequently linked to cortico-cerebellar than cortico-striatal pathways (Doyon and Benali 2005).
Previous studies have identified the engagement of premotor, prefrontal, temporal, and parietal cortical regions, as well as the cerebellum and bilateral basal ganglia in visuomotor adaptation (Clower et al. 1996; Ghilardi et al. 2000; Imamizu et al. 2000; Krakauer et al. 2004; Seidler et al. 2006). Our failure to observe a DRD2 genotype association with the rate of visuomotor adaptation can potentially be explained by the duration and difficulty of our design; we only assessed learning in a single session. A previous study reported an association between BDNF genotype and visuomotor adaptation but only at retention (after 24 h) and not during the initial adaptation (Joundi et al. 2012). These authors discussed the important role of duration and difficulty of the task in the magnitude of genetic effects; however, their gene of interest was not DRD2. They suggested that the size of the effect was associated with the size of the visuomotor distortion, as significantly larger direction errors are found for an 80° perturbation than for 60°. We used a 30° rotation in the current study, which could further point towards a role of task difficulty as a moderating effect of the genotype impact on visuomotor adaptation.
Studies in Patients with Parkinson's Disease
Parkinson's disease (PD) studies have provided invaluable information for evaluating the effect of dopamine activity on motor sequence learning and visuomotor adaptation tasks (Cools et al. 2001). Some studies have demonstrated impaired implicit sequence learning in patients with PD (Doyon et al. 1997; Shin and Ivry 2003), while others showed that this impairment is not evident in the early stages of PD (Muslimović et al. 2007). We have shown previously that PD patients who are carriers for the low-performance alleles (DRD2 T) show higher levels of early sequence learning improvement with medication than carriers of high-performance alleles (DRD2 G) who initially have higher striatal dopamine availability (Kwak et al. 2013). We speculate that the absence of association between sequence learning and DRD2 genotype in the current study is due to redundant and compensatory systems in healthy young adults.
As for the sensorimotor adaptation task, the results of PD studies suggest a compensatory mechanism of the cerebellum (due to striatal impairments) and a role for dopaminergic transmission less in early adaptation and more in the retention phase (Marinelli et al. 2009; Kapogiannis et al. 2011; Leow et al. 2012).
Working Memory Capacity
We have shown previously that visuospatial working memory capacity is associated with the rate of both sequence learning (Bo and Seidler 2009) and sensorimotor adaptation (Anguera et al. 2009, 2010). Given that dopaminergic pathways underlie working memory (Cools et al. 2007, 2008; Moustafa et al. 2008; de Frias et al. 2010; Cools and D'Esposito 2011), we measured genotype effects on working memory capacity to determine whether this might be the route by which genotypes for genes involved in dopaminergic transmission impact motor learning. However, we did not find evidence supporting a genetic association with working memory capacity. Also, we found no correlation between the working memory capacity and sensorimotor learning, in the two variant loci measured. Our findings suggest that the effect of dopaminergic genotypes on sequence motor learning and visuomotor adaptation is not through their effect on working memory capacity. Previous studies have also reported mixed results on genotype association with working memory. Many studies have failed to find such an effect (Tsai et al. 2003; Bilder et al. 2004; Ho et al. 2005;), while others reported a reliable COMT effect on working memory and executive function in older adults but not in young adults (Nagel et al. 2008, Harris et al. 2005; de Frias et al. 2004, 2005). These studies suggest that there is an important role of age in unmasking genotype effects. That is, there may be either ceiling effects or intact compensatory pathways that are present in young but not older adults (Nagel et al. 2008).
Additive Effects of High-Performance Alleles at Two Genes
Although we found a main effect of the NHPA on overall reaction time in the sequence learning task, the learning extent in all three learning phases was not associated with the NHPA factor. Those individuals who were carriers of only one high-performance allele showed slower response times (compared to carriers of 4 high-performance alleles), and higher rates of errors (compared to carriers of 4, 3, or 2 high-performance alleles) in performing the sequence learning task. These results suggest that higher dopamine levels may be associated with performance but not learning in the motor sequence learning task.
In the visuomotor adaptation task, we found a strong correlation between the rate of adaptation (indicated by the size of DE) and NHPA, suggesting a more facilitated motor adaptation for those individuals who are carriers of three or four HP alleles compared with carriers of just one or two HP alleles. The fact that we found this correlation between the rate of adaptation and NHPA, while we did not find any correlation between learning extent in the motor sequence learning task with NHPA provides support for the hypothesis of task specific genotype effects. Taking the underling neural substrates of each task into consideration, it has been suggested that motor adaptation is more dependent on cerebellar, parietal, and prefrontal circuits, with COMT variation having its effects predominantly on the latter.
Limitations
As expected based on population distributions, we observed unequal numbers of individuals in groups assigned by genotype. However, we found a dissociation in which we detected associations between genotype and performance but not between genotype and learning (for the sequence task), thus bolstering the notion that we had a sufficient sample size for detecting effects. Nevertheless, future studies could benefit from larger sample sizes that provide even higher statistical power to detect genotype-phenotype associations. Additionally, we employed gender and ethnicity exclusion criteria for our participants based on previous literature suggesting interactions between these factors and genotype effects, which prevents us from generalizing our results to other ethnicities and healthy young males. Another caveat of this study was the definition of three learning phases in the sequence-learning task, which was relative to the duration of our task (30–40 min of total practice time). It is important to consider that our definition of learning phases is task specific and cannot be applied to different motor learning paradigms.
Conclusion
In summary, our results support a role of genetic polymorphisms of COMT val158met and DRD2 G>T in explaining interindividual differences in motor performance and a role of COMT in association with sensorimotor adaptation. Our findings were task specific and were not correlated with an individual's working memory capacity.
GRANTS
This work was supported by a pilot grant (to R. D. Seidler) from the University of Michigan National Institutes of Health Claude D. Pepper Older Americans Independence Center Grant AG-024824.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.N., N.B.B., J.H., and D.T.B. analyzed data; F.N., D.T.B., M.L.M., N.I.B., and R.D.S. interpreted results of experiments; F.N. prepared figures; F.N. and R.D.S. drafted manuscript; F.N., Y.K., D.T.B., M.L.M., N.I.B., and R.D.S. edited and revised manuscript; F.N., N.B.B., Y.K., J.H., D.T.B., M.L.M., N.I.B., and R.D.S. approved final version of manuscript; N.B.B., Y.K., and J.H. performed experiments; Y.K., D.T.B., M.L.M., N.I.B., and R.D.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Joshua West for assistance in genotype analysis.
REFERENCES
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22: 1917–1930, 2010 [DOI] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci 23: 11–25, 2011 [DOI] [PubMed] [Google Scholar]
- Anguera JA, Russell CA, Noll DC, Seidler RD. Neural correlates associated with intermanual transfer of sensorimotor adaptation. Brain Res 1185: 136–151, 2007 [DOI] [PubMed] [Google Scholar]
- Anguera JA, Seidler RD, Gehring WJ. Changes in performance monitoring during sensorimotor adaptation. J Neurophysiol 102: 1868–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual–motor associations. Neuroimage 47: 1701–1710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, Di Giorgio A, Taurisano P, Papp A, Pinsonneault J, Wang D, Nardini M, Popolizio T, Sadee W. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain 132: 417–425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Taurisano P, Pisciotta NM, Blasi G, Fazio L, Romano R, Gelao B, Bianco Lo L, Lozupone M, Di Giorgio A, Caforio G, Sambataro F, Niccoli-Asabella A, Papp A, Ursini G, Sinibaldi L, Popolizio T, Sadee W, Rubini G. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS One 5: e9348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-o-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis, and neuropsychiatric phenotypes. Neuropsychopharmacology 29, 1943–1961.2004 [DOI] [PubMed] [Google Scholar]
- Bo J, Seidler RD. Visuospatial working memory capacity predicts the organization of acquired explicit motor sequences. J Neurophysiol 101: 3116–3125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Buch ER. Effects of Parkinson's disease on visuomotor adaptation. Exp Brain Res 150: 25–32, 2003 [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: 1136–1143, 2001 [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69: e113–e125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28: 1208–1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27: 5506–5514, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Hutchison KE, Frank MJ. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J Neurosci 31: 6188–6198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15: 161–167, 2005 [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Castonguay M, Bedard PJ, Bédard F, Bouchard JP, Bouchard JP. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn 34: 218–245, 1997 [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41: 252–262, 2003 [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol 21: 478–483, 2008 [DOI] [PubMed] [Google Scholar]
- Duara R, Barker WW, Lopez-Alberola R, Loewenstein DA, Grau LB, Gilchrist D, Sevush S, George-Hyslop PH. Alzheimer's disease Interaction of apolipoprotein E genotype, family history of dementia, gender, education, ethnicity, and age of onset. Neurology 46: 1575–1579, 1996 [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Influence of the COMT genotype on working memory and brain activity changes during development. Biol Psychiatry 70: 222–229, 2011 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci 2: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, Van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA 278: 1349–1356, 1997 [PubMed] [Google Scholar]
- Fazio L, Blasi G, Taurisano P, Papazacharias A, Romano R, Gelao B, Ursini G, Quarto T, Lo Bianco L, Di Giorgio A, Mancini M, Popolizio T, Rubini G, Bertolino A. D2 receptor genotype and striatal dopamine signaling predict motor cortical activity and behavior in humans. Neuroimage 54: 2915–2921, 2011 [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SGJ, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA. Planning ability in Parkinson's disease is influenced by the COMT val158met polymorphism. Mov Disord 19: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci 12: 1062–1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frias CM, Marklund P, Eriksson E, Larsson A, Öman L, Annerbrink K, Bäckman L, Nilsson LG, Nyberg L. Influence of COMT gene polymorphism on fMRI-assessed sustained and transient activity during a working memory task. J Cogn Neurosci 22: 1614–1622, 2010 [DOI] [PubMed] [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet 34: 533–539, 2004 [DOI] [PubMed] [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J Cogn Neurosci 17: 1018–1025, 2005 [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F. Visual feedback has differential effects on reaching movements in Parkinson's and Alzheimer's disease. Brain Res 876: 112–123, 2000 [DOI] [PubMed] [Google Scholar]
- Harris SE, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The functional COMT polymorphism, Val 158 Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci Lett 385: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol–methyl-transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology, and frontal cerebral blood flow. Mol Psychiatry 10: 229, 287–298 2005 [DOI] [PubMed] [Google Scholar]
- Huang CS, Chern HD, Chang KJ, Cheng CW, Hsu SM, Shen CY. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17 CYP1A1, and COMT: a multigenic study on cancer susceptibility. Cancer Res 59: 4870–4875, 1999 [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci USA 100: 5461–5466, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195, 2000 [DOI] [PubMed] [Google Scholar]
- Joundi RA, Lopez-Alonso V, Lago A, Brittain JS, Fernandez-del-Olmo M, Gomez-Garre P, Mir P, Jenkinson N, Cheeran B, Brown P. The effect of BDNF val66met polymorphism on visuomotor adaptation. Exp Brain Res 223: 43–50, 2012 [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci 12: 231–242, 2011 [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mooshagian E, Campion P, Grafman J, Zimmermann TJ, Ladt KC, Wassermann EM. Reward processing abnormalities in Parkinson's disease. Mov Disord 26: 1451–1457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol 91: 924–933, 2004 [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. In: Progress in Motor Control. New York: Springer, 2009, p. 405–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer UM, Cunillera T, Càmara E, Marco-Pallarés J, Cucurell D, Nager W, Bauer P, Schüle R, Schöls L, Rodriguez-Fornells A, Münte TF. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci 27: 14190–14198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Bohnen NI, Müller M, Dayalu P, Burke DT, Seidler RD. Task-dependent interactions between Dopamine D2 receptor polymorphisms and l-DOPA in patients with Parkinson's disease. Behav Brain Res 245: 128–136, 2013 [DOI] [PubMed] [Google Scholar]
- Kwak Y, Müller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. J Neurophysiol 103: 942–949, 2010 [DOI] [PubMed] [Google Scholar]
- Leow LA, Loftus AM, Hammond GR. Impaired savings despite intact initial learning of motor adaptation in Parkinson's disease. Exp Brain Res 218: 295–304, 2012 [DOI] [PubMed] [Google Scholar]
- Leow LA, De Rugy A, Loftus AM, Hammond G. Different mechanisms contributing to savings and anterograde interference are impaired in Parkinson's disease. Front Hum Neurosci 7: 55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Hum Neurosci 2: 234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 159: 652–654, 2002 [DOI] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Parkinsonism Relat Disord 15: 6–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Exp Brain Res 213: 415–422, 2011 [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Cohen MX, Sherman SJ, Frank MJ. A role for dopamine in temporal decision making and reward maximization in parkinsonism. J Neurosci 28: 12294–12304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimović D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson's disease. Brain 130: 2887–2897, 2007 [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, Von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2: 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet F, Bedard MA, Levesque M, Tremblay PL, Lemay M, Blanchet PJ, Scherzer P, Chouinard S, Filion J. Sensorimotor adaptation in Parkinson's disease: evidence for a dopamine dependent remapping disturbance. Exp Brain Res 185: 227–236, 2008 [DOI] [PubMed] [Google Scholar]
- Patterson CE, Todd SA, Passmore AP. Effect of apolipoprotein E and butyrylcholinesterase genotypes on cognitive response to cholinesterase inhibitor treatment at different stages of Alzheimer's disease. Pharmacogenomics J 11: 444–450, 2011 [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Bäckman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage 50: 1303–1312, 2010 [DOI] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, Hagler DJ, Lyons MJ, Neale MC, Pacheco J, Perry ME, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Kremen WS, Dale AM. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry 67: 493–499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res 175: 544–555, 2006 [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson's disease or cerebellar lesions. J Cogn Neurosci 15: 1232–1243, 2003 [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Yu YW, Chen TJ, Chen JY, Liou YJ, Chen MC, Hong CJ. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett 338: 123–126, 2003 [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature 428: 748–751, 2004 [DOI] [PubMed] [Google Scholar]
- Wiener M, Lohoff FW, Coslett HB. Double dissociation of dopamine genes and timing in humans. J Cogn Neurosci 23: 2811–2821, 2011 [DOI] [PubMed] [Google Scholar]
- Xu H, Kellendonk CB, Simpson EH, Keilp JG, Bruder GE, Polan HJ, Kandel ER, Gilliam TC. DRD2 C957T polymorphism interacts with the COMT Val158Met polymorphism in human working memory ability. Schizophr Res 90: 104–107, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA 104: 20552–20557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]