Abstract
Rhythmically bursting olfactory bulb external tufted (ET) cells are thought to play a key role in synchronizing glomerular network activity to respiratory-driven sensory input. Whereas spontaneous bursting in these cells is intrinsically generated by interplay of several voltage-dependent currents, bursting strength and frequency can be modified by local intrinsic and centrifugal synaptic input. Activation of metabotropic glutamate receptors (mGluRs) engages a calcium-dependent cation current (ICAN) that increases rhythmic bursting, but mGluRs may also modulate intrinsic mechanisms involved in bursting. Here, we used patch-clamp electrophysiology in rat olfactory bulb slices to investigate whether mGluRs modulate two key intrinsic currents involved in ET cell burst initiation: persistent sodium (INaP) and hyperpolarization-activated cation (Ih) currents. Using a BAPTA-based internal solution to block ICAN, we found that the mGluR1/5 agonist DHPG enhanced INaP but did not alter Ih. INaP enhancement consisted of increased current at membrane potentials between −60 and −50 mV and a hyperpolarizing shift in activation threshold. Both effects would be predicted to shorten the interburst interval. In agreement, DHPG modestly depolarized (∼3.5 mV) ET cells and increased burst frequency without effect on other major burst parameters. This increase was inversely proportional to the basal burst rate such that slower ET cells exhibited the largest increases. This may enable ET cells with slow intrinsic burst rates to pace with faster sniff rates. Taken with other findings, these results indicate that multiple neurotransmitter mechanisms are engaged to fine-tune rhythmic ET cell bursting to context- and state-dependent changes in sniffing frequency.
Keywords: olfaction, glomerulus, bursting, electrophysiology, rat
in the olfactory bulb, glutamatergic external tufted (ET) cells play important functional roles in the intra- and interglomerular networks. ET cells rhythmically burst from approximately 0.5 to 10 Hz, matching the theta frequency range characteristic of rodent sniffing rates (Hayar et al. 2004a; Liu and Shipley 2008b; Wachowiak and Shipley 2006). ET cell bursts entrain olfactory nerve input and in turn provide monosynaptic dendrodendritic input to periglomerular and short axon cell inhibitory interneurons, most of which are themselves devoid of direct olfactory nerve input (Hayar et al. 2004b; Shao et al. 2009). Activation of periglomerular cells by ET cell bursts provides a major source of feedback and feedforward inhibition that shapes the temporal structure of intraglomerular activity with rhythmic respiration (Shao et al. 2009, 2012; Wachowiak and Shipley 2006). ET cell input to short axon cells, by contrast, drives an interglomerular inhibitory circuit (Aungst et al. 2003; Liu et al. 2013; Whitesell et al. 2013). Dendritic glutamate release from ET cells is thought to generate long-lasting depolarizations that amplify olfactory nerve input onto, and synchronize spike output among, mitral/tufted cells associated with the same glomerulus (Carlson et al. 2000; De Saint Jan et al. 2009; Gire and Schoppa 2009; Hayar et al. 2005; Najac et al. 2011; Shao et al. 2012).
The bursting properties of ET cells thus reinforce or strengthen the rhythmic nature of olfactory input onto the glomerular network. ET cell bursting arises spontaneously and is dependent on a number of intrinsic voltage-dependent channels. Thus ET cell burst generation and frequency are unperturbed when ionotropic glutamate and GABA receptors are blocked (Hayar and Ennis 2007; Hayar et al. 2004a; Liu and Shipley 2008b). However, ET cell bursting can be potently regulated by several classes of modulatory neurotransmitter receptors. Serotonin, for example, increases ET cell burst frequency by enhancing a nonselective cation current in these cells (Liu et al. 2012). More recently, dopamine was found to increase ET cell burst frequency by positive modulation of the hyperpolarization-activated cation current (Ih) that plays a key role in intrinsic bursting (Liu et al. 2013).
ET cells express several modulatory metabotropic glutamate receptor (mGluR) subtypes, in particular, high levels of group I mGluRs (i.e., mGluR1 and mGluR5; Heinbockel et al. 2004; Romano et al. 1995; Sahara et al. 2001; Shigemoto et al. 1992). These receptors can be engaged directly by olfactory nerve input and also by glutamate spillover from the apical dendrites of ET and mitral/tufted cells (De Saint Jan and Westbrook 2007; Dong et al. 2009; Ennis et al. 2006; Johnston and Delaney 2010; Schoppa and Westbrook 2001; Yuan and Knöpfel 2006). mGluR1/5 activation triggers a calcium-dependent nonselective cation current (ICAN) that robustly increases both the strength and frequency of ET cell bursting. However, mGluR-evoked effects on bursting were incompletely reduced by nonselective cation channel antagonists. This suggests that mGluR activation may enhance ET cell excitability by modulation of intrinsic currents involved in burst initiation. The goal of this study therefore was to investigate whether mGluRs modulate intrinsic ET cell currents involved in spontaneous burst initiation using patch-clamp electrophysiology in rat olfactory bulb slices.
MATERIALS AND METHODS
Slice preparation.
Sprague-Dawley rats (18–30 days old of either sex) were decapitated in accordance with an approved Institutional Animal Care and Use Committee protocol and National Institutes of Health guidelines. The olfactory bulbs were removed as previously described (Dong et al. 2009) and immersed in oxygenated sucrose artificial cerebrospinal fluid (ACSF) composed of (in mM): 62 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 3 KCl, 4 MgSO4, 0.1 CaCl2, 15 glucose, and 120 sucrose; pH 7.3, 310 mOsm. Horizontal slices were cut with a Vibratome 3000 (Vibratome, St. Louis, MO) at thickness of 400 μm. After recovery at 33°C for 20 min, slices were then incubated until used at room temperature (22°C) in normal ACSF equilibrated with carbogen (95% O2-5% CO2) and composed of (in mM): 126 NaCl, 26 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, and 20 glucose; pH 7.3, 310 mOsm. For voltage-clamp experiments, cadmium and nickel were added to the ACSF, and NaH2PO4 was excluded. For recording, a single slice was placed in a recording chamber and continuously perfused with carbogen-saturated ACSF at the rate of 1.5 ml/min.
Electrophysiology.
All recordings were performed at 30°C. Neurons were visualized using an upright microscope (BX50WI; Olympus, Tokyo, Japan) equipped with epifluorescence and near-infrared differential interference contrast optics. Whole cell voltage-clamp recordings were made with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). The pipette resistance was 6–8 MΩ. Only neurons with access resistance <30 MΩ were included in this study. Unless otherwise noted, voltage-clamp recordings from ET cells were made with pipettes containing (in mM): 125 cesium methanesulfonate (CsMeSO3), 1 NaCl, 10 phosphocreatine di(tris) salt, 3 MgATP, 0.3 Na2GTP, 0.5 EGTA, 10 HEPES; pH 7.3, 290 mOsm. The intracellular solution for current-clamp recording was composed of (in mM): 124 potassium gluconate, 1 NaCl, 10 phosphocreatine di(tris) salt, 3 MgATP, 0.3 Na2GTP, 0.5 EGTA, and 10 HEPES; pH 7.3, 290 mOsm. Lucifer yellow (0.02%) was added to the intracellular solution in all experiments for in situ labeling.
Analog signals were low-pass filtered at 2 kHz (MultiClamp 700B) and digitized at 5 kHz using a Digidata 1440A interface and pClamp 10 software (Molecular Devices). Current-voltage (I–V) relationships of persistent sodium currents (INaP) were studied with a voltage-ramp protocol (−80 to −40 mV, 26 mV/s, 1.5-s duration) from a holding potential (HP) of −60 mV with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM), dl-2-amino-5-phosphonovaleric acid (APV; 50 μM), gabazine (SR-95531; 10 μM), tetraethylammonium chloride (TEA; 10 mM), cadmium (200 μM), and nickel (1 mM) in the ACSF. The ICAN elicited by 3,4-dihydroxyphenylglycine (DHPG) requires internal store calcium release and persists in the presence of the voltage-gated calcium channel blockers cadmium and nickel (Dong et al. 2009). In some experiments, I–V curves for INaP were converted to conductance according to the formula g = I/(Vt − Vr), where Vt = membrane test potential and Vr = the estimated reversal potential for sodium ions. To estimate the voltage at which half of the current was activated (Va), the curves of conductance vs. voltage (g–V) were fitted with the Boltzmann equation: 1/{1 + exp[(Va − Vt)/k]}. Membrane resistance was also calculated from currents elicited by negative voltage pulses (−20 mV, 100-ms duration) from a −60-mV HP. In current-clamp, ET cell resting or minimum membrane potential and the depolarizing envelope amplitude and duration were defined and measured as previously described (Dong et al. 2009; Liu and Shipley 2008b). Spike detection was performed offline using Mini Analysis software (Synaptosoft, Decatur, GA), and custom-designed algorithms were used to measure parameters of ET cell spike bursts as previously described (Dong et al. 2009): firing frequency, burst frequency (defined as the number of bursts per second), spikes per burst, and burst duration (defined as the time interval between the 1st and the last spike in a burst). A spike burst was defined as a series of 2 or more consecutive spikes that had interspike time intervals of <75 ms as previously reported (Hayar et al. 2004a). Group data I–V curves were plotted with a sigmoidal function (Origin 8.5; MicroCal, Northampton, MA). Data, expressed as means ± SE, were statistically analyzed using ANOVA followed by Newman-Keuls post hoc comparisons and with t-tests.
ET cells were identified by their characteristic electrophysiological and morphological characteristics (Hayar and Ennis 2007; Hayar et al. 2004a,b, 2005; Liu and Shipley 2008b). Electrophysiologically, they exhibited spontaneous rhythmic bursting in the cell-attached or current-clamp recordings. Rhythmic bursting was typically confirmed in extracellular recordings before switching to whole cell voltage-clamp mode. Additionally, ET cells exhibited randomly occurring (i.e., nonbursting) spontaneous excitatory postsynaptic currents in voltage-clamp recordings (HP = −60 mV; Hayar et al. 2004a,b, 2005). Morphologically, all recorded ET cells were characterized by soma location within the glomerular layer and by a primary dendrite with a tuftlike arborization that ramifies within a single glomerulus. They also lacked secondary dendrites. Cells that lacked these characteristics were discarded.
Drugs and solutions.
In most experiments, drugs and solutions were applied to the perfusion solution with a three-way valve system. Recording media, gabazine, CNQX, APV, TEA, cadmium chloride, nickel(II) chloride hexahydrate, and BAPTA were obtained from Sigma-Aldrich (St. Louis, MO). DHPG, L-CCG-I, LY 367385, 2-methyl-6-(phenylethynyl)-pyridine (MPEP), l-2-amino-4-phosphonobutyric acid (AP4), riluzole, and TTX were purchased from Tocris Bioscience (Ellisville, MO).
RESULTS
Activation of group I mGluRs enhance INaP.
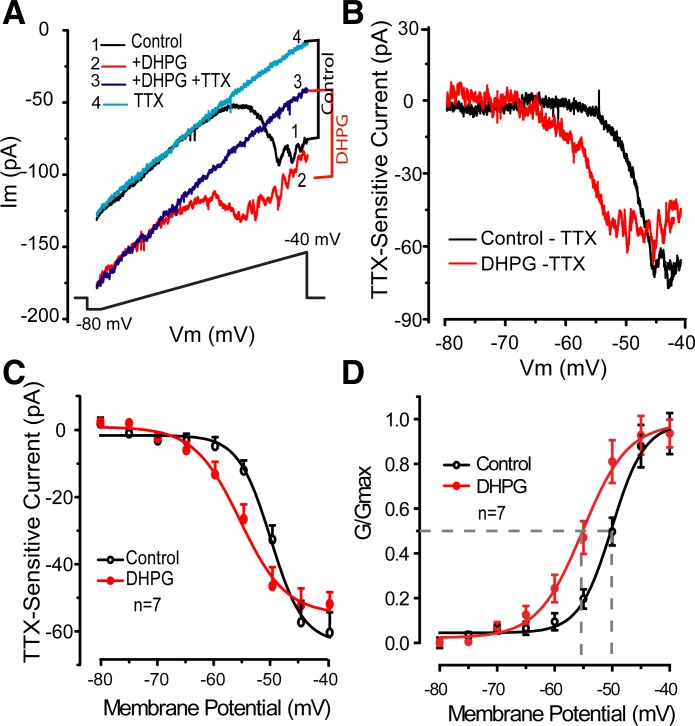
Data are reported from voltage- or current-clamp recordings obtained from 54 ET cells in 51 slices from 31 rats. Previous findings indicate that activation of mGluR1/5 by DHPG elicits a robust ICAN at voltages corresponding to the resting or interburst membrane potential of ET cells (Dong et al. 2009). Block of this current reduced but did not abolish DHPG-evoked increases in ET cell bursting. We therefore investigated potential effects of DHPG on intrinsic ET cell membrane currents, focusing on currents that are essential to burst initiation and/or control burst frequency, namely INaP and Ih. Both are active at ET cell resting membrane potential (Hayar et al. 2004a; Liu and Shipley 2008b) and are therefore potential candidates for DHPG effects on bursting. To investigate whether DHPG modulates INaP, we applied a slow voltage-ramp (see materials and methods) in the presence of CNQX (10 μM), APV (50 μM), gabazine (10 μM), TEA (10 mM), cadmium (200 μM), and nickel (1 mM) in the ACSF to block ionotropic glutamate and GABA receptors and voltage-gated calcium and potassium channels. As previously reported (Hayar et al. 2004a; Liu and Shipley 2008b), slow depolarizing ramps elicited a TTX-sensitive inward current, INaP, developing at −65 to −60 mV and increasing with depolarization to approximately −40 mV (Fig. 1A). As shown in Fig. 1, DHPG (30 μM) elicited an inward current shift at all voltages and also appeared to increase INaP superimposed on this current. Application of TTX (1 μM) blocked INaP, leaving a residual linear current identical to the previously described TTX-insensitive ICAN evoked by DHPG (Dong et al. 2009). The ICAN was associated with a significant decrease in membrane resistance measured with negative voltage steps as previously reported (−50 ± 8.8 MΩ, n = 7; P = 0.002, paired t-test). Subtraction of the TTX-sensitive component (Fig. 1, B–D) revealed a significant impact of DHPG on INaP (n = 7; P < 0.05, 2-way ANOVA). Compared with control values, INaP amplitude was increased in the range of −65 to −50 mV with a peak increase of −15.6 ± 5.8 pA at −55 mV (P < 0.05, ANOVA followed by Newman-Keuls test; Fig. 1C). Boltzmann equation fits of g-V curves (Fig. 1D) showed that DHPG shifted the activation threshold of INaP to more negative voltages with half-maximal activation values of −50.2 ± 0.9 and −55.8 ± 1.3 mV in control and DHPG (n = 7; P = 0.001, paired t-test). Comparable results were obtained by determining significant increases in INaP from −80 mV, which identified values of −50 and −60 mV in control and DHPG (n = 7; P < 0.05, ANOVA followed by Newman-Keuls tests). At potentials positive to approximately −40 mV, the magnitude of INaP in the presence of DHPG was equivalent or slightly less than in control. Thus DHPG shifts the overall window of INaP toward hyperpolarizing potentials.
Fig. 1.
3,4-Dihydroxyphenylglycine (DHPG) elicits calcium-dependent cation current (ICAN) and enhances persistent sodium current (INaP). A: inset indicates order of drug application. Voltage-clamp recordings from an example external tufted (ET) cell in control (trace 1) show that a slow depolarizing voltage-ramp (shown at bottom) elicits a TTX-sensitive INaP that developed at approximately −60 mV. Im, membrane current. DHPG (30 μM, trace 2) elicited a linear TTX-insensitive current, ICAN, across all membrane potentials (Vm) and also increased the magnitude of INaP. Note that DHPG shifts the activation threshold of INaP toward more negative potentials. Subsequent addition of 1 μM TTX (trace 3) eliminated INaP but did not affect ICAN. B: current-voltage (I–V) profiles of INaP from data in A in control and DHPG calculated by subtracting the currents in each condition in the presence and absence of TTX. Note that DHPG produced a leftward shift to decrease the INaP activation threshold. C: group subtraction data as in B from 7 ET cells show INaP in control and DHPG conditions. D: group data conductance vs. voltage plot showing the leftward shift in INaP activation threshold; dotted lines denote half-maximal activation values in control and DHPG.
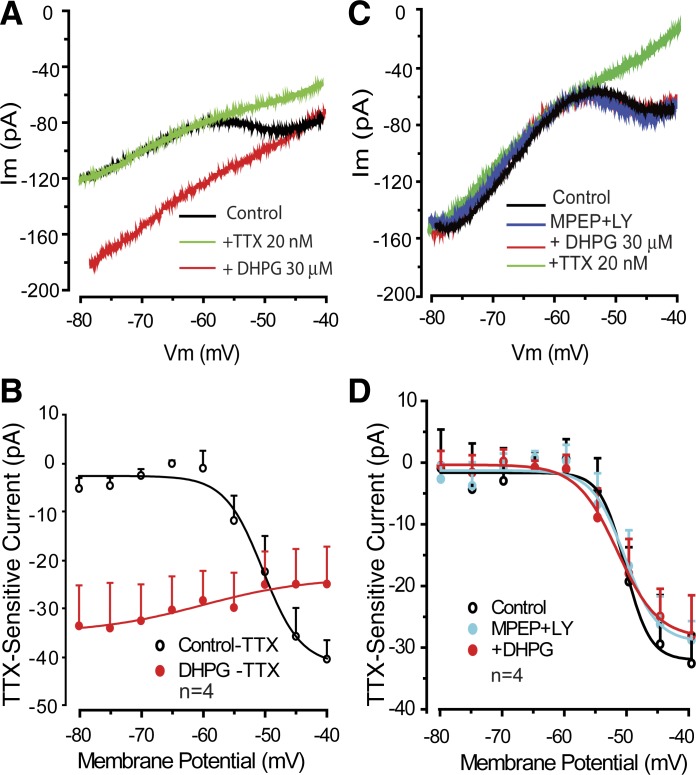
Low concentrations of TTX (e.g., 10–20 nM) and riluzole have been reported as effective blockers of sustained or persistent sodium currents, including INaP (Cramer et al. 2007; D'Ascenzo et al. 2009; Pace et al. 2007). TTX at 20 nM eliminated INaP (n = 8) and its enhancement by DHPG (n = 4) but did not alter ICAN (Fig. 2). Riluzole (30 μM) had similar effects (n = 2, data not shown). Group I mGluRs include mGluR1 and mGluR5. Both subtypes are expressed by neurons in the glomerular layer and by ET cells specifically (Dong et al. 2009; Fotuhi et al. 1993; Heinbockel et al. 2004; Romano et al. 1995; Sahara et al. 2001; Shigemoto et al. 1992; Van den Pol et al. 1995). Activation of ICAN and the enhancement of INaP were prevented when DHPG (30 μM) was applied in the presence of the mGluR1 and mGluR5 antagonists LY 367385 (100 μM) and MPEP (50 μM), respectively (Fig. 2, C and D, and Fig. 3). We did not attempt to determine the relative contribution of mGluR1 and mGluR5.
Fig. 2.
Low concentrations of TTX and group I metabotropic glutamate receptor (mGluR) antagonists block DHPG-evoked enhancement of INaP. A and B: individual cell (A) and group (B) data subtraction plot show that 20 nM TTX blocks INaP in control and the presence of DHPG but does not alter ICAN (red plot in B). C and D: in the presence of 2-methyl-6-(phenylethynyl)-pyridine (MPEP; 50 μM) and LY 367385 (LY; 100 μM), DHPG did not alter INaP or evoke ICAN.
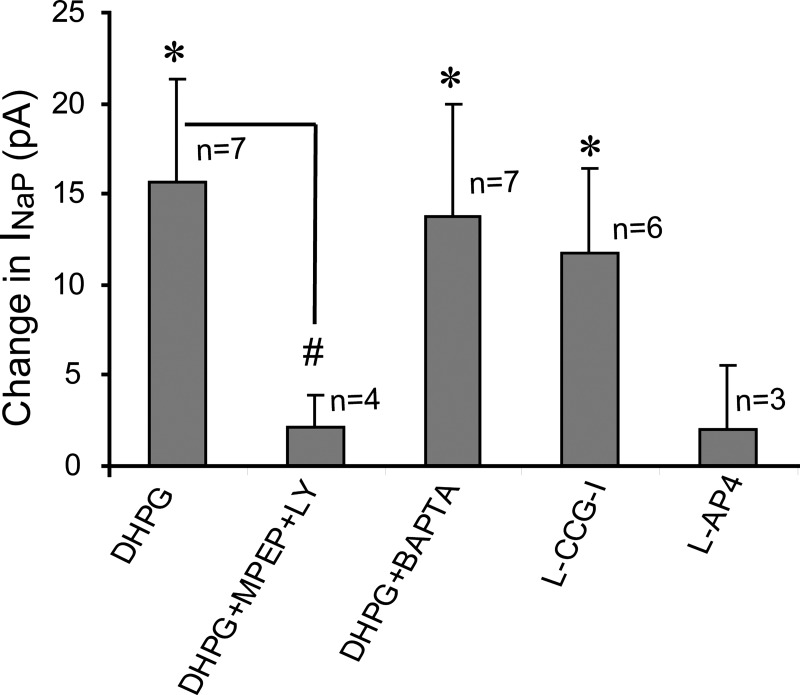
Fig. 3.
Bar graph comparing the magnitude of INaP at −55 mV in different experimental conditions. L-AP4, l-2-amino-4-phosphonobutyric acid. *P < 0.05 compared with respective control values, paired t-tests. #P < 0.05, unpaired t-test.
ET cells express group II and III mGluRs (Dong et al. 2009; Hayar and Ennis 2007), and we next investigated whether agonists for these mGluR classes alter ICAN or INaP. We found that the group II mGluR agonist L-CCG-I (10 μM) enhanced INaP in a manner similar to DHPG (n = 6; Fig. 3). Unlike DHPG, L-CCG-I did not evoke an ICAN, consistent with a lack of effect on ET cell input resistance measured with negative voltage steps (−9.3 ± 17.4 MΩ change, n = 6; P > 0.24, paired t-test). The group III mGluR agonist AP4 (10 μM) did not elicit ICAN, alter INaP (Fig. 3; n = 3; P = 0.45), or alter input resistance (−21.3 ± 46 MΩ change; P > 0.17).
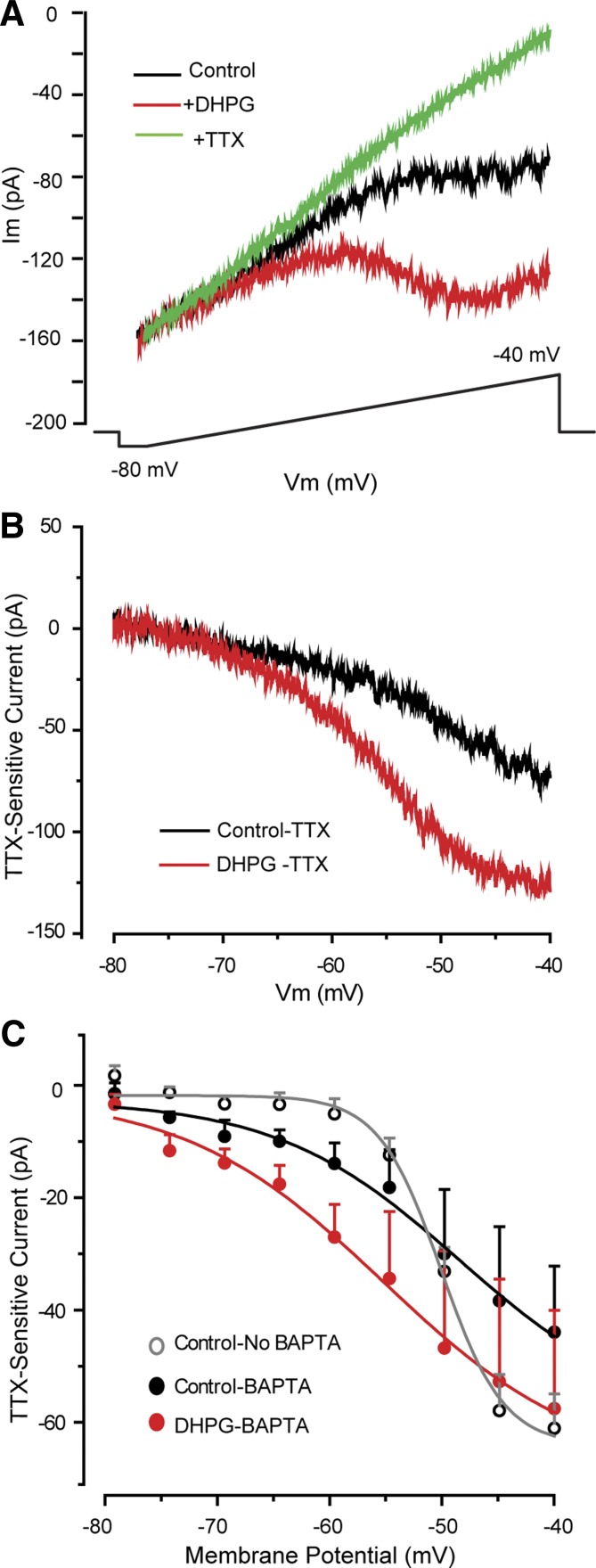
To investigate DHPG modulation of INaP further, recordings were made with BAPTA-based pipettes to block the DHPG-evoked ICAN in ET cells (Dong et al. 2009). BAPTA alone tended to increase slightly the basal INaP magnitude at hyperpolarized potentials (−80 to −55 mV) and to increase INaP at more depolarized potentials (e.g., −50 to −40 mV; Fig. 4C). As shown in Fig. 4, with BAPTA pipettes, the DHPG-evoked ICAN was absent, whereas INaP was enhanced. At −55 mV, DHPG increased INaP amplitude by −13.7 ± 6.2 pA (n = 7; Figs. 3 and 4C), a value that did not differ from that obtained with standard pipettes (−15.6 ± 5.8 pA; P > 0.6, unpaired t-test). DHPG did not alter membrane resistance measured with negative voltage steps (−6.6 ± 18.2 MΩ change, n = 7; P > 0.9, paired t-test), further consistent with the absence of ICAN.
Fig. 4.
DHPG enhances INaP when ICAN is blocked. A: voltage-ramps elicit the TTX-sensitive INaP in this example ET cell using a BAPTA-based recording pipette (see main text). Note that DHPG (30 μM) enhances INaP, whereas the DHPG-evoked ICAN is absent in this condition (compare with Figs. 1A and 2A). B and C: single cell (B) and group (C; n = 7) data subtraction plots show the DHPG-evoked enhancement of INaP. C includes the I–V profile of INaP obtained with the standard recording solution (Control-No BAPTA). Note that INaP magnitude is larger at depolarized potentials (−45 to −40 mV) compared with that obtained with a BAPTA-based internal solution.
ET cells exhibit an Ih that is active at resting membrane potential and cooperates with INaP to initiate ET cell bursts (Ennis and Hayar 2008; Liu and Shipley 2008b). However, we found no evidence for modulation of Ih by DHPG (n = 6; P > 0.15, 2-way repeated-measures ANOVA). Ih was eliminated by bath application of the selective Ih blocker ZD 7288 (200 μM, n = 4; P < 0.001, 2-way ANOVA).
Does DHPG modulation of INaP enhance rhythmic bursting?
Activation of group I mGluRs by DHPG enhances ET cell rhythmic bursting (Dong et al. 2009). This is mediated in part by activation of ICAN. Does the enhancement of INaP contribute to rhythmic bursting following mGluR activation? To investigate this question, we initially tested whether blockade of INaP by 10 nM TTX or 30 μM riluzole could attenuate DHPGs effects on bursting in current-clamp recordings. Consistent with previous studies (Liu and Shipley 2008b), we found that 10 nM TTX (n = 3) and riluzole (n = 2) invariably and completely eliminated spontaneous bursting; bursting could not be restored by depolarizing current injection (data not shown). As an alternative approach, we tested the effects of DHPG in the presence of APV-CNQX-gabazine using BAPTA-based pipettes, a condition that abolishes DHPG-evoked ICAN. Compared with recordings with standard pipettes in similar conditions [n = 11 ET cells, data from Dong et al. (2009)], BAPTA did not significantly alter ET cell resting membrane potential or membrane resistance (n = 11; P > 0.5 and P > 0.7, unpaired t-tests). DHPG (30 μM) depolarized the membrane potential by 3.6 ± 0.8 mV (n = 11; P < 0.006, paired t-test), a value significantly less than that obtained with standard recording pipettes in similar conditions [7.3 ± 1.1 mV, n = 11 ET cells from Dong et al. (2009); P = 0.012, unpaired t-test]. The mean 3.6-mV depolarization is similar to the 2.8-mV value predicted by the 13.7-pA mean inward current evoked by DHPG at −55 mV and the average ET cell input resistance (201 ± 11 MΩ, n = 11). DHPG did not alter the depolarizing envelope amplitude or duration (P > 0.18 and P > 0.4, respectively, paired t-tests).
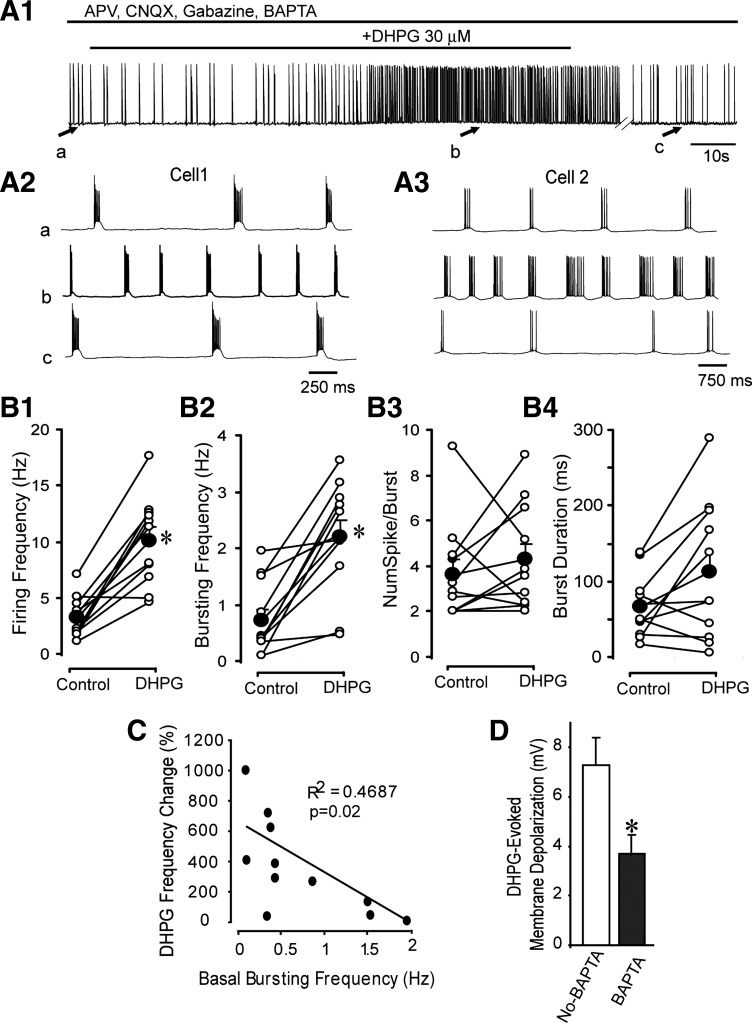
As shown in Fig. 5, DHPG significantly increased the firing rate and burst frequency by approximately 252 and 243%, respectively, but had no effect on the number of spikes per burst or burst duration. DHPG did not alter the intraburst frequency (40.4 ± 8.6 vs. 37.7 ± 8.9 Hz, n = 11; P = 0.32, paired t-test). Thus the increased firing rate is due to a faster burst rate rather than an increase in the number of spikes per burst or the intraburst frequency. We asked whether the effect of DHPG depended on the basal burst properties, finding an inverse relationship between the basal burst rate and the increase evoked by DHPG (Fig. 5; n = 11; r2 = 0.47, P = 0.02). Thus DHPG evoked the largest increase in ET cells with the slowest basal burst rates. By contrast, the increase in burst frequency was unrelated to the basal number of spikes per burst (r2 = 0.005, P > 0.8) or the magnitude of the DHPG-evoked depolarization (r2 = 0.002, P > 0.8). It should be noted that the burst frequencies recorded with BAPTA were slower than in previous extracellular recordings studies or via whole cell current-clamp recordings with a standard internal solution (approximately 0.5–11 Hz; Hayar and Ennis 2007; Hayar et al. 2004a; Liu and Shipley 2008b). This suggests that BAPTA reduces ET cell burst rate, perhaps by modulating calcium-dependent intracellular processes.
Fig. 5.
DHPG enhances rhythmic bursting in presence of BAPTA. A1: current-clamp recording with a BAPTA-based recording solution shows that DHPG increases ET cell burst frequency in the presence of dl-2-amino-5-phosphonovaleric acid (APV; 50 μM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM), and gabazine (10 μM). A2: faster time-scale records taken from the 3 points indicated in A1. Note increase in burst frequency and decrease in number of spikes per burst with DHPG. A3: in another ET cell, DHPG increases burst frequency and the number of spikes per burst. B1–B4: scatterplots showing the effects of DHPG on burst parameters in 11 ET cells. Filled circles denote means ± SE. *P < 0.05, paired t-tests. Note increase in firing frequency and burst frequency. NumSpike/Burst, number of spikes per burst. C: scatterplot of the relation between the basal (intrinsic) burst frequency and the increase in frequency elicited by DHPG. Note the larger enhancement of burst frequency in cells with slower basal burst rates. D: graph shows that the DHPG-evoked depolarization is significantly reduced in ET cells recorded with a BAPTA-based internal solution. *P = 0.012, unpaired t-test.
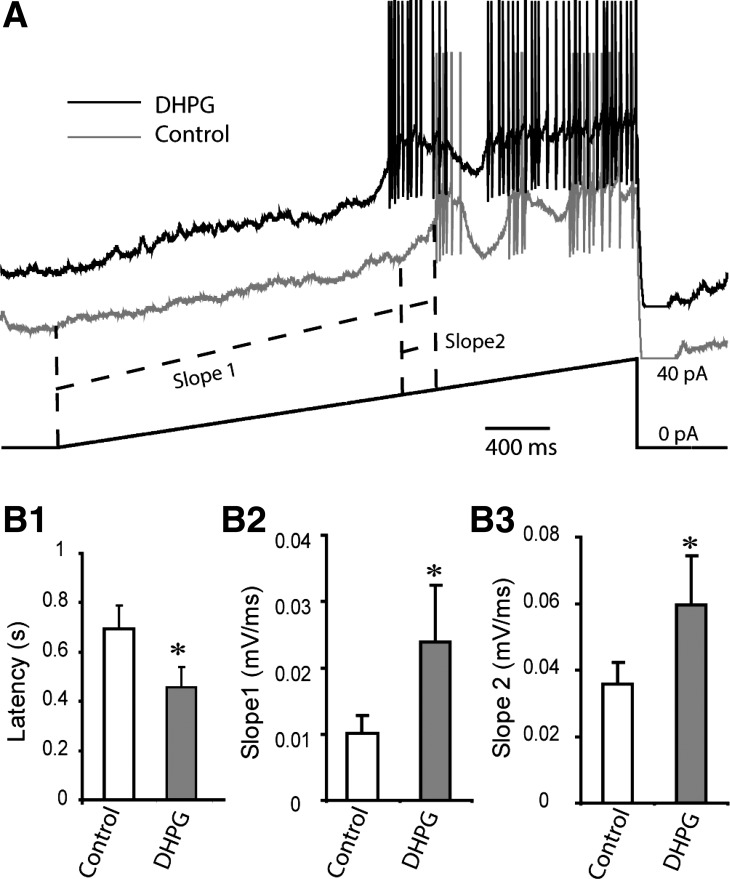
INaP activation drives the ET cell membrane potential toward the threshold of the depolarizing envelope underlying spike bursts (Liu and Shipley 2008b). DHPG facilitation of INaP is consistent with the observed increase in burst rate. However, the modest depolarization by DHPG could also underlie the increased burst rate (Hayar et al. 2004a). To address this, we prevented spontaneous bursting and compensated for the DHPG-evoked depolarization with negative current injection and then applied a slow current ramp (1.5-s duration, 20 pA) to evoke a burst. As shown in Fig. 6, DHPG reduced the burst latency from 0.69 ± 0.09 to 0.46 ± 0.08 s (n = 6; P < 0.02, paired t-test). DHPG also increased the slope between the ramp onset and first burst as well as the slope of the depolarizing envelope.
Fig. 6.
DHPG hastens evoked ET cell bursting. A: a slow current ramp (shown at bottom) was applied to evoke a burst in ET cells recorded with BAPTA-based internal solution in the presence of APV-CNQX-gabazine. DHPG (10 μM) shortens the delay to the 1st burst and increases the slope from ramp onset to the burst depolarizing envelope onset as well as the rising phase of the depolarizing envelope. B1–B3: bar graphs of group data from 6 ET cells showing changes in burst latency (B1), slope to 1st burst (B2), and rising slope of depolarizing envelope. *P = 0.017 (B1), P = 0.046 (B2), P = 0.047 (B3), paired t-tests.
DISCUSSION
The present results reveal that group I mGluR activation enhances ET cell excitability via positive modulation of INaP, a key intrinsic current involved in rhythmic burst generation in these cells. The facilitation of INaP elicited a modest depolarization and an increase in the rate of rhythmic bursting without effect on other major parameters of ET cell bursting. The increase in burst frequency evoked by DHPG was most pronounced in cells with slower basal burst rates, suggesting that mGluR activation may preferentially enable ET cells with slow intrinsic bursting to pace with faster sniffing frequencies.
ET cell burst initiation and frequency are largely determined by Ih, INaP, and low voltage-activated (LVA) calcium currents (Hayar et al. 2004a; Liu and Shipley 2008b). In each burst cycle, postburst hyperpolarizations engage Ih, which drives the membrane potential to activate INaP and subsequently LVA currents. The present results demonstrate that a major effect of DHPG is facilitation of INaP, consisting of a hyperpolarizing shift in the activation threshold together with an increased current magnitude in the range of −60 to −50 mV. Comparable effects of DHPG and group I mGluR agonists on INaP have also been reported in neocortical pyramidal cells and nucleus accumbens neurons (Carlier et al. 2006; D'Ascenzo et al. 2009). The impact of mGluR activation on INaP would be predicted to allow Ih to engage a more vigorous INaP at more hyperpolarized voltages. As observed here, burst frequency would therefore increase by facilitating INaP activation at an earlier stage in the interburst cycle. Since depolarization increases ET cell burst rate (Hayar et al. 2004a), the depolarization elicited by DHPG alone would also be expected to increase burst rate. However, when this depolarization was compensated by negative current injection, the latency of evoked bursts decreased. The rate of rise for evoked bursts and the increased slope of the rising phase of the depolarizing envelope are also consistent with facilitation of INaP. As previously reported (Dong et al. 2009) and observed here, DHPG also elicited a BAPTA-sensitive ICAN that robustly depolarizes ET cells and also increases burst frequency, burst duration, and number of spikes per burst. Taken together with these results (Dong et al. 2009), the present findings suggest that mGluR enhancement of ICAN and INaP cooperatively increase burst rate, whereas ICAN facilitation also increases burst strength, i.e., number of spikes per burst and burst duration.
Our results indicate that the other key current involved in burst initiation, Ih, is unaffected by DHPG and therefore unlikely to contribute to the observed modulation of burst frequency. Ih modulation would also be expected to alter burst duration and the number of spikes per burst (Liu and Shipley 2008b), which were not altered by DHPG in the presence of BAPTA. We cannot exclude possible effects of DHPG on LVA currents. However, modulation of LVA currents alters the number of spikes per burst as well as burst duration (Liu and Shipley 2008b), effects incompatible with the lack of effect of DHPG on these burst properties. It is conceivable that DHPG may modify other currents, such as the large conductance BK-type calcium-dependent potassium current that shapes ET cell burst duration and spikes per burst (Liu and Shipley 2008b), but these properties were not altered by DHPG. Additionally, the BAPTA concentration used in this study is known to block IBK (Schwindt et al. 1992; Velumian and Carlen 1999), and the observed effect of DHPG on bursting is not consistent with modulation of this current.
Electrical and chemical synapses made by ET cells play a robust role in coordinating intraglomerular network activity and amplifying output from mitral/tufted cells (De Saint Jan et al. 2009; Gire et al. 2012; Gire and Schoppa 2009; Hayar et al. 2004b, 2005; Najac et al. 2011; Shao et al. 2009, 2012). The intrinsic bursting properties of these cells are thought to reinforce the rhythmic, sniff-driven temporal pattern of sensory input onto the glomerular network. As a population, ET cells spontaneously burst at frequencies encompassing the approximately 1- to 12-Hz theta range of rodent sniffing (for review, see Wachowiak 2011; Welker 1964). Although the spontaneous burst rate in individual ET cells is remarkably stable, bursting can synchronize to olfactory nerve input delivered at rates faster than the intrinsic burst frequency (Hayar et al. 2004a). Olfactory nerve input therefore can override intrinsic burst frequency, perhaps allowing these cells to tune to the prevailing rhythmic sniffing rate. However, burst entrainment to olfactory nerve input begins to fail in the upper theta range (approximately >5 Hz), characteristic of active or investigational sniffing. Context- and state-dependent increases in sniffing (Kay and Laurent 1999; Wachowiak 2011), however, may engage mechanisms to offset burst failures. The activity of brain-stem serotonergic cell groups that innervate the glomerular layer including ET cells increases with arousal (Jacobs and Azmitia 1992; McLean and Shipley 1987). Serotonin engages a nonselective cation current that increases ET cell burst frequency (Liu et al. 2012). Notably, similar to the present results, this occurred preferentially in cells with slower intrinsic burst rates. Dopamine released from glomerular interneurons was recently found to increase ET cell burst frequency by enhancing Ih (Liu et al. 2013). mGluR activation in the olfactory bulb is preferentially engaged by high-frequency olfactory nerve input (De Saint Jan and Westbrook 2007; Ennis et al. 2006; Johnston and Delaney 2010; Yuan and Knöpfel 2006). INaP plays a key role in boosting the strength of subthreshold excitatory input to ET cells, particularly at high frequencies (Liu and Shipley 2008a). Thus the mGluR enhancement of INaP and burst frequency reported here may operate at the upper theta range of sniff-driven sensory input to offset burst failure. Taken together with previous findings, our results suggest that modulation of ET cell excitability by neurotransmitters intrinsic to the glomerular circuitry as well as extrinsic centrifugal inputs allow fine-tuning of ET cell bursting to state- and context-dependent changes in sniff rates.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant DC-003195.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.D. and M.E. conception and design of research; H.D. performed experiments; H.D. analyzed data; H.D. and M.E. interpreted results of experiments; H.D. prepared figures; H.D. drafted manuscript; H.D. and M.E. edited and revised manuscript; H.D. and M.E. approved final version of manuscript.
REFERENCES
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003 [DOI] [PubMed] [Google Scholar]
- Carlier E, Sourdet V, Boudkkazi S, Déglise P, Ankri N, Fronzaroli-Molinieres L, Debanne D. Metabotropic glutamate receptor subtype 1 regulates sodium currents in rat neocortical pyramidal neurons. J Physiol 577: 141–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20: 2011–2021, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NP, Li Y, Keller A. The whisking rhythm generator: a novel mammalian network for the generation of movement. J Neurophysiol 97: 2148–2158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J Physiol 587: 3233–3250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL, Charpak S. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29: 2043–2052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint JD, Westbrook GL. Disynaptic amplification of metabotropic glutamate receptor 1 responses in the olfactory bulb. J Neurosci 27: 132–140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Hayar A, Callaway J, Yang XH, Nai Q, Ennis M. Group I mGluR activation enhances Ca(2+)-dependent nonselective cation currents and rhythmic bursting in main olfactory bulb external tufted cells. J Neurosci 29: 11943–11953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Hayar A. Physiology of the main olfactory bulb. In: The Senses: A Comprehensive Reference, Volume 4: Olfaction and Taste, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer W, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, Kaas JH, Gardner E. San Diego, CA: Academic Press, 2008, p. 641–686 [Google Scholar]
- Ennis M, Zhu M, Heinbockel T, Hayar A. Olfactory nerve-evoked, metabotropic glutamate receptor-mediated synaptic responses in rat olfactory bulb mitral cells. J Neurophysiol 95: 2233–2241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci 13: 2001–2012, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32: 2964–2975, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Ennis M. Endogenous GABA and glutamate finely tune the bursting of olfactory bulb external tufted cells. J Neurophysiol 98: 1052–1056, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci 24: 1190–1199, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinbockel T, Heyward P, Conquet F, Ennis M. Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol 92: 3085–3096, 2004 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 72: 165–229, 1992 [DOI] [PubMed] [Google Scholar]
- Johnston J, Delaney KR. Synaptic activation of T-type Ca2+ channels via mGluR activation in the primary dendrite of mitral cells. J Neurophysiol 103: 2557–2569, 2010 [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci 2: 1003–1009, 1999 [DOI] [PubMed] [Google Scholar]
- Liu S, Aungst JL, Puche AC, Shipley MT. Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J Neurophysiol 107: 473–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci 33: 2916–2926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Intrinsic conductances actively shape excitatory and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci 28: 10311–10322, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci 28: 1628–1635, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci 7: 3016–3028, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, De Saint JD, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBötzinger Complex neurons and respiratory rhythm generation. J Physiol 580: 485–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355: 455–469, 1995 [DOI] [PubMed] [Google Scholar]
- Sahara Y, Kubota T, Ichikawa M. Cellular localization of metabotropic glutamate receptors mGluR1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci Lett 312: 59–62, 2001 [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron 31: 639–651, 2001 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Effects of intracellular calcium chelation on voltage-dependent and calcium-dependent currents in neocortical neurons. J Neurophysiol 47: 571–578, 1992 [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Liu S, Shipley MT. Intraglomerular inhibition shapes the strength and temporal structure of glomerular output. J Neurophysiol 108: 782–793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol 322: 121–135, 1992 [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol 362: 134–150, 1995 [DOI] [PubMed] [Google Scholar]
- Velumian AA, Carlen PL. Differential control of three after-hyperpolarizations in rat hippocampal neurones by intracellular calcium buffering. J Physiol 517: 201–216, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron 71: 962–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol 17: 411–423, 2006 [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour 22: 233–244, 1964 [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci 33: 1552–1563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Knöpfel T. Olfactory nerve stimulation-evoked mGluR1 slow potentials, oscillations, and calcium signaling in mouse olfactory bulb mitral cells. J Neurophysiol 95: 3097–3104, 2006 [DOI] [PubMed] [Google Scholar]