Abstract
Temporal pole (TP) cortex is associated with higher-order sensory perception and/or recognition memory, as human patients with damage in this region show impaired performance during some tasks requiring recognition memory (Olson et al. 2007). The underlying mechanisms of TP processing are largely based on examination of the visual nervous system in humans and monkeys, while little is known about neuronal activity patterns in the auditory portion of this region, dorsal TP (dTP; Poremba et al. 2003). The present study examines single-unit activity of dTP in rhesus monkeys performing a delayed matching-to-sample task utilizing auditory stimuli, wherein two sounds are determined to be the same or different. Neurons of dTP encode several task-relevant events during the delayed matching-to-sample task, and encoding of auditory cues in this region is associated with accurate recognition performance. Population activity in dTP shows a match suppression mechanism to identical, repeated sound stimuli similar to that observed in the visual object identification pathway located ventral to dTP (Desimone 1996; Nakamura and Kubota 1996). However, in contrast to sustained visual delay-related activity in nearby analogous regions, auditory delay-related activity in dTP is transient and limited. Neurons in dTP respond selectively to different sound stimuli and often change their sound response preferences between experimental contexts. Current findings suggest a significant role for dTP in auditory recognition memory similar in many respects to the visual nervous system, while delay memory firing patterns are not prominent, which may relate to monkeys' shorter forgetting thresholds for auditory vs. visual objects.
Keywords: rhesus macaque, working memory, short-term memory, suppression, single-unit, delayed matching-to-sample
processing functions of temporal polar cortex, the rostral portion of the temporal lobe, are not well known compared with other higher-order sensory cortical areas (Olson et al. 2007), including inferior temporal cortex (ITC), rostral superior temporal gyrus, parietal cortex, and prefrontal cortex within the dual-stream cortical network model for spatial and nonspatial information processing in nonhuman primates (Hackett 2010; Mishkin et al. 1983; Poremba and Mishkin 2007) and cats (Lomber and Malhotra 2008). The temporal pole is linked to functions involving highly processed sensory information, e.g., recognition of faces and voices, species-specific vocalizations, recognition memory, semantic memory and social/emotional processing (Andics et al. 2010; Belin et al. 2002; Belin 2006; Fritz et al. 2005; Jimura et al. 2009; Nakamura et al. 2001; Olson et al. 2007; Patterson et al. 2007; Poremba et al. 2004; Tranel 2006). Lesions to higher-order auditory regions along the superior temporal gyrus, including temporal pole, severely impair auditory perception, discrimination of complex sounds (e.g., species-specific vocalizations), and short-term recognition memory (Colombo et al. 1990, 1996; Dewson et al. 1969, 1970; Fritz et al. 2005; Heffner and Heffner 1984, 1986; Iversen and Mishkin 1973; Kupfer et al. 1977; Leff et al. 2009; Weiskrantz and Mishkin 1958. The respective temporal pole neural mechanisms of auditory encoding and memory remain a mystery.
Dorsal temporal pole (dTP), consisting of granular and dysgranular areas, has extensive connections with auditory and auditory-related regions, e.g., superior temporal gyrus, parabelt areas, limbic thalamus, amygdala, hippocampus, and lateral, orbital and medial prefrontal cortices (Barbas et al. 1999; Ding et al. 2009; Kondo et al. 2005; Markowitsch et al. 1985; Moran et al. 1987; Romanski et al. 1999; Saleem et al. 2008; Yeterian and Pandya 1989), and it lies at the ventral-most portion of the proposed auditory object identification pathway (Poremba et al. 2003; Rauschecker and Scott 2009). The dTP appears analogous to ITC and ventral temporal pole (vTP), both higher-order visual cortical areas situated along the ventral “what” stream for visual object processing, showing neural correlates of visual analysis and identification through stimulus selectivity to complex objects (Desimone et al. 1984; Nakamura et al. 1994; Tanaka 1996). These visual areas also exhibit sustained firing activity within memory delays, reflecting retention of information for visual working/recognition memory (Colombo and Gross 1994; Miller et al. 1991 1993; Miyashita and Chang 1988; Nakamura and Kubota 1995, 1996). Considering the anatomical and functional analogies between visual and auditory nervous systems, dTP is a potential candidate to support complex analyses of sounds and mediate auditory recognition memory.
A few recording studies reveal stimulus-specific activity related to auditory encoding and sometimes working-memory-related activity, in the auditory cortex (Gottlieb et al. 1989; Sakurai 1994) and the prefrontal cortex (Bodner et al. 1996; Romanski et al. 2005; Russ et al. 2008). However, prior studies only use a pair of tone stimuli during auditory memory tasks, and the behavioral paradigms are sometimes simply delayed stimulus-response tasks. Neural activity revealed across auditory cortical regions may not be on par with those shown by visual electrophysiological studies, in terms of task complexity, difficulty and engagement. The present study thus examines activity of dTP neurons when monkeys perform an auditory delayed matching-to-sample (DMS) task wherein two sounds, separated by a memory delay, are the same or different. A wide range of auditory stimuli is also utilized, ranging from pure tones and band-passed noises to human and monkey vocalizations. The present study assesses response profiles of single dTP neurons to discrete task events, e.g., sound presentation, memory delay, possible decision period, and behavioral response. Expectations include verification of dTP as a cortical region in which significant neuronal activity is evoked by sound stimuli, and that encoding of DMS task events, including cues, memory delays, and neural correlates of recognition memory are observed.
MATERIALS AND METHODS
Subjects and Surgical Methods
Two adult rhesus macaque monkeys (Macaca mulatta) were used, a male and a female, weighing 10 and 6 kg, respectively. They were individually housed in Spence Laboratories at the University of Iowa (12:12-h light-dark cycle). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa. Monkey biscuits (Harlan Teklad, Madison, WI) were fed to animals daily with fruits, vegetables, and treats scheduled throughout the week. Monkeys had access to water ad libitum in home cages equipped with environmental enrichment. Each animal's weight was maintained above 85% of his or her starting weight with controlled daily feeding schedules, and weight was adjusted upward based on age. Prior to surgery, each monkey was scanned with magnetic resonance imaging (MRI; 2T Sigma unit; GE Medical Systems) to locate the precise coordinates of temporal pole and to verify the placement of electrodes within the chamber grid and dTP after surgery. Placements of recording chambers were initially performed at the National Institute of Mental Health (Bethesda, MD). Monkeys were sedated with ketamine (10 mg/kg im) and anesthetized with isoflurane (1–2%). Using a stereotaxic apparatus (David Kopf Instruments), an angled 45° recording chamber (Crist Instruments) was implanted on the skull of the left hemisphere, centered at −2 mm posterior and −23 mm lateral of stereotaxic 0,0 (Saleem and Logothetis 2007), and its position was secured with titanium screws and dental acrylic. A stainless steel headpost was attached tightly against the backside of the skull for restraining head movement during electrode recordings. Antibiotics and analgesics were given to the animals after surgery. Recording chambers were cleaned routinely with antiseptics to inhibit infection after the bone was removed for insertion of recording electrodes.
Auditory Stimuli
Each auditory stimulus, 220–500 milliseconds (ms) long, was digitized and processed with a sampling frequency of 44,100 Hz and consisted of 8-bit mono-recorded sound clips. All auditory stimuli were presented through a front loudspeaker (flat: ±3 dB; frequency response: 75 Hz to 20 kHz) placed ∼40 cm from the head region, at 80-decibels (dB) standard sound pressure level. A collection of 96 standard stimuli was used and classified into 8 sound types in a manner similar to Ng et al. (2009). Animal vocalizations (n = 12) included sounds recorded from birds and domestic animals (e.g., cat and dog). Human vocalizations (n = 12) included speech sounds (e.g., “girl,” “thank you,” “good morning”) and nonspeech sounds (e.g., laughing, crying, sneezing) generated from unknown male and female speakers. Monkey vocalizations (n = 12) were coos, grunts, screams, shrill barks, and harmonic arches recorded in a natural monkey reserve of South Carolina (by the author A. Poremba). Music clips (n = 12) contained notes (e.g., harmonics) and sound clips (e.g., extracts of orchestra symphonies and melodies of TV commercials) generated from various musical instruments (e.g., violin, flute, trumpet). Natural sounds (n = 12) included recorded samples of natural phenomena such as fire burning, water rippling, stream flowing, wind breezing, hurricane, and thunder. Pure tones (n = 12) were digitally generated and normalized with root-mean-square methods, and their frequencies ranged between 500 and 12,000 Hz. Synthesized clips (n = 12) consisted of digitally generated sounds (e.g., rhythmic notes and frequency-modulated sweeps) and recordings of man-made environmental sounds (e.g., engine noise) and sounds resulting from metallic bombardment (e.g., coins in a machine). White noise stimuli (n = 12) were band-passed noise created with different low- and high-pass filters (lower and upper frequency limits between 10 and 10,000 Hz). The 96 standard stimuli were then sorted into 12 sound folders with each folder containing 1 sound sample from the 8 sound types.
Twenty-one additional exemplars of pure tones (250-ms long) were used for sampling spike activity of dTP neurons across different frequency at 80 dB during passive listening. They covered the frequency range between 100 Hz to 20 kHz (100–1,000 Hz at 100-Hz incremental steps; 1–10 kHz at 1-kHz incremental steps; 10–20 kHz at 5-kHz incremental steps). These 21 sinusoidal pure tones, as well as those 12 pure tones used in the behavioral task, were digitally generated under the environment of MATLAB programming (Math Works, Natick, MA), and normalized with root-mean-square methods by Adobe Audition (Adobe, San Jose, CA).
Experimental Procedures
Passive listening.
Recording sessions were conducted in a double-walled acoustic chamber (background noise: 35 dB; Industrial Acoustic). The animal sat in a monkey chair with its head fixed, facing a free-field loudspeaker (see above). Ninety-six standard sounds (divided into 12 sound folders) and 21 pure-tone stimuli were presented in a series of blocks. For each recording session, the animal listened to only one sound folder of eight preselected sounds (1 stimulus per sound type), and each stimulus was repeated at least eight times. A given sound folder was thus repeatedly used every 12 recording sessions. The order of sound presentations within each block was randomized with the LabView program. Randomized interstimulus intervals were 1, 1.2 and 1.5 s during passive listening. Once a unit was isolated, the 8 preselected sounds presentations were followed by a block of 21 pure tone stimuli. The animal was not required to respond, and no food reward was given throughout the passive listening experiment. Then the animal performed the auditory DMS task with the same eight sound stimuli from this pretask passive listening.
Auditory DMS task.
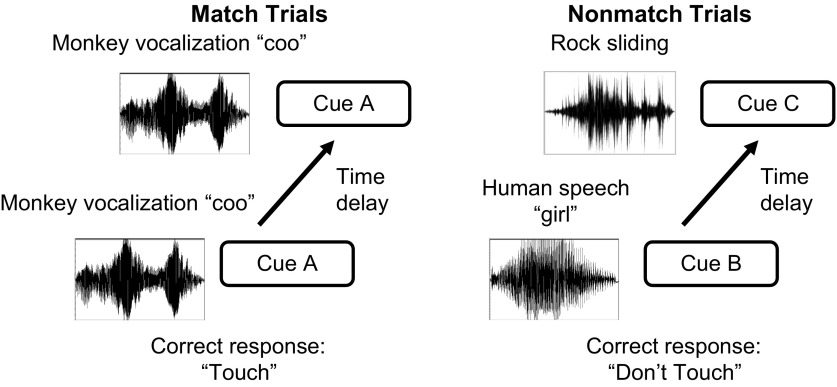
Each animal was trained on the DMS task with auditory stimuli as described in Ng et al. (2009). The task employed go/no-go response rules for the auditory DMS task (Fig. 1). Eight preselected sounds, used during passive listening in the same recording session, were used during the DMS task. The ratio of match to nonmatch trials was 1, after being pseudorandomly controlled by the LabView software program (National Instruments, Austin, TX). In a given trial, cue 1 (a sample stimulus) was first presented, followed by a memory delay before cue 2 (a test stimulus) was presented. After presentation of cue 2, subjects were required to wait 1 s before having an opportunity to make their response, i.e., wait time. Then, on each trial, the Plexiglas response button was lit from behind to signal the possible response period. The memory delay, inserted between two sound stimuli (i.e., interstimulus intervals), was always 5 s long per trial. On match trials, the two sounds were the same, and a correct response was made by touching the button (i.e., a go response) and a small chocolate candy reward was delivered. On nonmatch trials, the two sounds presented were different, and a correct response was scored if the monkey avoided touching the button (i.e., a no-go response), with no subsequent food delivery. Thus the current DMS task, employing go/no-go rules, used an asymmetric reinforcement contingency. On both match and nonmatch trials, the touch-sensitive button was lit up for a maximum of 1.5 s (i.e., the possible response period). Once a button-press was recorded during this 1.5-s period, the light was extinguished, and the trial ended whether or not the response was correct. If the animal responded to five nonmatch trials in a row, a prolonged intertrial interval (ITI) (up to 30 s long) was introduced after the last incorrect button press to help correct overresponding by the animal. A mild air puff (300–500 ms in duration) directed toward the general head region of each animal was delivered if the animal erroneously pressed the button on 10 consecutive nonmatch trials. The ITI was randomized across sets of 6, 8 and 10 s and 8, 10 and 12 s for the monkeys OP and AB, respectively. A premature response during the ITI or during 5-s memory delays reset the same trial type but with a different stimulus pair. There were no more than three consecutive trials of match or nonmatch trials. Positions of sound presentations on nonmatch trials (i.e., cue 1 or cue 2) were completely counterbalanced among the eight sound types. Each sound stimulus had equal probability of appearing on match and nonmatch trials, as well as becoming the sample (cue 1) or test (cue 2) stimulus. The behavioral task contained 200 trials to yield 8–10 repetitions of each sound stimulus at each trial and event condition. Trials were sometimes excluded for data analysis, for example, due to premature behavioral responses during ITIs or interstimulus intervals, poor memory performance, presentation error during sounds and events due to an occasional computer glitch, and movement artifact. A minimum of 10 trials was used for any trial-type analysis.
Fig. 1.
Schematic diagram depicting the auditory delayed matching-to-sample task. Recording sessions contain 200 trials, with equal numbers of match and nonmatch trials. During match trials, the first sound, followed by a 5-s delay, was same as the second sound. The correct response was a touch (go-response), and the animal was then rewarded. During nonmatch trials, the two sounds were different and also separated by a 5-s delay, and the correct response was to not touch. The correct no-go response was not rewarded, and thus the study utilized asymmetric reinforcement contingency. An erroneous touch response during nonmatch trials resulted in an extended intertrial interval before the next trial started.
Recording procedures.
Single-unit activity was recorded in dTP cortex by lowering tungsten microelectrodes (130–140 mm long, 1–3 MΩ; FHC, Bowdoin, ME) at appropriate angles, starting above the parietal cortex to the dorsal region of temporal pole. Verification of the well placement for angled movement of the electrodes through a chamber grid was verified with subsequent MRIs, wherein capillary tubes filled with vitamin E were lowered to the dura surface through the chamber grid to calculate X-, Y-, and Z-coordinates for dTP electrode placement from the MRI images. Verification of depth coordinates and medial-lateral positioning was additionally verified by noting the positions of the rostral bone cavity containing the dTP under the eye orbit when electrode tracks were finished. In the present study, the definition of dTP, consisting of dysgranular (TGdd) and granular layers (TGdg), adapted from the scheme used by Carmichael and Price (1995), Ding et al. (2009), Galaburda and Pandya (1983), Hackett et al. (1998), Kondo et al. (2003), Moran et al. (1987), and Poremba et al. (2003). Microelectrodes were inserted into a 23-g sterile guide cannula that was held by an x–y grip positioner attached to a micromanipulator. Electrode advancement was accomplished by a computer-controlled electrode drive system (NAN Instruments, Nazareth, Israel). Spike activity was isolated, amplified, and discriminated in real-time by the Multichannel Acquisition Processor with the SortClient program (contour sorting method, Plexon, TX). Corresponding data, as well as timelines of stimulus and behavioral events, were saved for offline analysis. Principal component analysis was used to distinguish different spike waveforms collected at a given x–y coordinate, and mediated cluster cutting. The study employed multivariate ANOVA (P ≤ 0.01) to assess the degree of separation when two or more clusters were revealed (Offline Sorter program, Plexon, TX). The verified cluster was then considered as a single unit for subsequent analyses. The x–y position of the recording site was recorded every time and compared with MRI coordinates to determine locations of recorded dTP cells. Recordings were conducted in the left hemisphere of dTP to maximize the probability of finding significant dTP activity during passive listening and the memory task, as consistent findings have shown a lateralization of higher-order auditory perception in the left hemisphere in terms of faster acquisition and robust neuronal activity (Heffner and Heffner 1984, 1986; Petersen et al. 1984; Poremba et al. 2004). To minimize sampling bias, no sound stimuli were presented to the animal when lowering the electrodes. Once an isolated dTP neuron was obtained, the experimenter commenced the recording session.
Data analysis.
Spike activity was recorded at a sampling frequency of 40 kHz. Each isolated unit was sorted into waveforms for offline analysis (Offline Sorter, Plexon, TX). On a neuron-by-neuron basis, the response profile of each dTP neuron was constructed for passive listening and the auditory DMS task in regard to different stimuli or task events. Peristimulus time histograms were created to visualize spike activity related to various stimuli and task events across time using NeuroExplorer (Nex Technologies, Littleton, MA). Spike activity was sampled using 10-ms intervals. Spike activity during the events was standardized by calculating a mean pretrial firing rate and its standard deviation for each unit across the 500-ms pretrial period of the DMS task. The pretrial firing mean was subtracted from the activity values for each event and divided by the standard deviation of the pretrial period. The resultant standardized values were then used for statistical analysis. Spike activity of each unit was compared with pretrial firing rate to determine whether it was responsive to sounds or task-relevant events. Pretrial firing rate (or prestimulus firing rate) was estimated from recording intervals, i.e., 500 ms prior to trial presentation during the DMS task (or stimulus presentation during passive listening). Additional details of data analysis are provided in the experiment sections for the auditory DMS task and the passive listening task. Data analysis was conducted within MATLAB programming. Mean firing rate of an event was defined as the number of action potentials divided by the length of a specific time interval. Binomial tests were used to examine if there was a significant number of evoked units for a given task event, relative to chance, among all recorded dTP units. The general critical probability level for all statistical analyses in the present study is 0.05, unless specified.
Auditory DMS task.
Percentage correct and response latency during match and nonmatch trials were collected with the LabView program. Single-unit activity from one or more units was isolated during 86 memory task sessions from two monkeys (OP: n = 42; AB: n = 44). Performance on each of these sessions reached at least 60% correct for match and nonmatch trials. For each recorded cell, mean firing rates regarding sound presentations of cue 1 (a sample stimulus) and cue 2 (a test stimulus), memory delay, wait, and response periods were calculated for each of these task events. To verify whether the neuronal activity was significantly different from pretrial firing rate for each task event, separate one-way ANOVAs were used with post hoc Tukey's HSD (honestly significant difference) tests for pairwise comparisons across intervals. Significant differences revealed evoked spike activity at least 2 SDs above or below the pretrial firing rate. Each trial was divided into a pretrial firing period (500 ms; before cue 1), cue 1 and cue 1 offset events (500 ms each), cue 2 and cue 2 offset events (500 ms each), delay period (4,500 ms), wait time (500 ms) and response periods (1,500 ms). Due to the dynamic nature of sound information, cue presentation (cues 1 and 2) and postcue event (cue offsets 1 and 2) were each binned into 100-ms intervals for ANOVAs to reveal fine activity change from pretrial firing rate. The other task-relevant events were divided into time intervals of 500 ms for ANOVAs; wait time (1 interval), response period (3 intervals) and delay period (9 intervals). It is necessary to conduct separate one-way ANOVAs for discrete task-relevant events during the memory task, because the nature of these events is qualitatively and quantitatively diverse. They differ in event duration (500 ms to 5 s long), presence or absence of auditory stimulation, stimulus placement within the trial, i.e., sample or test stimulus, and behavioral responses. The cue 1 and cue 2 periods are auditory events presented to the animal subjects with corresponding offset periods. The 5-s delay periods are potentially related to memory and/or attention processes in the absence of auditory stimulation. After the cue 2 offset period, the wait time period (right before the animal was allowed to produce responses) is potentially related to match/nonmatch decision-making and/or motor plans associated with the respective decision outcomes. The neuronal activity during the response periods will be assessed for any relationship to behavioral outcome, i.e., correct or incorrect performance. Additionally, reward-related activity may be observed when comparing trials during which a response was made on match-correct and nonmatch-incorrect trials but rewarded on match-correct trials only. There are four possible trial types present in the current memory task that are defined by a combination of learning rule and behavioral outcome: 1) match-correct trials, where the subjects correctly pushed the button after two matching stimuli; 2) match-incorrect trials, where the subjects failed to produce button-press responses after two matching stimuli; 3) nonmatch-correct trials, where the subjects withheld button-press responses when the two stimuli were different sounds; and 4) nonmatch-incorrect trials, where the subjects erroneously pushed the button when the two sound stimuli were different rather than matching.
The population results are based on all 225 recorded units. For each event, spike activity of each unit for a given time interval was used as a single data point. The resultant standardized values of a unit for a given event were then used for population analyses. Although this method may combine dissimilar units across various task events, it provides an overall summary of how a population of dTP neurons responds to discrete task events by trial type. Evoked activity across various task events at the population level was then examined in a similar manner to those used in single-unit analyses (one-way ANOVAs and Tukey's HSD). To examine trial influence on population activity across similar task-relevant events, repeated-measures ANOVAs were used to evaluate whether dTP neurons would encode the matching rules (match vs. nonmatch trials), behavioral outcome (correct vs. incorrect), or a combination of both factors. Unless otherwise specified, post hoc comparisons for population analyses were conducted by paired sample t-tests with the Bonferroni procedure and Keppel's modification to correct for multiple comparisons (Keppel 1982). The product, of the number of degrees of freedom and the standard α-level of 0.05, was divided by the number of t-test comparisons. The resultant value was then used as the adjusted critical probability level. For example, the study examined if population activity changes during cue 1 varied among match-correct, nonmatch-correct and nonmatch-incorrect trials. The adjusted critical probability level would be 0.033 (0.05 “α-level” × 2 “degree of freedom” divided by 3 “number of comparisons”).
Passive listening.
Each isolated unit was tested first with one-way ANOVAs to determine whether it was auditory responsive relative to prestimulus firing rate. Post-hoc Tukey's HSD tests were used with procedures similar to those used previously to control errors due to multiple comparisons. Data analysis was performed in sets of five intervals during sound presentations to capture fine changes of spike activity within dTP. Spike activity during stimulus period and stimulus offset period was then binned into 100-ms intervals (i.e., 5–100 ms intervals each). In contrast to the 96 standard sounds, 21 pure-tone stimuli were only 250 ms long and analyzed with 50-ms intervals (i.e., 5–50 ms intervals each) for comparable one-way ANOVA tests.
RESULTS
Recording Placement
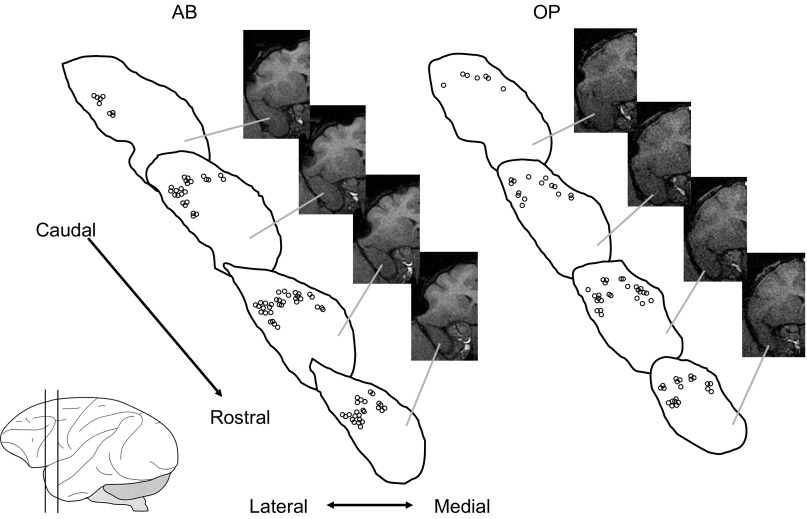
Electrode placements inside the recording chamber are illustrated in Fig. 2. All units were located between 0 and +3.6 mm from bregma (Paxinos et al. 1999). Of the 225 units, 120 were located on the lateral side of dTP, and 105 on the medial side of dTP. The recording sites were mainly located in TGdd and TGdg, using the nomenclatures for subdivisions of temporal pole (Kondo et al. 2003, 2005; Saleem et al. 2008). The majority of the collected units are more rostral along the rostral superior temporal plane than the units in the recent study of Kikuchi et al. (2010), and the present findings reveal a similar continuation and extension of the stimulus selectivity of the rostrocaudal axis along the superior temporal gyrus in primates (see results in Passive listening). Of the total recorded units from the lateral and medial regions of dTP, respectively, 80% and 79% were responsive to one or more task-relevant events during the DMS task. Table 1 illustrates response profiles of evoked dTP units across various task events separated by anatomical locations. Within the lateral area of dTP, more units tended to be responsive during response periods 1–3 (34% or above; binomial tests, P ≤ 0.05). Within the medial area of dTP, the majority of units (40%) were evoked during response period 2, compared with other events (29% on average). Unit responsiveness to sounds was similar between lateral and medial regions of dTP during passive listening (lateral 23% vs. medial 24%) and the memory task (lateral 38% vs. medial 31%).
Fig. 2.
Electrode placements at dorsal temporal pole (dTP) in the two monkeys, AB and OP. Magnetic resonance images were obtained from the two monkeys at the left hemisphere of dTP (slice thickness is 1 mm). Schematic diagrams show recording sites at dTP, between 0 and +3.6 mm from bregma, represented by circles (lateral: N = 120; medial: N = 105). [Adpated from Paxinos et al. 1999].
Table 1.
Percentage of units responsive to discrete task events during the delayed matching-to-sample task (cue 1, cue 2 and their offset periods, memory delay, wait time and response periods 1, 2 and 3), separated by anatomical location within the dTP
| Cue 1 | Cue 1 Offset | Cue 2 | Cue 2 Offset | Delay | Wait | Response 1 | Response 2 | Response 3 | |
|---|---|---|---|---|---|---|---|---|---|
| Lateral | 28 | 20 | 29 | 18 | 22 | 29 | 43 | 34 | 34 |
| Medial | 24 | 22 | 30 | 31 | 19 | 28 | 32 | 40 | 31 |
Lateral, N = 120; medial, N = 105. The dorsal temporal pole (dTP) unit percentage is evoked above chance (binomial tests, P ≤ 0.05) for each given task event. Data were pooled together across all sound stimuli and trial types for each recorded unit.
Auditory DMS Task
The monkeys performed the DMS task, on average, with accuracy of 78% (SE 1.4%) at match trials and of 66% (SE 1.1%) at nonmatch trials from all recording sessions. A similar bias toward “go” responding has been observed in other animal experiments using go/no-go paradigms in which correct “go” responses are rewarded (Bigelow and Poremba 2013). Using the overall go response rate of 57% to determine chance level accuracy for the behavioral task, chance performance of the monkeys were 57% and 43% at match and nonmatch trials accordingly. Response latency was shorter during match-correct trials (181 ± 9 ms), than during nonmatch-incorrect trials (209 ± 13 ms) (paired-sample t-tests, P < 0.001). The study collected 225 units at the left-hemisphere dTP while subjects were participating in the memory task (monkey OP: N = 112; monkey AB: N = 113). The majority of these recorded cells (80%) was responsive to at least one of the task-relevant events and considered generally task related.
Single-Unit Analysis
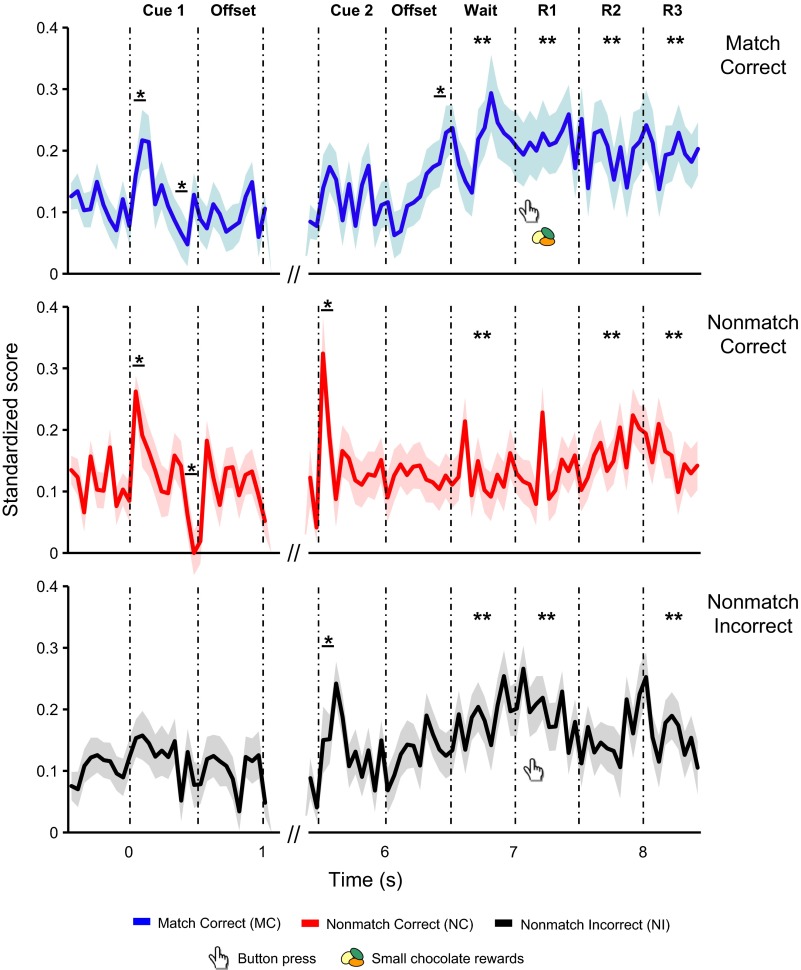
During the memory task, neurons of dTP were primarily active (27–39% of total cells) during cue presentations, when sound cue information might be encoded, during the wait period, when subjects might be deciding whether or not to produce a behavioral response, and during the response periods (Tables 1 and 2; Data were pooled together across all sound stimuli and trial types for each recorded unit). More neurons exhibited significantly increased activity than decreased activity to these events. Over one-half of all recorded units showed evoked activity during match-correct trials, relative to nonmatch trial types (nonmatch-correct: 35%; nonmatch-incorrect: 36%; binomial tests, P ≤ 0.05). However, only 20% of recorded units showed evoked activity during match-incorrect trials. Table 3 summarizes the percentage of units showing activity change during discrete task events by trial type, and Fig. 3 illustrates examples of task-related activity by trial type.
Table 2.
Percentage of units showing a significant activity change from pretrial firing rate, either increasing (+), decreasing (−) or a combination of both changes (both) for a given task event for at least one trial type
| Task Events | + | − | Both | Total |
|---|---|---|---|---|
| Cue 1 | 16 | 8 | 3 | 27 |
| Cue 1 offset | 13 | 5 | 2 | 20 |
| Memory delay | ||||
| Early | 11 | 9 | 20 | |
| Middle | 9 | 11 | 20 | |
| Late | 13 | 9 | 22 | |
| Cue 2 | 17 | 9 | 3 | 29 |
| Cue 2 offset | 14 | 6 | 4 | 24 |
| Wait | 16 | 12 | 28 | |
| Response 1 | 21 | 18 | 39 | |
| Response 2 | 22 | 15 | 37 | |
| Response 3 | 19 | 14 | 33 |
N = 225. The total dTP unit percentage is evoked above chance (binomial tests, P ≤ 0.05) for each given task event. Data were pooled together across all sound stimuli and trial types for each recorded unit.
Table 3.
Percentage of units showing a significant activity change from pretrial firing rate, either increasing, decreasing or a combination of both changes for a given task event for each of the four trial types
| Task Events | MC | NC | NI | MI |
|---|---|---|---|---|
| Cue 1 | 13* | 11* | 9* | 5 |
| Cue 1 offset | 8* | 8* | 7 | 6 |
| Cue 2 | 16* | 12* | 6 | 8* |
| Cue 2 offset | 10* | 10* | 7 | 6 |
| Delay | 10* | 7 | 7 | 6 |
| Wait | 17* | 8* | 7 | 5 |
| Response 1 | 26* | 11* | 13* | 5 |
| Response 2 | 28* | 8* | 8* | 5 |
| Response 3 | 21* | 11* | 10* | 5 |
N = 225. MC, match correct; NC, nonmatch correct; NI, nonmatch incorrect; MI, match incorrect.
For a given task event, the percentage of dTP units evoked above chance (binomial tests, P ≤ 0.05). Data were pooled together across all sound stimuli for each recorded unit.
Fig. 3.
Various examples of dTP neurons responsive to discrete task-relevant events during the memory task. Raster plots and peristimulus time histograms show spike activity aligned to onset of cue 1 (time = 0). Asterisks denote significant activity change against pretrial firing rate during a given 100-ms interval or a given 500-ms interval. Each bin is 100 ms. A: unit (no. 1021092a) encoded the events of cue presentations and offsets, wait and response periods during match and nonmatch trials. Compared with nonmatch-correct (NC) trials, the unit increased firing rate during wait period of match-correct (MC) and nonmatch-incorrect (NI) trials before eventual responses were produced by the subject. Significant activity change of the unit was associated with response initiation. This unit had sustained firing throughout response periods 1–3 during MC trials only. This effect was modulated by presence of food rewards. B: unit (no. 0420102b) was responsive to the events of cue 1, cue 2, and response periods during the three trial types. Compared with NI trials, this unit increased firing to cue 1 and 2 only for MC and NC trials, reflecting auditory encoding linked to accurate memory performance. C: unit (no. 0521101c) was associated with wait and response periods during MC, NC and NI trials. The unit had sustained firing from cue 2 offset toward the wait period during NI trials in contrast to sustained firing along the entire response interval during MC trials. The effect may be due to behavioral outcomes associated with these two trial types (correct/error or reward/no reward).
Cues and cue offsets.
About one-third of the neurons showed significantly evoked responses to the sound cues with a general drop off to 20%, on average, of neurons during the sound offset period, except in the medial portion of the dTP which remained evoked during the cue 2 offset period (Tables 1 and 2). These overall percentages are consistent with other higher-order auditory regions, such as rostral superior temporal plane (Kikuchi et al. 2010) and prefrontal cortex (Plakke et al. 2013).
Delay.
Among the 21% of total recorded units showing activity changes during memory delays, the majority had significant activity present in only one 500-ms interval during the early, middle or late delay periods (Table 4); additionally, these changes were rather short-lived, intermittent, and rarely maintained at successively longer time intervals (Fig. 4, A and B). Delay-related activity revealed in dTP was different from that in higher-order visual cortical areas that sometimes show sustained delay activity along the ventral “what” pathway for visual object processing, as in ITC and vTP (Miyashita and Chang 1988; Miller and Desimone 1994; Nakamura and Kubota 1995, 1996; Woloszyn and Sheinberg 2009).
Table 4.
Percentage of units showing a significant activity change from pretrial firing rate, either increasing or decreasing, across early, middle and late periods of fixed 5-s delays during the delayed matching-to-sample task
| Trial Type | 1 Interval | 2 Intervals | 3 Intervals |
|---|---|---|---|
| Early delay | |||
| MC | 29* | 11* | 6 |
| MI | 39* | 6 | |
| NC | 50* | 8* | 4 |
| NI | 33* | 4 | 4 |
| Middle delay | |||
| MC | 34* | 11* | 3 |
| MI | 39* | 6 | |
| NC | 50* | 12* | |
| NI | 38* | 8* | |
| Late delay | |||
| MC | 51* | 9* | 3 |
| MI | 39* | 22* | |
| NC | 23* | 31* | |
| NI | 33* | 13* | 8* |
Each delay period consisted of 3 intervals of 500-ms intervals. Significant activity changes were either present in 1, 2 or 3 successive intervals for each delay period.
For a given task event, the percentage of dTP units evoked above chance (binomial tests, P ≤ 0.05). Data were pooled together across all sound stimuli for each recorded unit.
Fig. 4.
Various examples of dTP neurons responsive to memory delays and button-press responses during the memory task with activity temporally aligned with the onset of behavioral responses. Asterisks indicate significant activity change from pretrial firing rate for 100-ms or 500-ms intervals. Each bin is 100 ms. Units [no. 0426101a (A) and no. 1005091b (B)] showed limited and intermittent activity during three periods of fixed 5-s memory delays. Evoked activity change only lasted for fragments of 500- to 1,000-ms intervals for each trial type. Note that these units also encoded other task events, mainly associated with auditory cue events, wait and response periods. These two examples, as well as the rest of the data, suggested that significant activity change of dTP during memory delays may not necessarily indicate the memory trace of a perceived sound. Button-press responses (black arrows) were followed by food rewards (gray arrows) at MC trials, compared with when an erroneous response was made on NI trials and no reward was presented. A food reward followed the first button-press during MC trials by ∼200 ms. C: unit (no. 1028091a) showed increases in firing that immediately followed responses (at time = 0) on both MC and NI trials, indicating the evoked activity is associated with go responses. However, the increased firing was continuous and maintained only during MC trials, which result in food rewards, suggesting that this unit may encode behavioral outcomes including the rewarding event. D: unit (no. 0426101a) increased firing 500 ms before button-press responses and then decreased firing during MC trials. A combination of response and reward modulated the activity of this unit.
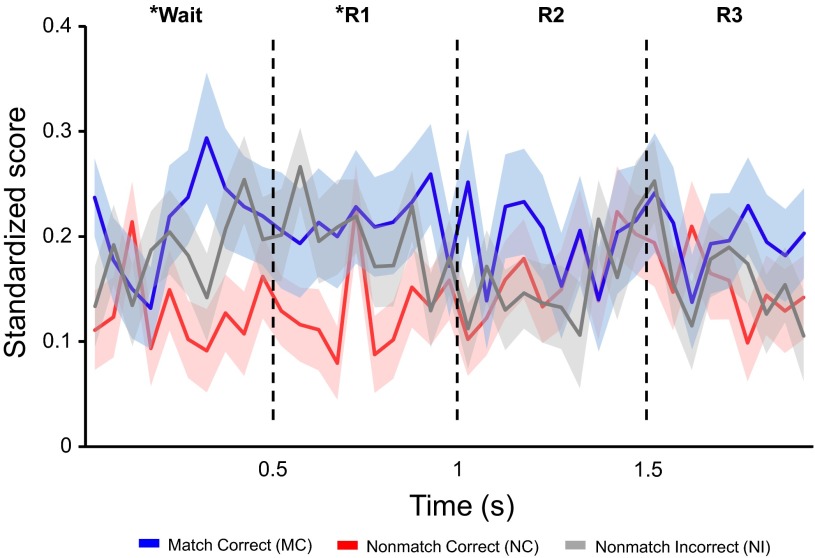
Wait and response.
During wait and response periods, about a quarter of units exhibited task-relevant activity at match-correct trials, relative to other trial types (binomial tests, P ≤ 0.05). This difference likely reflects a combination of factors e.g., the upcoming response preparation, button-press response, and the actual presence of reward after the subjects successfully responded to the match condition, relative to other trial types. Overall, spike activity was often low within dTP units during incorrect performance on match trials, which rarely occurred in these highly train subjects. This may be associated with a low level of attention on the subjects' part during the behavioral task.
The go/no-go learning rule of the memory task allowed the study to differentiate spike activity changes due to response, reward or both across match-correct (presence of response and reward), nonmatch-correct (absence of response and reward) and nonmatch-incorrect trials (presence of response only). Three example units, displayed in Fig. 3, all show significant changes in activity with regard to behavioral responses during match-correct and nonmatch-incorrect trials, and additionally reveal activity changes related to rewards present during match-correct trials only. The present study also examined if dTP activity was related to button-press responses during match-correct and nonmatch-incorrect trials. The firing rate of each unit was assessed in relationship to the exact timing of the button press. Intervals were taken from before and after button-press during four 500-ms intervals (preresponse period and postresponse periods 1, 2 and 3). Spike data were rearranged and aligned with the first button-press produced during match-correct trials (correct go-response) and nonmatch-incorrect trials (erroneous go-response on no-go trials). Although both trial conditions involved button pressing, there were more responsive units present for match-correct than for nonmatch-incorrect trials across the preresponse period and postresponse periods 1 and 2 (Table 5; binomial tests, P ≤ 0.05). This is congruent with the shorter response latencies on match-correct vs. nonmatch-incorrect trials (paired-sample t-tests, P < 0.001; Fig. 4, C and D) and is also consistent with the previous study using the same auditory DMS task with six monkeys (Ng et al. 2009). Differences in evoked activity during response events between match-correct and nonmatch-incorrect trials also suggest information coding of reward outcomes in dTP when subjects correctly identified two matching sound stimuli.
Table 5.
Percentage of units showing a significant activity change from pretrial firing rate, either increasing (+) or decreasing (−), during the occurrence of button pressing (MC and NI trials)
| Preresponse | Postresponse 1 | Postresponse 2 | Postresponse 3 | |
|---|---|---|---|---|
| MC | ||||
| + | 10 | 13 | 13 | 11 |
| − | 9 | 10 | 12 | 10 |
| Total | 19 | 23 | 25 | 21 |
| NI | ||||
| + | 6 | 8 | 6 | 6 |
| − | 3 | 3 | 4 | 3 |
| Total | 9 | 11 | 10 | 9 |
N = 225. The total dTP unit percentage is evoked above chance (binomial tests, P ≤ 0.05) for each given task event. Data were pooled together across all sound stimuli for each recorded unit.
Responsiveness across events.
In general, more than one-half of the responsive units encoded at least one out of the nine events, excluding delay, during the memory task. The majority of task-related units encoded 1–3 events (58%), others, 4–6 (30%), and less often, 7 or more (12%). When linking cue-related activity with the other three major task events (delay, wait and response), 16% of active units in dTP (N = 179; binomial tests, P ≤ 0.05) encoded both cue/response, and only 2% a combination of cue/delay or cue/wait. There were 6% and 8% of active units, respectively, encoding cue/wait/response or cue/delay/response combinations (binomial tests, P ≤ 0.05).
Sound-evoked activity of dTP.
Among all recorded units, nearly one-third were auditory responsive to at least one of the eight sound stimuli during passive listening (30%) or the memory task on cue 1 (34%). The number of auditory responsive units was distributed similarly across the eight sound types (3–9%), and the majority increased their firing rate from pretrial firing rate. These auditory units were selective as they mainly responded to only one of the eight stimuli (passive listening: 60%; DMS task: 83%). During pure tones presentations of the passive listening, only 20% were responsive to one pure-tone stimulus, and another 12% were responsive to two or more pure tones where the multiple frequencies that effectively evoked spike activity for a given unit were not contiguous and had many intervening frequencies between them. This small subpopulation also covered most of the frequencies presented (0.1–20 kHz), so that in this small region of cortex there was at least one neuron firing to each of the 21 pure tones even if they responded to multiple pure tones. During the memory task, only 7% of recorded units were responsive to pure tones. Three units were responsive to pure tones during both passive listening and the memory task, but even these responded to different frequency levels of pure tones during the separate tasks. Therefore, there is no clear evidence to suggest neurons of dTP exhibit stable frequency preferences in the current experiment.
Comparisons of sound-evoked activity across experimental contexts.
Of the 176 units held during both passive listening and the memory task, 54% were sound responsive; and 87% of this sound responsive population showed changes in response profiles by being responsive in only one of the experimental contexts or by responding to different sounds across the two contexts. Only 13% of units were consistently responsive to the same sound stimulus across contexts. The majority of auditory responsive units in dTP tended to respond to different sound stimuli when switching between experimental conditions perhaps flexibly encoding auditory information depending on the experimental context and possibly influenced by task difficulty, demand, and/or attention.
Population Analysis
Cue.
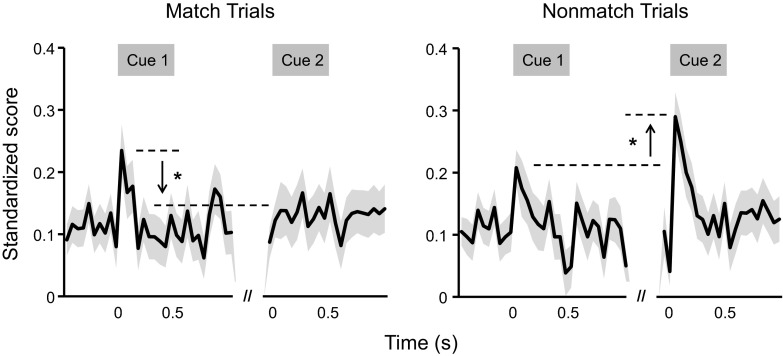
Population analyses of dTP units revealed discrete trial-type differences between cue 1 and cue 2 that primarily occurred in the first 100-ms interval (Fig. 5; see Supplemental Table S1 for full analyses details; supplemental materials are available online at the Journal website); therefore, further population analyses divided the first 100-ms period into two 50-ms intervals to focus on the dynamic portion of the cue presentation periods for dTP (Supplemental Table S2). During match trials, population activity was higher at cue 1 than cue 2 [main effect of cue: F(1, 2,143) = 5.77, P ≤ 0.05] with the opposite occurring on nonmatch trials [main effect of cue: F(1, 2,248) = 3.97, P ≤ 0.05; Fig. 6].
Fig. 5.
Population activity of 225 units across discrete task events for the three trial types. Underlined single asterisks denote significant activity change against pretrial firing rate during a 100-ms interval of cue presentation or cue offset. Double asterisks denote significant activity change against pretrial firing rate during a 500-ms interval of a target event (i.e., wait time, response periods R1, R2, and R3). Shaded areas behind the darker curve for each trial type illustrate the range of standard error for its respective averaged population mean (±1 standard error of mean). Standardized spike data shown on the graph were binned into 50-ms intervals. Time 0 denote the onset of a cue 1. Note that button-press responses were present during response period R1 of MC trials (correct go-response), which were followed by a food reward. Behavioral responses were also present during the NI trials (erroneous go-response). Note that population activity during match-incorrect (MI) trials was not significantly different from pretrial firing rate and had significantly less activity than the other three trial types. Population activity during MI is thus not depicted to facilitate visual comparison of the other three trial types on the same graph.
Fig. 6.
Cue effects associated with spike activity changes during the cue 1 and cue 2 presentations. Population spike activity of the two trial types was highlighted during cue presentations. Correct and incorrect trials were collapsed across match trials and nonmatch trials, respectively. Horizontal lines, arrows and asterisks denote significant differences between cue 1 and cue 2 during the first 100-ms interval of cue presentations. While suppression on population activity was present at cue 2 during match trials compared with cue 1, enhancement on population activity was found at cue 2 during nonmatch trials compared with cue 1.
To directly compare the trial types and determine response latency within the early part of the cue response, population activity during the first 90-ms was resampled into three 30-ms intervals for detailed analyses to capture the peak activity differences. Population data for match- and nonmatch-correct trials during the cue 1 presentation were combined (cue 1 - MCNC) due to similar increases in firing rate. Combined cue 1 were was compared with match- and nonmatch-correct trials during cue 2 (cue 2 - MC and cue 2 - NC) with paired-sample t-tests (Supplemental Table S3). At the second 30-ms interval of cue presentation (31–60 ms from cue start), cue 2 activity was higher on nonmatch trials compared with match (P = 0.019 for the adjusted critical probability level of 0.033; Fig. 7). These findings suggest that a significantly reduced population response was present upon identical sound presentations during match-correct trials (e.g., individual example in Fig. 7A), and 9% of the individually recorded units showed significantly reduced activity between cue 1 and cue 2. This phenomenon, sometimes termed match suppression, has been similarly observed in the visual object identification pathway (e.g., ITC and vTP) located ventrally of dTP (vTP: Nakamura and Kubota 1995, 1996; ITC: Baylis and Rolls 1987; Miller et al. 1993; Miller and Desimone 1994). This trial difference regarding the activity change during presentations of cue 2 occurred rapidly and transiently, within 31–60 ms of the start of cue 2. Mechanisms such as response fatigue within dTP itself cannot explain the present finding, as population activity differences between the two correct trial types did not occur until at least 31–60 ms from the start of cue 2 presentations; additionally, as is consistent with suppression effects in nonhuman primate visual studies (Liu et al. 2009), population trial differences were time-limited, ceasing within the first 100 ms of cue 2 presentations.
Fig. 7.

Match suppression effect upon identical sound presentations present in dTP during MC trial conditions. A: an example of a unit (no. 1028091c) showing match suppression when the monkey correctly identified two matching sounds. Asterisks and brackets denote significant activity difference between cue 1 and cue 2 (paired sample t-test, *P <0.05). Each interval is 100 ms. Red bars represent sound presentations, and black ticks represent action potentials in a raster plot of spike activity. The unit was responsive to four sound stimuli during the delayed matching-to-sample task: an animal vocalization, a human vocalization, a monkey vocalization, and a synthesized clip. Match suppression occurred when the two matching sound were an animal vocalization or a human vocalization, but not the other two sounds. Compared with visual studies showing match suppression (e.g., Miller et al. 1993; Nakamura et al. 1994; Nakamura and Kubota, 1995), the present study does not use a priori selection criterion, which involves assessing the most effective stimuli. This may account for the selective nature of the suppression response at the level of individual units. B: detailed analysis of population activity (N = 225) during cue 2 between trials of MC, NC and NI. The brackets and asterisks denote a significant trial difference in discrete 30-ms intervals (P < 0.033). Results suggest that active suppression may be present during presentations of cue 2 during MC trials. The effect was very early and transient during the cue 2 presentations, within 31–60 ms from cue onset.
A detailed analysis, similar to that of cue 2 directly above, was also used to clarify trial type differences at cue 2 offset, where changes occurred toward the end of the interval (Supplemental Table S2). All spike data during the last 90 ms of cue 2 offset were resampled into 30-ms time intervals for match-correct, nonmatch-correct, and nonmatch-incorrect trials. Activity at match-correct trials was higher than the two nonmatch trial types during the first and last 30-ms intervals (paired-sample t-tests, P = 0.022 for the adjusted critical probability level of 0.033; Supplemental Table S3).
There were also significant differences between the activity on correct and incorrect trials (Supplemental Tables S1 and S2). In general, activity was higher on correct trials than incorrect trials, particularly during cue 1 with the opposite occurring during cue 2 offset. This suggested that robust encoding of the first cue may be important for correct trial performance; however, no significant correlation with correct trial types was observed (Pearson correlation with critical probability levels of 0.05). Another striking difference was that spike firing during match-incorrect trials was low and irregular across trials within a session compared with the other three trial types, with nearly 80% of total units associated with this trial type nonresponsive to task events on match-incorrect trials. This low firing-rate activity may suggest a lack of attention during these trials and is in sharp contrast with the activity pattern on the nonmatch-incorrect trials on which the subjects make an erroneous behavioral response and activity is closer to match-correct trials.
Delay.
Population activity was not significantly different from pretrial firing rate during the memory delay period for any of the four trial types individually (one-way ANOVAs). However, population analyses on trial type showed that activity during the delay is significantly higher on incorrect trials than correct trials [repeated-measures ANOVAs; within-subject factors: delay periods (early, middle and late) and time intervals (three 500 ms per interval per period)] for match trials [F(2, 20,398) = 14.43, P ≤ 0.05] and nonmatch trials [F(2, 20,398) = 14.18, P ≤ 0.05] respectively (Supplemental Table S4).
Wait and response.
After processing the cue 2 presentations during the cue and offset periods, the subject was required to wait to make a response during the possible response and reward periods. Evoked activity change was associated with the involvement of behavioral responses and reward during wait and response periods. During match-correct trials [F(4, 56,249) = 14.85], increased spike activity was present for all wait and response intervals. For the nonmatch-correct and nonmatch-incorrect trials, significant activity change was found at both the wait period and two out of three response periods during nonmatch-correct trials [F(4, 56,249) = 3.77, P ≤ 0.05] and nonmatch-incorrect trials [F(4, 56,249) = 9.03, P ≤ 0.05]. There was no significant activity change from pretrial firing rate in any wait or response periods during match-incorrect trials.
To examine effects of choice behavior and reward, four events (wait period and response periods 1–3) and trial type were used as within-subject and between-subject factors, respectively, for repeated-measure ANOVAs. Overall, trials associated with behavioral responses (match-correct and nonmatch-incorrect trials) robustly evoked stronger population activity of dTP than those without (match-incorrect and nonmatch-correct trials). The resultant effects occurred as early as the wait period and extended into some portion of the three response periods. Activity during match-correct trials at the wait period and response periods 1 and 2 was higher than that of match-incorrect [trial × event interaction: F(3, 61,194) = 2.65, P ≤ 0.05], but for nonmatch-correct trials the effect stopped after response period 1 [trial × event interaction: F(3, 61,194) = 8.01, P ≤ 0.05; Fig. 8].
Fig. 8.
Trial effects associated with population activity change during wait and response periods. Each event was 500 ms long, and data shown on the graph were binned into 50-ms intervals. Button-press responses were followed by food rewards at MC trials, compared with when an erroneous response was made on NI trials and no reward was presented. Asterisks denote a significant increase in firing rate for particular 500-ms intervals during MC and NI trials (blue and black lines), relative to NC trials (red line) and MI trials (data not shown). During both the wait and response period 1, results show that population activity for trials associated with the presence of button-press responses (i.e., MC and NI) was higher than those without (i.e., MI and NC). Note that population activity during MI trials was not significantly different from the pretrial firing rate and had significantly less activity than the other three trial types. Population activity during MI is thus not depicted to facilitate visual comparison of the other three trial types on the same graph.
Sound type.
Population analysis of dTP units across the eight different sound types was conducted by combining population responses during cue 1 presentations between match-correct and nonmatch-correct trials, as there was no difference in population response to cue 1 between these two trial types. In repeated-measures ANOVAs, sound type was the between-subject factors, and interval (five 100-ms intervals) was the within-subject factors. There was a main effect of interval [F(4, 71,968) = 10.82] showing that population activity during the first 100-ms interval of combined cue 1 presentations was the highest among the entire interval of cue presentations (paired-sample t-tests, two-tailed, P < 0.001). There was also a main effect of sound type [F(7, 17,992) = 2.35, P ≤ 0.05]. Responses to human vocalizations were significantly higher than those of natural sounds and synthesized clips (paired-sample t-tests, two-tailed, P = 0.011), and pure tones or white noises were significantly higher than those of synthesized clips (paired-sample t-tests, two-tailed, P = 0.013). When the eight sound types were reorganized into simple (i.e., pure tones) vs. complex (the rest of the seven types) based on their acoustical features and properties, population analysis did not reveal any significant difference between simple and complex sound types.
A mixture of coos, grunts, harmonic arches, warbles, shrill barks and screams composed the sound stimuli for the monkey vocalization sound type. Population analysis was examined among these subtypes of vocalizations, because dTP may be crucial for socio-emotional processing in humans and monkeys (Olson et al. 2007; Zahn et al. 2007). The initial analysis did not reveal any significant effect of monkey vocalization subtypes (repeated-measures ANOVAs with three trial types: combined cue 1 across match- and nonmatch-correct trials, cue 2 of match-correct trials, and cue 2 of nonmatch-correct trials), but significant findings could have been obscured by a wide range of biological/ethological significances of stimulus subtypes (e.g., from food-related scenarios, social grooming to agonistic encounters between monkeys). A population analysis of coos vs. screams only, representing highly divergent communication subtypes in terms of social and ethological significance (positive vs. negative valence), showed that dTP activity during screams was significantly higher than during coos at cue 2 during match-correct trials [F(1,728) = 8.96, P ≤ 0.05]. Future studies will need to assess responses to hetero-specific calls and perhaps backward or scrambled calls to ascertain the significance of this observed difference.
DISCUSSION
Neurons in dTP encode auditory stimuli and a number of memory task events, i.e., decision wait time, response period, and reward delivery; however, evoked activity during memory delays was limited within the region. With nearly one-third of dTP units responsive to cue presentations across trial types, auditory encoding is a robust feature of dTP with population activity during the sample sound associated with correct memory performance. Neural dTP encoding of the sample sound, and other task-related neural correlates in dTP in addition to recognition memory correlates, are likely to be crucial for mediating auditory analysis and identification facilitating accurate recognition memory performance.
In the DMS paradigm, sustained delay activity and modulated activity on identical stimulus presentations (match suppression or enhancement) are often present in the visual nervous system and are considered neural correlates of working and recognition memory, respectively (Desimone 1996; Miller et al. 1996; Nakamura and Kubota 1996). Here the study reveals that reduced population activity during a repeated sound presentation (i.e., cue 2 during match trials) was present, and the resultant magnitude was significantly lower than that when the animals correctly distinguished two different sounds (i.e., nonmatch-correct trials). Similar to vTP and ITC along the visual object identification pathway (Baylis and Rolls 1987; Miller et al. 1993; Miller and Desimone 1994; Nakamura and Kubota 1995; Persson et al. 2002; Woloszyn and Sheinberg 2009), dTP shows match suppression on identical sound presentations, suggesting a robust signature of recognition memory within the auditory nervous system. Humans also exhibit reduced activity in anterior superior temporal gyrus when subjects passively listened to repeated stimuli or performed short-term memory tasks with verbal stimuli (Buchsbaum and D'Esposito 2009; Buchsbaum et al. 2011; Dehaene-Lambertz et al. 2006). Suppression on auditory cortical responses is also revealed in human auditory cortex during a nonverbal, auditory DMS task, relative to passive listening and number-counting conditions. Cortical suppression occurs during the late delay period and the first 100 ms of the second sound presentation, and the effect is enhanced when using sounds with increased acoustic structure (Rong et al. 2011). For any given trial of the current task, match suppression on identical sound stimuli may indicate that a recent, repeated sound is familiar within a single trial context and is more quickly and efficiently processed than a relatively novel or different sound during nonmatch trials, sometimes referred to as bottom-up processing (Desimone 1996; Grill-Spector et al. 2006). Although mechanisms underlying reduced activity patterns are still under debate (Liu et al. 2009; McMahon and Olson 2007; Persson et al. 2002; Sawamura et al. 2006). Additionally, there was an increase in dTP activity to cue 2 when a different sound was presented on nonmatch trials, which may be an additional processing pattern to differentiate between match and nonmatching trials.
Compared with higher-order visual areas, the memory-processing component of dTP is largely reflected by its match suppression effect rather than its intermittent activity during delay periods, which is far less substantial and reliable than in vTP and ITC during visual working memory paradigms (Miller and Desimone 1994; Miyashita and Chang 1988; Nakamura and Kubota 1995, 1996; Woloszyn and Sheinberg 2009). Approximately one-fifth of dTP units exhibit delay-related activity changes; however, there is a lack of consistent spike activity during the delay period correlated with behavioral performance, and sustained delay period activity is rare (seldom lasting longer than 1.5 s) with no significant population response above pretrial firing rate. In addition, increased population activity during memory delays at incorrect trials, regardless of trial types, may suggest a neural correlate associated with poor information encoding, failure to attend to the DMS task or a combination of both. The nature of delay-related activity in the auditory nervous system of nonhuman primates remains unclear. Few studies show sustained delay activity at delays longer than 1 s in the primary auditory cortex of monkeys and rats (e.g., Gottlieb et al. 1989; Sakurai 1994). In an acoustic flutter discrimination task, neuronal activity of A1 in monkeys is related to stimulus rate of acoustic flutter; although sustained, memory-related activity is not present during the 3-s delay periods (Lemus et al. 2009). Neurons of ITC and vTP, by contrast, show sustained delay activity when monkeys performed a visual DMS task (Miller and Desimone 1994; Nakamura and Kubota 1996; Woloszyn and Sheinberg 2009), wherein one-third of vTP units show robust, steady delay firing activity up to 5 s correlated with response selectivity of visual stimuli and correct memory performance (Nakamura and Kubota 1995). In humans, imaging studies demonstrate better, substantial evidence of auditory memory-related activation in the superior temporal gyrus. Gamma-band responses during memory delays of an auditory DMS task are correlated with “preferred” and “nonpreferred” sound stimuli and sometimes linked to performance accuracy of human subjects (Brechmann et al. 2007; Kaiser et al. 2008; Leiberg et al. 2006; Lutzenberger et al. 2002). Stimulus-specific activations in the superior temporal gyrus are suggested to be stimulus-relevant maintenance for short-term memory (Kaiser et al. 2009).
The use of auditory stimuli with no a priori assessment of the neurons' preference, although typical during visual studies, may account for this difference. Other possibilities include distributed encoding patterns of memory-related activity (Zhou et al. 2007), or activity patterns during the delay may instead be related to task progression. Significant dTP population response during the memory delay period immediately preceding the test stimulus suggests possible temporal processing of trial information. On a functional level, less robust memory performances and shorter forgetting thresholds for auditory stimuli compared with visual have been observed in nonhuman primates (Buffalo et al. 1999; D'Amato and Colombo 1985; Fritz et al. 2005; Scott et al. 2012; Wright 1998, 1999). The observed transient, intermittent delay-related dTP activity may be a potential factor related to less robust behavioral performance on auditory recognition tasks and lowered performance at longer delays (Funahashi 2006). On the other hand, delay activity revealed in ITC is more susceptible to intervening stimuli presented during visual memory delays compared with prefrontal cortices (Desimone 1996); therefore, sustained activation may not be the optimal mechanism to maintain memory traces in these higher-order sensory regions, reflecting instead an attention/expectation aspect of objects relevant to a behaviorally engaging task. The prefrontal cortex may also be a potential candidate for auditory encoding that maintains a cue-induced memory trace during delay periods (Bodner et al. 1996; Desimone 1996; Funahashi 2006; Fuster and Jervey 1982; Miller et al. 1996; Plakke et al. 2013). Current findings suggest that evoked activity of dTP during memory delays of an auditory DMS task may not necessarily link to the memory trace of a perceived sound. Whether or not a weak capacity exists for maintaining a task-relevant cue, the present study suggests encoding differences for delay activity in dTP relative to visual studies, but not in recognition memory encoding.
A combination of decision-making processes and behavioral responses play a significant role in dTP activity modulation, as single-unit and population analyses yielded robust activity changes during wait and response periods. The go/no-go rule with asymmetric reinforcement contingency allows evaluation of distinct effects for button-press responses (match-correct and nonmatch-incorrect trials) and food rewards (match-correct trials only) with activity of many dTP units robustly strengthened or weakened by food rewards. Increased population activity of dTP is always present in trials when button-press responses were produced, regardless of correct or incorrect performance. The increased activity of dTP may occur immediately after presentations of a test stimulus (i.e., cue 2 offset and/or wait periods during match trials), in which decision-making is initiated and followed by a button-press response. Reward expectation and outcome also complementarily influence population encoding of dTP during the auditory DMS task.
Prior studies consistently show influence of response behavior and reward contingency robustly modulating neuronal activity of other auditory primary cortical areas in nonhuman primates. The primary auditory cortex exhibit response-related activity associated with bar press and release in monkeys (Brosch et al. 2005). These neuronal activities are sometimes correlated to choice behavior when animal subjects correctly discriminate auditory stimuli based on changes in amplitude-modulation (Niwa et al. 2012). Neuronal activity of the primary auditory cortex and posterior belt fields is remarkably modulated by reward size, reward expectance and mismatch between expected and delivered reward (Brosch et al. 2011). Positive and negative reinforcement notably increase or decrease neuronal activity of A1 when ferrets discriminate between noise and pure tone in the same task with different behavioral contingencies (David et al. 2012). Evidence of task-relevant activity during reward and response periods suggest a top-down feedback to the auditory nervous system, perhaps facilitating auditory learning and memory. The present findings also reveal that dTP shows differential population responses across the eight sound types not explained by the purely acoustical properties of the sounds (e.g., simple vs. complex), but in some instances may contrast the ethological significance (e.g., coos vs. screams). The human temporal pole is sensitive to auditory and visual stimuli associated with social and/or emotional content (Jimura et al. 2009; Liu et al. 2007; Royet al. 2000; Zahn et al. 2007), and lesions to primate temporal pole, amygdala, and/or orbitofrontal cortex induce symptoms of Kluver-Bucy syndrome (Olson et al. 2007). A neural network connecting these three areas may therefore mediate information processing of reward outcome and emotion (Barbas et al. 1999; Höistad and Barbas 2008; Reser et al. 2009; Saleem et al. 2008). With some connections between dTP and striatum (Kondo et al. 2003), motor information as well as reward information may be transmitted to dTP and mediate goal-directed behaviors. Present findings parallel the proposed role of temporal pole in processing socio-emotional information and reward outcome (Olson et al. 2007). During auditory object processing, temporal pole neurons may help in facilitating recognition and retrieval using episodic or emotional details to familiar stimuli.
This first memory survey of auditory responsive units in dTP suggests these neurons are highly stimulus selective, parallel to a recent study reporting auditory selectivity within the adjacent rostral superior temporal plane (Kikuchi et al. 2010). Compared with Kikuchi et al. (2010), present electrode placements are more rostral, mainly within TGdd and TGdg, with no rostro-caudal or medial-lateral differences in dTP response profiles. Our recent study (Ng et al. 2010) suggests that dTP neurons do not faithfully encode specific frequencies, when the same animal subjects passively listened to a set of 129 sounds, ranging from pure tones, band-passed noises to human and monkey vocalizations. Although some neurons respond to multiple pure tones, these frequency levels are often far apart from each other and are not adjacent, as might be expected in traditional auditory processing regions (Kaas and Hackett 2000). Although our study did not specifically aim to assess dTP spike activity to visual objects or images, no units responded to the lighted response button used on all trials for signaling the possible response period, suggesting this area may be auditory selective. Future recording studies using a variety of sensory stimuli will elucidate dTP sensory selectivity.
Among the auditory responsive units, many of their response-encoding profiles were modified across experimental contexts but remained consistent within either the passive presentations or the memory task. Units sometimes encoded additional stimuli when the memory task started, or dropped some or all of the sounds to which they previously responded, or switched firing preferences to different sound stimuli. These findings suggest that the majority of dTP neurons show flexible encoding across contexts and may be influenced by task demand, complexity, and/or attention. Effects of context on the auditory system have been documented to discern whether behavioral engagement influences neuronal activity of auditory primary regions. Tasks requiring attention and behavioral responses from animals robustly modulated, either by increasing or decreasing spike activity changes compared with passive conditions (Otazu et al. 2009; Scott et al. 2007; Sutter and Shamma 2011). These current observations that the memory task evokes more dTP neurons than the passive listening context in a region beyond secondary auditory cortex processing is supported by previous findings in primary cortical regions. This finding also contrasts with those for visual stimuli and recordings in vTP in which preferences and firing patterns are more stable, and normally an a priori criterion is often applied to use preferred visual stimuli; whereas, in the present study, sound stimuli and the memory task were presented to every neuron identified.
Understanding of recognition memory is historically based on studies that have examined behavioral and neural correlates of memory functions from the visual nervous system in monkeys and humans. Interactions between higher-order visual cortical areas and the medial temporal lobe system govern how universal models for neural substrates of memory functions would extend across other sensory modalities. However, the sensory nature of stimulus quality is different between the visual and auditory modalities; in the latter, temporal components become pivotal for auditory processing (Wang et al. 2008). Auditory functions manifest in ways that are both behaviorally and neuronally different from their visual counterparts in the short-delay memory domain; yet there are strong similarities between all other aspects of recognition memory, i.e., cue, match suppression, decision, reward, and response encoding, albeit processed in different cortical regions. Continued examination of these sensory processing pathways will provide a more unified theory of recognition memory and stimulus processing, which are critical subcomponents of communication processing.
GRANTS
This work was supported by funding awarded to A. Poremba from University of Iowa Startup Funds and National Institute of Deafness and Other Communication Disorders Grant DC-0007156.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.W.N., B.P., and A.P. conception and design of research; C.W.N. performed experiments; C.W.N. analyzed data; C.W.N. interpreted results of experiments; C.W.N. prepared figures; C.W.N. drafted manuscript; C.W.N. and A.P. edited and revised manuscript; C.W.N., B.P., and A.P. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Mortimer Mishkin, Dr. Richard Saunders, and Megan Malloy for invaluable support and contribution to this research.
REFERENCES
- Andics A, McQueen JM, Petersson KM, Gál V, Rudas G, Vidnyánszky Z. Neural mechanisms for voice recognition. Neuroimage 52: 1528–1540, 2010 [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol 410: 343–367, 1999 [DOI] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET. Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Exp Brain Res 65: 614–622, 1987 [DOI] [PubMed] [Google Scholar]
- Belin P. Voice processing in human and non-human primates. Philos Trans R Soc Lond B Biol Sci 361: 2091–2107, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Ahad P. Human temporal-lobe response to vocal sounds. Brain Res Cogn Brain Res 13: 17–26, 2002 [DOI] [PubMed] [Google Scholar]
- Bigelow J, Poremba A. Auditory memory in monkeys: costs and benefits of proactive interference. Am J Primatol 75: 425–434, 2013 [DOI] [PubMed] [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport 7: 1905–1908, 1996 [DOI] [PubMed] [Google Scholar]
- Brechmann A, Gaschler-Markefski B, Sohr M, Yoneda K, Kaulisch T, Scheich H. Working memory specific activity in auditory cortex: potential correlates of sequential processing and maintenance. Cereb Cortex 17: 2544–2552, 2007 [DOI] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci 25: 6797–6806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Representation of reward feedback in primate auditory cortex. Front Syst Neurosci 5: 5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. Repetition suppression and reactivation in auditory-verbal short-term recognition memory. Cereb Cortex 19: 1474–1485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Padmanabhan A, Berman KF. The neural substrates of recognition memory for verbal information: spanning the divide between short- and long-term memory. J Cogn Neurosci 23: 978–991, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem 6: 572–599, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641, 1995 [DOI] [PubMed] [Google Scholar]
- Colombo M, D'Amato MR, Rodman HR, Gross CG. Auditory association cortex lesions impair auditory short-term memory in monkeys. Science 247: 336–338, 1990 [DOI] [PubMed] [Google Scholar]
- Colombo M, Gross CG. Responses of inferior temporal cortex and hippocampal neurons during delayed matching to sample in monkeys (Macaca fascicularis). Behav Neurosci 108: 443–455, 1994 [DOI] [PubMed] [Google Scholar]
- Colombo M, Rodman HR, Gross CG. The effects of superior temporal cortex lesions on the processing and retention of auditory information in monkeys (Cebus apella). J Neurosci 16: 4501–4517, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato MR, Colombo M. Auditory matching-to-sample in monkeys (Cebus apella). Anim Learn Behav 13: 375–382, 1985 [Google Scholar]
- David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci U S A 109: 2144–2149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Anton JL, Campagne A, Ciuciu P, Dehaene GP, Denghien I, Jobert A, Lebihan D, Sigman M, Pallier C, Poline JB. Functional segregation of cortical language areas by sentence repetition. Hum Brain Mapp 27: 360–371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A 93: 13494–13499, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci 4: 2051–2062, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson JH, III, Cowey A, Weiskrantz L. Disruptions of auditory sequence discrimination by unilateral and bilateral cortical ablations of superior temporal gyrus in the monkey. Exp Neurol 28: 529–548, 1970 [DOI] [PubMed] [Google Scholar]
- Dewson JH, III, Pribram KH, Lynch JC. Effects of ablations of temporal cortex upon speech sound discrimination in the monkey. Exp Neurol 24: 579–591, 1969 [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol 514: 595–623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Mishkin M, Saunders RC. In search of an auditory engram. Proc Natl Acad Sci U S A 102: 9359–9364, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience 139: 251–261, 2006 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Jervey JP. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J Neurosci 2: 361–375, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol 221: 169–184, 1983 [DOI] [PubMed] [Google Scholar]
- Gottlieb Y, Vaadia E, Abeles M. Single unit activity in the auditory cortex of a monkey performing a short term memory task. Exp Brain Res 74: 139–148, 1989 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10: 14–23, 2006 [DOI] [PubMed] [Google Scholar]
- Hackett TA. Information flow in the auditory cortical network. Hear Res 271: 133–146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol 394: 475–495, 1998 [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J Neurophysiol 56: 683–701, 1986 [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science 226: 75–76, 1984 [DOI] [PubMed] [Google Scholar]
- Höistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage 40: 1016–1033, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Comparison of superior temporal and inferior prefrontal lesions on auditory and non-auditory tasks in rhesus monkeys. Brain Res 55: 355–367, 1973 [DOI] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Miyashita Y. Temporal pole activity during perception of sad faces, but not happy faces, correlates with neuroticism trait. Neurosci Lett 453: 45–48, 2009 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A 97: 11793–11799, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Heidegger T, Wibral M, Altmann CF, Lutzenberger W. Distinct gamma-band components reflect the short-term memory maintenance of different sound lateralization angles. Cereb Cortex 18: 2286–2295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Decker C, Wibral M, Rahm B. Task- and performance-related modulation of domain-specific auditory short-term memory representations in the gamma-band. Neuroimage 46: 1127–1136, 2009 [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook (2nd Ed.). Englewood Cliffs, NJ: Prentice-Hall, 1982, chapt. 8 [Google Scholar]
- Kikuchi Y, Horwitz B, Mishkin M. Hierarchical auditory processing directed rostrally along the monkey's supratemporal plane. J Neurosci 30: 13021–13030, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 493: 479–509, 2005 [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 465: 499–523, 2003 [DOI] [PubMed] [Google Scholar]
- Kupfer K, Jurgens U, Ploog D. The effect of superior temporal lesions on the recognition of species-specific calls in the squirrel monkey. Exp Brain Res 30: 75–87, 1977 [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 132: 3401–3410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiberg S, Kaiser J, Lutzenberger W. Gamma-band activity dissociates between matching and nonmatching stimulus pairs in an auditory delayed matching-to-sample task. Neuroimage 30: 1357–1364, 2006 [DOI] [PubMed] [Google Scholar]
- Lemus L, Hernández A, Romo R. Neural codes for perceptual discrimination of acoustic flutter in the primate auditory cortex. Proc Natl Acad Sci U S A 106: 9471–9476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci 27: 4587–4597, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Murray SO, Jagadeesh B. Time course and stimulus dependence of repetition-induced response suppression in inferotemporal cortex. J Neurophysiol 101: 418–436, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of “what” and “where” processing in auditory cortex. Nat Neurosci 11: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Ripper B, Busse L, Birbaumer N, Kaiser J. Dynamics of gamma-band activity during an audiospatial working memory task in humans. J Neurosci 22: 5630–5638, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primate's temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol 242: 425–458, 1985 [DOI] [PubMed] [Google Scholar]
- McMahon DB, Olson CR. Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. J Neurophysiol 97: 3532–3543, 2007 [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science 263: 520–522, 1994 [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science 254: 1377–1379, 1991 [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci 13: 1460–1478, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6: 414–417, 1983 [Google Scholar]
- Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature 331: 68–70, 1988 [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol 256: 88–103, 1987 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Nagumo S, Kubota K, Fukuda H, Ito K, Kojima S. Neural substrates for recognition of familiar voices: a PET study. Neuropsychologia 39: 1047–1054, 2001 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J Neurophysiol 74: 162–167, 1995 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res 77: 53–77, 1996 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto K, Mikami A, Kubota K. Visual response properties of single neurons in the temporal pole of behaving monkeys. J Neurophysiol 71: 1206–1221, 1994 [DOI] [PubMed] [Google Scholar]
- Ng CW, Plakke B, Opheim R, Poremba A. Neuronal population encoding of auditory recognition memory within the primate temporal polar cortex. Program No. 405.2 In: 2010 Neuroscience Meeting Planner (Online) San Diego, CA: Society for Neuroscience http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=6e90c8b2-e1be-437b-bf63–8614a983bcdc&cKey=41781f47-c7ee-46a8-a419–1f888860b477&mKey={E5D5C83F-CE2D-4D71–9DD6-FC7231E090FB} 2010 [Google Scholar]
- Ng CW, Plakke B, Poremba A. Primate auditory recognition memory performance varies with sound type. Hear Res 256: 64–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Johnson JS, O'Connor KN, Sutter ML. Activity related to perceptual judgment and action in primary auditory cortex. J Neurosci 32: 3193–3210, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130: 1718–1731, 2007 [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci 12: 646–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8: 976–987, 2007 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1999 [Google Scholar]
- Persson J, Habib R, Nyberg L. Decreased activity in inferotemporal cortex during explicit memory: dissociating priming, novelty detection, and recognition. Neuroreport 13: 2181–2185, 2002 [DOI] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Green S, Marler PR, Moody DB, Stebbins WC. Neural lateralization of vocalizations by Japanese macaques: communicative significance is more important than acoustic structure. Behav Neurosci 98: 779–790, 1984 [DOI] [PubMed] [Google Scholar]
- Plakke B, Ng CW, Poremba A. Neural correlates of auditory recognition memory in primate lateral prefrontal cortex. Neuroscience 244: 62–76, 2013 [DOI] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey's temporal poles. Nature 427: 448–451, 2004 [DOI] [PubMed] [Google Scholar]
- Poremba A, Mishkin M. Exploring the extent and function of higher-order auditory cortex in rhesus monkeys. Hear Res 229: 14–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Saunders RC, Crane AM, Cook M, Sokoloff L, Mishkin M. Functional mapping of the primate auditory system. Science 299: 568–572, 2003 [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci 12: 718–724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser DH, Burman KJ, Richardson KE, Spitzer MW, Rosa MG. Connections of the marmoset rostrotemporal auditory area: express pathways for analysis of affective content in hearing. Eur J Neurosci 30: 578–592, 2009 [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol 93: 734–747, 2005 [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol 403: 141–145, 1999 [DOI] [PubMed] [Google Scholar]
- Rong F, Holroyd T, Husain FT, Contreras-Vidal JL, Horwitz B. Task-specific modulation of human auditory evoked response in a delayed-match-to-sample task. Front Psychol 2: 85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci 20: 7752–7759, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Ackelson AL, Baker AE, Cohen YE. Coding of auditory-stimulus identity in the auditory non-spatial processing stream. J Neurophysiol 99: 87–95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. J Neurosci 14: 2606–2623, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol 506: 659–693, 2008 [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain. San Diego, CA: Academic, 2007 [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron 49: 307–318, 2006 [DOI] [PubMed] [Google Scholar]
- Scott BH, Malone BJ, Semple MN. Effect of behavioral context on representation of a spatial cue in core auditory cortex of awake macaques. J Neurosci 27: 6489–6499, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BH, Mishkin M, Yin P. Monkeys have a limited form of short-term memory in audition. Proc Natl Acad Sci U S A 109: 12237–12241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter ML, Shamma SA. The relationship of auditory cortical activity to perception and behavior. In: The Auditory Cortex, edited by Winer JA, Schreiner CE. New York: Springer, 2011, p. 617–641 [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annu Rev Neurosci 19: 109–139, 1996 [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology 20: 1–10, 2006 [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience 157: 484–494, 2008 [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Mishkin M. Effects of temporal and frontal cortical lesions on auditory discrimination in monkeys. Brain 81: 406–414, 1958 [DOI] [PubMed] [Google Scholar]
- Woloszyn L, Sheinberg DL. Neural dynamics in inferior temporal cortex during a visual working memory task. J Neurosci 29: 5494–5507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA. Auditory list memory and interference processes in monkeys. J Exp Psychol Anim Behav Process 25: 284–296, 1999 [PubMed] [Google Scholar]
- Wright AA. Auditory list memory in rhesus monkeys. Psychol Sci 9: 91–98, 1998 [Google Scholar]
- Yeterian EH, Pandya DN. Thalamic connections of the cortex of the superior temporal sulcus in the rhesus monkey. J Comp Neurol 282: 80–97, 1989 [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A 104: 6430–6435, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Ardestani A, Fuster JM. Distributed and associative working memory. Cereb Cortex 17: i77–i87, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.