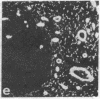
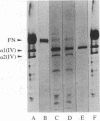
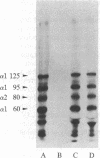
Abstract
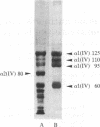
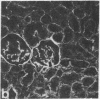
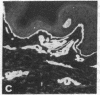
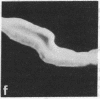
Monoclonal antibodies (mAbs) have been prepared against type IV collagen isolated from human kidney. Two mAbs, designated CIV 22 and CIV 16, were extensively characterized. CIV 22 reacted only with native type IV collagen, whereas CIV 16 also bound to fragments derived from the alpha 1(IV) chain after reduction and alkylation of the molecule. Therefore, CIV 22 recognizes a conformational epitope on the triple helical type IV collagen, whereas CIV 16 binds to a sequential determinant in the carboxyl-terminal half of the alpha 1(IV) chain. By immunofluorescence, typical basement membrane structures were stained with both mAbs on frozen sections of different human organs. The mAbs were used to investigate the chain composition of type IV collagen. Radiolabeled type IV collagen bound to CIV 22, proving its triple helical configuration. These native probes, containing both the alpha 1(IV) and the alpha 2(IV) chains, also bound to CIV 16. Since CIV 16 does not react with the isolated alpha 2(IV) chain, both chains must be arranged in a single triple helical molecule (heterotrimer).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Vaheri A., Krieg T., Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980 Aug;109(1):247–255. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Mainardi C. L., Beachey E. H., Kang A. H. 7S domain constitutes the amino-terminal end of type IV collagen: an immunochemical study. Coll Relat Res. 1983 May;3(3):263–269. doi: 10.1016/s0174-173x(83)80008-9. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Seyer J. M., Kang A. H. Biochemical and immunochemical characterization and internal alignment of pepsin-derived collagenous fragments of the alpha 1(IV) chain from bovine kidney cortices. J Biol Chem. 1982 May 10;257(9):4864–4868. [PubMed] [Google Scholar]
- Dixit S. N. Type-IV collagens. Isolation and characterization of two structurally distinct collagen chains from bovine kidney cortices. Eur J Biochem. 1980 May;106(2):563–570. doi: 10.1111/j.1432-1033.1980.tb04604.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fitch J. M., Gibney E., Sanderson R. D., Mayne R., Linsenmayer T. F. Domain and basement membrane specificity of a monoclonal antibody against chicken type IV collagen. J Cell Biol. 1982 Nov;95(2 Pt 1):641–647. doi: 10.1083/jcb.95.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch J. M., Mayne R., Linsenmayer T. F. Developmental acquisition of basement membrane heterogeneity: type IV collagen in the avian lens capsule. J Cell Biol. 1983 Sep;97(3):940–943. doi: 10.1083/jcb.97.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foellmer H. G., Madri J. A., Furthmayr H. Methods in laboratory investigation. Monoclonal antibodies to type IV collagen: probes for the study of structure and function of basement membranes. Lab Invest. 1983 May;48(5):639–649. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbisa S., Liotta L. A., Tryggvason K., Siegal G. P. Antibodies to collagenase-resistant terminal regions of pro-type IV collagen recognize whole basement membrane and 7 S collagen. FEBS Lett. 1981 May 18;127(2):257–262. doi: 10.1016/0014-5793(81)80219-0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Heathcote J. G., Orkin R. W. Current concepts of basement-membrane structure and function. Biosci Rep. 1981 Nov;1(11):819–842. doi: 10.1007/BF01114816. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Fitch J. M., Schmid T. M., Zak N. B., Gibney E., Sanderson R. D., Mayne R. Monoclonal antibodies against chicken type V collagen: production, specificity, and use for immunocytochemical localization in embryonic cornea and other organs. J Cell Biol. 1983 Jan;96(1):124–132. doi: 10.1083/jcb.96.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Mayne R., Sanderson R. D., Wiedemann H., Fitch J. M., Linsenmayer T. F. The use of monoclonal antibodies to fragments of chicken type IV collagen in structural and localization studies. J Biol Chem. 1983 May 10;258(9):5794–5797. [PubMed] [Google Scholar]
- Mayne R., Wiedemann H., Irwin M. H., Sanderson R. D., Fitch J. M., Linsenmayer T. F., Kühn K. Monoclonal antibodies against chicken type IV and V collagens: electron microscopic mapping of the epitopes after rotary shadowing. J Cell Biol. 1984 May;98(5):1637–1644. doi: 10.1083/jcb.98.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Risteli J., Schuppan D., Glanville R. W., Timpl R. Immunochemical distinction between two different chains of type IV collagen. Biochem J. 1980 Nov 1;191(2):517–522. doi: 10.1042/bj1910517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Furthmayr H. A new method of iodinating collagens for use in radioimmunoassay. Anal Biochem. 1979 Jul 15;96(2):489–499. doi: 10.1016/0003-2697(79)90611-0. [DOI] [PubMed] [Google Scholar]
- Sage H., Woodbury R. G., Bornstein P. Structural studies on human type IV collagen. J Biol Chem. 1979 Oct 10;254(19):9893–9900. [PubMed] [Google Scholar]
- Sakai L. Y., Engvall E., Hollister D. W., Burgeson R. E. Production and characterization of a monoclonal antibody to human Type IV collagen. Am J Pathol. 1982 Sep;108(3):310–318. [PMC free article] [PubMed] [Google Scholar]
- Scheinman J. I., Tsai C. Monoclonal antibody to type IV collagen with selective basement membrane localization. Lab Invest. 1984 Jan;50(1):101–112. [PubMed] [Google Scholar]
- Schuppan D., Timpl R., Glanville R. W. Discontinuities in the triple helical sequence Gly-X-Y of basement membrane (type IV) collagen. FEBS Lett. 1980 Jun 30;115(2):297–300. doi: 10.1016/0014-5793(80)81191-4. [DOI] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V., Schmidt J., Häring P. High frequencies of antigen-specific hybridomas: dependence on immunization parameters and prediction by spleen cell analysis. J Immunol Methods. 1980;32(3):297–304. doi: 10.1016/0022-1759(80)90194-5. [DOI] [PubMed] [Google Scholar]
- Sundarraj N., Willson J. Monoclonal antibody to human basement membrane collagen type IV. Immunology. 1982 Sep;47(1):133–140. [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Wick G., Gay S. Antibodies to distinct types of collagens and procollagens and their application in immunohistology. J Immunol Methods. 1977;18(1-2):165–182. doi: 10.1016/0022-1759(77)90168-5. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Gröbli B., Spiess M., Odermatt B. F., Winterhalter K. H. Basement membrane (type IV) collagen is a heteropolymer. J Biol Chem. 1982 May 10;257(9):5239–5245. [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- von Der Mark K., Ocalan M. Immunofluorescent localization of type V collagen in the chick embryo with monoclonal antibodies. Coll Relat Res. 1982 Nov;2(6):541–555. doi: 10.1016/s0174-173x(82)80008-3. [DOI] [PubMed] [Google Scholar]