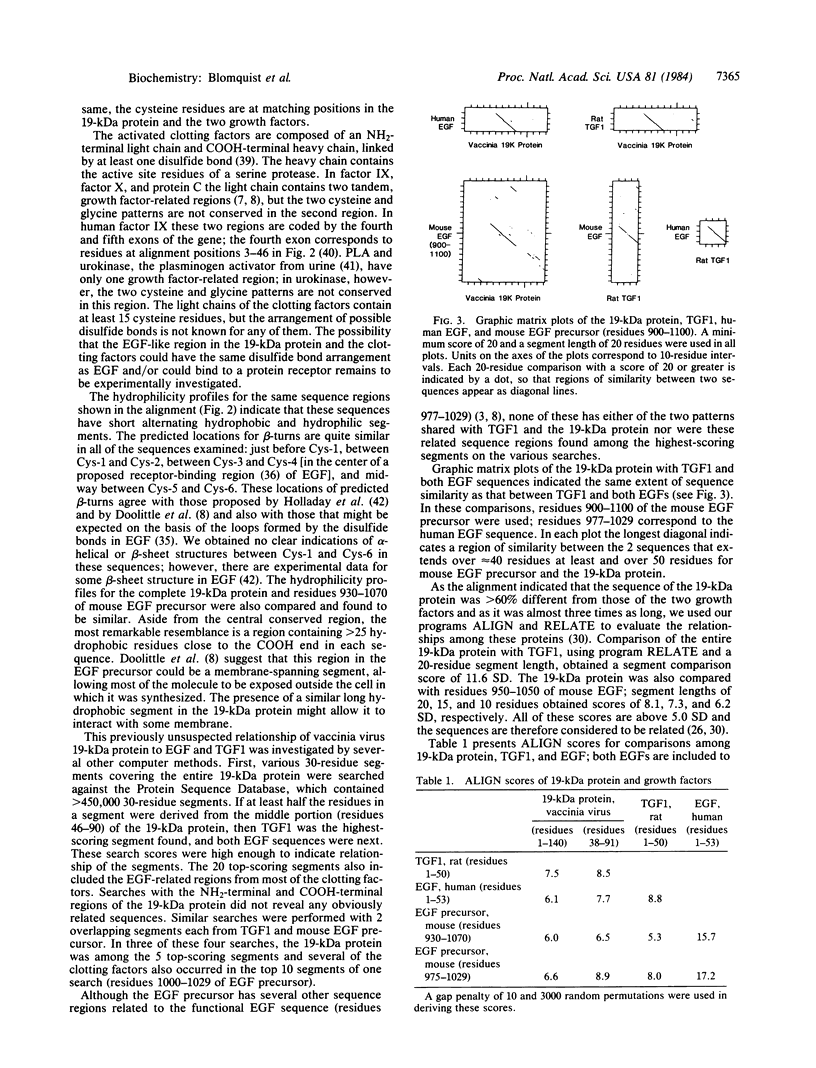
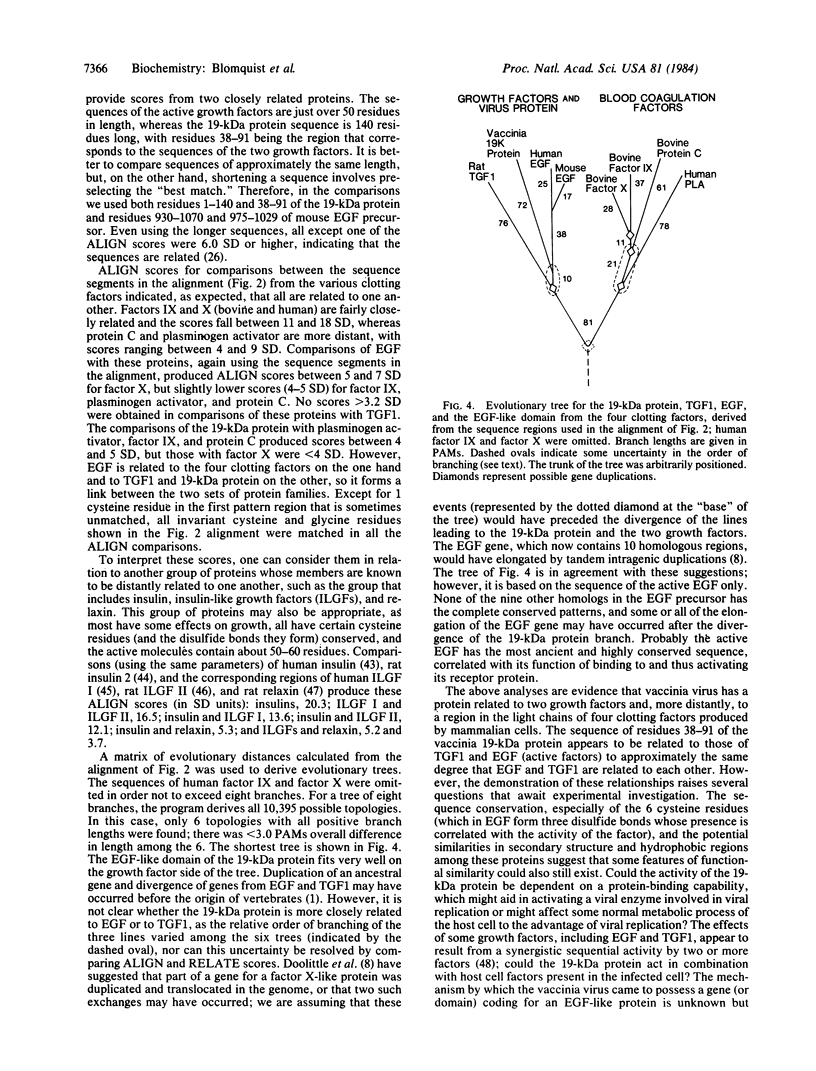
Abstract
We observed two unusual patterns of cysteine and glycine residues in transforming growth factor type 1 (TGF1); our computer search found only one other protein, the 19-kilodalton early protein of vaccinia virus, having both patterns. The sequences of epidermal growth factor (EGF) and of the light chains from several components of the blood coagulation system also have one of the patterns, but gaps are required to adjust conserved cysteine and glycine residues in the second pattern. We used several computer analyses to confirm these relationships; the 19-kilodalton protein appears to be related to TGF1 and EGF to the same degree that they are related to each other; all three are more distantly related to the coagulation factors. An evolutionary scheme is presented for these proteins. We suggest that the conservation of cysteine residues, which form the disulfide bonds present in the active EGF molecule, may extend to conservation of disulfide bonds in these other proteins. We also suggest that the structural similarities may be correlated with a protein-binding capability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O. The origin and evolution of protein superfamilies. Fed Proc. 1976 Aug;35(10):2132–2138. [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Fujikawa K., Walsh K. A., Neurath H., Titani K. Amino acid sequence of the light chain of bovine factor X1 (Stuart factor). Biochemistry. 1980 Feb 19;19(4):659–667. doi: 10.1021/bi00545a009. [DOI] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Amino acid sequence of the light chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12170–12179. [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin. The 29,000-dalton NH2-terminal domain. J Biol Chem. 1983 Oct 25;258(20):12670–12674. [PubMed] [Google Scholar]
- George D. G., Yeh L. S., Barker W. C. Unexpected relationships between bacteriophage lambda hypothetical proteins and bacteriophage T4 tail-fiber proteins. Biochem Biophys Res Commun. 1983 Sep 30;115(3):1061–1068. doi: 10.1016/s0006-291x(83)80043-6. [DOI] [PubMed] [Google Scholar]
- Gregory H., Preston B. M. The primary structure of human urogastrone. Int J Pept Protein Res. 1977;9(2):107–118. doi: 10.1111/j.1399-3011.1977.tb03470.x. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Savage C. R., Jr, Cohen S., Puett D. Conformation and unfolding thermodynamics of epidermal growth factor and derivatives. Biochemistry. 1976 Jun 15;15(12):2624–2633. doi: 10.1021/bi00657a023. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P., Haley J., Cronk M., Shine J., Niall H. Molecular cloning and characterization of cDNA sequences coding for rat relaxin. Nature. 1981 May 14;291(5811):127–131. doi: 10.1038/291127a0. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Cellular responsiveness to growth factors correlates with a cell's ability to express the transformed phenotype. Cell. 1983 Jul;33(3):931–938. doi: 10.1016/0092-8674(83)90036-3. [DOI] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoriya A., Hortsch M., Meyers C., Smith M., Kanety H., Schlessinger J. Biologically active synthetic fragments of epidermal growth factor: localization of a major receptor-binding region. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1351–1355. doi: 10.1073/pnas.81.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J., Henderson L. E., Oroszlan S. Purification and primary structure of a polypeptide with multiplication-stimulating activity from rat liver cell cultures. Homology with human insulin-like growth factor II. J Biol Chem. 1981 Jul 10;256(13):6859–6865. [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W., Sasagawa T., Howald W. N., Kwa E. Y., Weinstein B. Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid. Biochemistry. 1983 Jun 7;22(12):2875–2884. doi: 10.1021/bi00281a016. [DOI] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper N. Instability and reiteration of DNA sequences within the vaccinia virus genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1614–1618. doi: 10.1073/pnas.78.3.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOL D. S., SMITH L. F. Amino-acid sequence of human insulin. Nature. 1960 Aug 6;187:483–485. doi: 10.1038/187483a0. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E., Sundelin J., Lind P., Peterson P. A. The cell attachment domain of fibronectin. Determination of the primary structure. J Biol Chem. 1982 Aug 25;257(16):9593–9597. [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Savage C. R., Jr, Hash J. H., Cohen S. Epidermal growth factor. Location of disulfide bonds. J Biol Chem. 1973 Nov 25;248(22):7669–7672. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Yarden Y., Schlessinger J. A non-mitogenic analogue of epidermal growth factor enhances the phosphorylation of endogenous membrane proteins. Biochem Biophys Res Commun. 1981 Jul 30;101(2):517–523. doi: 10.1016/0006-291x(81)91290-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P. Amino acid sequence of the heavy chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12180–12190. [PubMed] [Google Scholar]
- Titani K., Fujikawa K., Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H. Bovine factor X1 (Stuart factor): amino-acid sequence of heavey chain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3082–3086. doi: 10.1073/pnas.72.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]



