Abstract
Strong and consistent evidence exists that physical activity reduces breast cancer risk by 10-25 %, and several proposed biologic mechanisms have now been investigated in randomized, controlled, exercise intervention trials. Leading hypothesized mechanisms relating to postmenopausal breast cancer include adiposity, endogenous sex hormones, insulin resistance, and chronic low-grade inflammation. In addition, other pathways are emerging as potentially important, including those involving oxidative stress and telomere length, global DNA hypomethylation, immune function, and vitamin D exposure. Recent exercise trials in overweight/obese postmenopausal women implicate weight loss as a mechanism whereby exercise induces favorable changes in circulating estradiol levels and other biomarkers as well. Still it is plausible that some exercise-induced biomarker changes do not require loss of body fat, whereas others depend on abdominal fat loss. We highlight the latest findings from randomized, controlled trials of healthy postmenopausal women, relating exercise to proposed biomarkers for postmenopausal breast cancer risk.
Keywords: Breast cancer, Postmenopausal women, Randomized trials, Physical activity, Exercise, Weight loss, Adiposity, Biomarkers, Sex hormones, Estrogen, Biomechanisms
Introduction
There is convincing epidemiologic evidence that body fatness, and probable evidence that adult weight gain, are associated with an increased risk of postmenopausal breast cancer [1]. One meta-analysis demonstrated that every 5 kg/m2 increase in body mass index (BMI) increases postmenopausal breast cancer risk by 12 % on average [2] with possible variation by tumor subtype [3]. In addition, after a breast cancer diagnosis, survival rates are decreased with higher BMI [4] by as much as 30 % [5].
It is of great interest, therefore, to prospectively study the effects of weight control on breast cancer risk and survival in overweight or obese postmenopausal women [6]. Yet first, understanding the contributions of energetic factors—i.e., physical activity and diet—is needed to determine the optimal weight control intervention. Regular physical activity is a widely accepted health-promoting behavior that is recommended for cancer prevention [1, 7], but the type and dose of activity that is optimal for postmenopausal breast cancer prevention remains unclear.
Physical activity and breast cancer
Convincing epidemiologic evidence suggests that physical activity of moderate-to-vigorous intensity reduces breast cancer risk by 10-25 % on average relative to inactivity [8•, 9] The dose of activity required for breast cancer prevention is unclear, but across observational studies, risk generally decreases with higher physical activity duration [8•] and intensity [9]. For cancer prevention overall, public health recommendations advise at least 30 minutes of moderate-intensity activity equivalent to brisk walking every day [1] or 30 minutes or more of moderate-to-vigorous activity at least 5 days per week [7] for adults.
Biomarker studies in healthy women
Given clear inverse relations between physical activity and future postmenopausal breast cancer risk, lifestyle modification for inactive women holds promise for breast cancer prevention. Whereas randomized, controlled trials (RCTs) examining breast cancer outcomes would best inform an exercise prescription, these trials have not been conducted because of the large sample size and time required for a prospective study. A more feasible approach is to study the impact of lifestyle change on breast cancer biomarkers using a RCT [10]. The number of exercise RCTs studying proposed biomarkers for breast cancer has escalated during the past 10 years, shedding light on: 1) exercise prescriptions that might impact breast cancer risk, and 2) underlying biologic mechanisms.
We provide an update on the epidemiologic evidence relating exercise to proposed biomarkers for postmenopausal breast cancer risk, without dietary modification. This review enhances our earlier reviews [8, 11] by focusing on the strongest, most up-to-date epidemiologic evidence (from randomized trials) relating exercise to estrogens and adiposity, the two most convincing biomarkers of postmenopausal breast cancer risk. We also update our biologic model that relates physical activity to breast cancer by incorporating newly hypothesized biomarkers. Our goal is to guide future clinical and mechanistic research surrounding physical activity and postmenopausal breast cancer prevention. The focus of this review will be on healthy women in whom biomarker profiles and the types and effects of prescribed exercise may differ from breast cancer survivors. Biomarker studies in breast cancer survivors are reviewed elsewhere [12•, 13].
Epidemiologic evidence relating exercise to proposed biomarkers
To simplify our discussion of the existing epidemiologic evidence, we have classified proposed biomarkers of breast cancer risk as “convincing” or “hypothesized.” Our classification of estrogens and adiposity as convincing biomarkers is based on the relatively strong and consistent body of epidemiologic evidence relating these markers to postmenopausal breast cancer risk. We summarize that evidence and describe in more detail the effects of exercise on estrogen levels (by systematically reviewing RCT evidence) and adiposity (citing recent reviews and large RCTs), specifically in postmenopausal women. We then provide a high-level overview of other, hypothesized biomarkers of postmenopausal breast cancer risk and possible relations with exercise.
Systematic review of RCTs relating exercise to estrogens
In September 2013, we searched the published literature (PubMed-NIH) for RCTs that studied the impact of exercise on estrogens. In brief, we identified all RCTs of long-term exercise (≥4 weeks) that compared exercise-only to a nonexercise control group in healthy postmenopausal women. Studies exclusive to hormone replacement therapy users were excluded as were studies in morbidly obese women (BMI >40 kg/m2) and trained athletes.
Convincing biomarkers for postmenopausal breast cancer risk
Adiposity
Body fatness is an accepted, convincing biomarker for increased postmenopausal breast cancer risk in healthy women [1, 2]. Multiple interrelated biologic pathways could mediate the association between adiposity and postmenopausal breast cancer, with sex hormones, insulin resistance, and low-grade chronic inflammation as leading hypotheses [14•, 15]. Furthermore, central adiposity may be particularly important. Recently in postmenopausal women, independently of BMI, waist circumference was positively associated with breast cancer risk [16] and abdominal fat was related to sex hormone bioavailability [17•], which is a strong biomarker of breast cancer risk. Therefore, with respect to postmenopausal breast cancer, there may be more benefit from exercise prescriptions that can effectively lower abdominal fat.
Exercise is publicly recommended for modest weight loss, for prevention of weight gain in overweight and obese adults, and for prevention of weight regain after weight loss [18, 19]. Exercise trials typically produce <3 % weight loss in adults, although more might be achieved with higher volumes of exercise, e.g., the American College of Sports Medicine recently proposed >250 minutes per week at moderate intensity [18]. However exercise-induced weight loss could vary by age. In a prospective, observational study of 58,610 postmenopausal women, whether body weight was lost, maintained, or gained with high levels of physical activity generally depended on the age of the women at baseline [20]. Also, exercise type could be relevant, e.g., aerobic may be preferable to resistance exercise with respect to weight loss [18] and lowering total abdominal fat [21•] in overweight adults. Indeed, the largest exercise RCTs of moderate-vigorous aerobic exercise in healthy postmenopausal women all showed decreases in intra-abdominal fat [22, 23] or waist circumference [24•, 25, 26] and overall body fat [22, 23, 24•, 25].
Sex hormones
Higher levels of endogenous estrogens and androgens and lower levels of circulating sex hormone binding globulin (SHBG) are related to an increased risk of postmenopausal breast cancer [27]. In addition, associations between postmenopausal breast cancer risk and hormone replacement therapy use [28] and effective use of antiestrogenic drugs to prevent breast cancer [29] firmly support a causal role for estrogens. Estrogens can decrease apoptosis and act as mitogens in the breast via estrogen receptor binding; moreover, oxidative estrogen metabolites act as mutagenic, genotoxic agents possibly contributing to breast cancer initiation [30].
A total of nine exercise-only RCTs in postmenopausal women that studied changes in estrogen-related biomarkers for breast cancer were identified in our systematic review of published literature (Table 1). The number of non-HRT users assigned to exercise-only or control groups ranged from 16 [31] to 320 [32]. All study populations were overweight or obese on average, and all but one RCT of women 65+ years [33] studied younger postmenopausal women with mean ages from 54–61 years. Roughly half of the trials, comprising the four largest RCTs [32, 34, 35, 36••] and one smaller RCT [37], involved 12-month interventions, whereas the remainder were 12 [31, 33] or 16 [38, 39] weeks duration. Most interventions were aerobic [32, 33, 35, 36••, 38] or combined aerobic/resistance training [34, 37]; two small RCTs focused on resistance training [31, 39]. Exercise prescriptions ranged from 150–225 minutes/week (except [31] where minutes per week were not reported) and were generally moderate-vigorous intensity (i.e., 60-85 % maximum heart rate).
Table 1.
Summary of randomized controlled trials of long-term exercise that studied estrogen changes in cancer-free, postmenopausal women
| Trial name/reference, country | Sample sizea | Study participants | Intervention arm prescription | Comparison group(s) |
|---|---|---|---|---|
| Figueroa et al., 2003, USA [37] |
n = 24 EX; n = 28 CTL |
• Mean body fat, 39 %; mean body weight, 67–71 kg • Inactive • No HRT use (HRT users analyzed separately) • Age 40–65 yr; mean, 57 yr |
• 12 mo • 60–75 min/day, 3 days/wk, supervised • Resistance and weight-bearing aerobic exercise • 7 resistance exercises, 2 sets @ 70-80 % 1-RM + 25 min aerobic exercise @ 50-80 % HRmax |
Maintained usual level of physical activity |
| Copeland et al., 2004, Canada [31] |
n = 8 EX; n = 8 CTL |
• Mean BMI 26 kg/m2 (EX); 32 kg/m2 (CTL) • No regular exercise in past year • No HRT use (HRT users analyzed separately) • Mean age, 54 yr |
• 12 wk • 3 days/wk, supervised • Resistance training • By 1 month, progressed to 8 exercises @ 3 sets,10 repetitions each |
Flexibility exercises 3 days/wk, unsupervised |
| Physical Activity for Total Health Study, USA [35] |
n = 87 EX; n = 86 CTL |
• BMI 25–40 kg/m2, mean 30 kg/m2; body fat >33 % • Previously <60 min/week exercise that caused sweating • No hormone use past 6 mo • Age 50–75 yr; mean, 61 yr • 86 % non-Hispanic white |
• 12 mo • 45 min/day, 5 days/wk (supervised and home-based) • Aerobic exercise • 60-75 % HRmax by wk 8 |
Stretching controls |
| Orsatti et al., 2008, Brazil [39] |
n = 22 EX; n = 21 CTL |
• Mean BMI 28–29 kg/m2, mean body fat 33-36 % • No previous leisure activity besides household • No hormone therapy past 6 mo • Age 40–70 yr; mean, 58–59 yr |
• 16 wk preceded by 4-wk low-load adaptation period • 50–60 min/day, 3 days/week, supervised • Resistance training • 8 exercises @ 3 sets, 8–12 repetitions each, 60-80 % 1-RM |
Asked not to change exercise habits |
| Sex Hormones and Physical Exercise (SHAPE) study, the Netherlands [34] |
n = 96 EX; n = 93 CTL |
• BMI 22–40 kg/m2, mean 27 kg/m2; mean body fat 40-41 % • <2 hr/wk moderate sport/recreational activity and not adherent to international physical activity recommendations • No HRT use past 6 mo • Age 50–69 yr; mean, 58–59 yr |
• 12 mo • 60 min/day, 2 days/wk supervised group session + 30 min/week home-based individual session • Supervised sessions: aerobic (20 min @ 60-85 % HRmax) and strength training (25 min) + warm-up, cool-down • Home-based sessions: brisk walking or cycling @ 60-80 % HRmax (30 min) |
Asked to retain habitual exercise patterns |
| Alberta Physical Activity and Breast Cancer Prevention (ALPHA) trial, Canada [32] |
n = 160 EX; n = 160 CTL |
• BMI 22–40 kg/m2, mean 29 kg/m2 • <90 min/wk recreational activity or if between 90–120 min/wk had maximal oxygen uptake <34.5 mL/kg/min • No hormone use • Age 50–74 yr; mean, 61 yr • 91 % white race |
• 12 mo • 45 min/day, 5 days/wk (supervised and home-based) • Aerobic exercise, mainly walking or cycling • At least half of each workout @ 70-80 % heart rate reserve; achieved by wk 12 |
Maintained usual level of activity |
| Yoo et al., 2010, South Korea [33] |
n = 11 EX; n = 10 CTL |
• Mean BMI, 25–27 kg/m2 • No hormone use • Age >65 yr; mean age 71 yr |
• 12 wk • 60 min/day, 3 days/wk, supervised • 45-min walking with two 1-kg ankle weights; 10-min warm-up + 5-min cool-down |
Asked to maintain usual physical activity routine |
| Kim et al., 2012, South Korea [38] |
n = 15 EX; n = 15 CTL |
• Mean BMI, 25 kg/m2; >32 % body fat, mean 36 % • <20 min exercise twice weekly • No hormone use • Mean age 54 yr |
• 16 wk • 60 min/day, 3 days/wk, supervised • Aerobic exercise • Line dancing, attained 70-80 % HRmax by wk 12 |
No exercise |
| Nutrition and Exercise for Women (NEW) Trial, USA [36••] |
n = 117 EX; n = 87 CTL; n = 118 DIET; n = 117 DIET + EX |
• BMI >25.0 kg/m2, mean 30.9 kg/m2, mean body fat 47.2 % • Moderate-intensity physical activity <100 min/week • No hormone use past 3 months • Age 50–75 yr; mean, 58 yr • 85 % non-Hispanic white |
• 12 mo • ≥ 45 min/day, 5 days/wk (3 supervised and 2 home-based) • Aerobic exercise with metabolic equivalent ≥ 4 •70-85 % HRmax for 45 min by wk 7 |
1)Reduced-calorie weight loss diet 2)Combined reduced-calorie weight loss diet + aerobic exercise 3)Requested not to change exercise or dietary habits |
Table 2 summarizes results for estradiol, the most biologically potent estrogen [15], and also for estrone and SHBG from RCTs in Table 1. Findings on other estrogen-related biomarkers are described below. Nearly all of the reports in our systematic review described circulating hormone levels [31–35, 36••, 37–39] with single articles describing analysis of urine [40] and adipose tissue [41]. Results across RCTs were remarkably consistent, generally showing average decreases in sex hormones and increases in SHBG levels in exercise groups, typically <10 % in magnitude. Yet only a few primary analyses demonstrated statistically significant differences between exercise and control groups with respect to change in total estradiol [32], free estradiol [32, 35], estrone [35, 36••], or SHBG [32, 38]. No statistically significant group differences were found in primary analyses of testosterone or androstenedione [31–34, 36••, 37, 39, 42]. In the SHAPE trial, however, a significant intervention effect was found for testosterone and androstenedione (decreased levels in exercisers versus controls) for the subgroup who lost >2 % body fat [34]. The Physical Activity for Total Health study [40] showed no significant differences after 12 months between exercise (n = 87) and control groups (n = 86) for changes in 2-hydroxyestrone, 16α-hydroxyestrone, or their ratio in urine. In an ancillary study of the NEW trial (n = 45) [41], subcutaneous adipose tissue was analyzed for expression of 82 candidate genes related to adipokines, proinflammatory cytokines, and sex hormones. Combining women from all four trial arms, greater weight loss after 6 months was associated with decreased gene expression related to estrogen biosynthesis, e.g., 17β-hydroxysteriod dehydrogenase 1, which converts estrone to estradiol.
Table 2.
Evidence from randomized controlled trials relating exercise to estrogens in healthy postmenopausal womena
| Proposed biomarker | Average biomarker change for exercise-only groupb | Evidence of adiposity change as a potential mediator of sex hormone change | Study reference |
|---|---|---|---|
| Circulating estradiol | −9.5%c; NS at 13 wk | ----- | [31] |
|
−7.7 %; NS at 3 mo −4.4 %; NS at 12 mo |
Stronger decreases in exercisers who lost 0.5 % + body fat | [35] | |
| No change at 12 mo | ----- | [37] | |
| −8 %; NS over 12 mo |
−11.7 % at 12 mo in exercise group who lost >2 % body fat Change in estradiol was significantly associated with change in % body fat |
[34] | |
| −12 % at 12 mo; p = 0.004 over 12 mo |
Exercise effect was slightly attenuated but remained significant after statistical adjustment for body weight change Statistical tests for mediation implied mediation by change in % body fat or total body fat, but not intra-abdominal fat |
[32, 54•] | |
| +20.5 % c; NS at 3 mo | ----- | [33] | |
| −4.9 %, NS at 12 mo |
The decrease in the DIET + EX group (−20.3 %) was significantly greater than for the EX group; p < 0.001 Significantly greater differences between change in EX versus CTL across subgroups of increasing weight loss (p-trend < 0.01) |
[36••] | |
| Circulating free estradiol |
−8.2 %, p = 0.02 at 3 mo −6.1 %; NS at 12 mo |
Stronger decreases in exercisers who lost 0.5 % + body fat | [35] |
| +7.9 % c; NS at 16 wk | ----- | [39] | |
| −7.3 %; NS over 12 mo |
−11.4 % at 12 mo in exercise group who lost >2 % body fat Change in free estradiol was significantly associated with change in %body fat |
[34] | |
| −12.9 % at 12 mo; p = 0.001 over 12 mo |
Exercise effect was slightly attenuated but remained significant after statistical adjustment for body weight change Statistical tests for mediation implied mediation by change in % body fat, total body fat, intra-abdominal fat |
[32, 54•] | |
| −4.7 %, NS at 12 mo |
The decrease in the DIET + EX group (−26 %) was significantly different than for the EX group; p < 0.001 Significantly greater differences between change in EX versus CTL across subgroups of increasing weight loss (p-trend = 0.001) |
[36••] | |
| Circulating estrone |
−3.8 %, p = 0.03 at 3 mo −1.8 %; NS at 12 mo |
Stronger decreases in exercisers who lost 0.5 % + body fat | [35] |
| No change at 12 mo | ----- | [37] | |
| −9.7 %; NS over 12 mo |
−23.7 % at 12 mo in exercise group who lost >2 % body fat Change in estrone was significantly associated with change in % body fat |
[34] | |
| −5.4 %; NS over 12 mo | Remained NS after statistical adjustment for body weight change | [32] | |
| −5.5 %, p = 0.01 at 12 mo |
The decrease in the DIET + EX group (−11.1 %) was significantly greater than for the EX group; p = 0.01 Significantly greater differences between change in EX versus CTL across subgroups of increasing weight loss (p-trend < 0.01) |
[36••] | |
| Circulating sex hormone binding globulin (SHBG)d |
+5.7 %; NS at 3 mo +8.8 %; NS at 12 mo |
Stronger increases in exercisers who lost 0.5 % + body fat | [35] |
| −0.7 %; NS over 12 mo | +2.0 % at 12 mo in exercise group who lost >2 % body fat | [34] | |
| +3.2 % at 12 months; p = 0.001 over 12 mo |
Effect of exercise was no longer statistically different from controls after statistical adjustment for body weight change Statistical tests for mediation implied mediation by % body fat, total body fat, intra-abdominal fat |
[32, 54•] | |
| +6.2%c; p < 0.001 at 16 wk | ----- | [38] | |
| −0.7 %, NS at 12 mo |
The change in the DIET + EX group (+25.8 %) was significantly different than for the EX group; p < 0.001 Greater difference between change in EX versus CTL groups with more weight loss, but p-trend NS |
[36••] |
aRCTs of long-term exercise-only interventions with results exclusively for healthy postmenopausal women
bNS indicates a nonstatistically significant difference between the change in the exercise group versus the control group
cPercent change in the exercise group was not reported in the article and therefore was approximated from published results, as follows:
[average sex hormone level at follow-up – average sex hormone level at baseline] / [average sex hormone level at baseline] x 100 %
dSHBG binding decreases estradiol and testosterone bioavailability
A mediating role for weight loss in the causal pathway between exercise and decreased estrogen levels is plausible given that adipose tissue is the primary source of endogenous estrogens post-menopause [15]. Evidence of mediation by adiposity change, described in Table 2, generally supports this hypothesis. Moreover, in the NEW trial, substantially greater decreases in total and free estradiol levels occurred, on average, within the diet + exercise arm than with exercise-only (−20.3 % vs. −4.9 % for estradiol; −26 % vs. −4.7 % for free estradiol) [36••]. In a smaller weight loss trial of obese postmenopausal women, a 14 % average decrease in total fat mass was associated with a 24 % decrease in estradiol levels in breast ductal fluid [43•]. In a recent prospective study, a 12.7 % decrease in total estradiol was estimated for every 1 kg/m2 decrease in BMI in 84 postmenopausal women who lost weight [44]. Interestingly in another recent study of 1,180 postmenopausal women [17•], waist circumference and waist-to-hip ratio—independently of BMI—were associated with circulating SHBG, free estradiol, and free testosterone levels, implicating abdominal fat as a specific target for breast cancer prevention.
Hypothesized biomarkers of postmenopausal breast cancer risk
Insulin resistance
Insulin resistance, characterized by hyperinsulinemia, is a major predictor of diabetes risk and of possible etiologic importance in breast cancer [45, 46]. Insulin receptor binding promotes mitosis and antiapoptotic effects in breast cancer cells, and also tumor cell migration and tumor-associated angiogenesis [47]. In addition, chronically elevated insulin can enhance estrogen bioactivity and promote activities of breast cancer-related adipokines [47] and IGF-1 [14•]. Metformin, a pharmacologic agent that improves insulin sensitivity, is undergoing clinical testing for improved breast cancer survival [14•, 48].
In the United States, at least 150 minutes per week of moderate-vigorous aerobic and resistance exercise is recommended for diabetes prevention in prediabetics [49]. In larger RCTs of postmenopausal women, long-term aerobic exercise groups experienced average decreases of approximately −4 % to −10.3 % in insulin [50, 51, 52•, 53], −2 % to −11.4 % in homeostatic model of assessment for insulin resistance (HOMA-IR) [50, 51, 52•], −3.7 % in C-peptide [52•], and a small decrease [53] or negligible change in circulating glucose [50, 51, 52•]. However, in the NEW trial [52•], HOMA-IR decreased an average of −24 % and −26 % in the diet and diet + exercise intervention groups, respectively, after 12 months, suggesting strongly that improved whole-body insulin sensitivity in the exercise-only group (−8.6 % decrease in HOMA-IR) resulted from weight loss and not exercise per se. Secondary analyses of ALPHA trial data [54•] implied partial mediation by change in intra-abdominal fat area, which is of etiologic relevance to insulin resistance [55], and by total (%) body fat but also mediation by other unidentified factors as well.
Changes in insulin sensitivity could vary with different exercise prescriptions. In large RCTs of postmenopausal women, the greatest improvement in HOMA-IR was found with aerobic exercise >225 minutes/week [50] and >130 minutes/week [51]. In the NEW trial, 225 minutes/week of prescribed aerobic exercise was associated with a regression to normal fasting glucose levels for those with impaired glucose tolerance at baseline [52•]. Resistance training could provide distinct benefits for glycemic control by altering the quality and quantity of skeletal muscle [56]. However, in an RCT comparing 8 months of aerobic versus resistance exercise in 155 overweight adults [21•], only aerobic exercise reduced HOMA and visceral fat. Similarly a RCT of obese postmenopausal women showed essentially no change in average insulin levels after 12 weeks of low-intensity resistance training [57].
Adipokines
Adipose tissue is an active endocrine organ, secreting bioactive factors known as adipokines [58] at abnormal levels in obesity. Some adipokines (TNF-α, IL-6) are mediators of a low-grade, systemic inflammatory state that is characteristic of obesity [59]. Adipokines, such as leptin, TNF-α, and IL-6, could mediate breast cancer development and progression directly by acting as mitogens in the breast, inhibiting apoptosis, and influencing tumor cell migration and invasion [15, 60]. They also may act indirectly, e.g., by enhancing estrogen bioactivity and promoting insulin resistance [15]. Conversely, adiponectin is an adipokine that occurs at lower levels in obesity and is anti-mitogenic, anti-inflammatory, and promotes insulin sensitivity. Epidemiologic findings relating adipokines in circulation to increased postmenopausal breast cancer risk are generally mixed, but suggestive, for increased leptin [61–63], decreased adiponectin [62–64], and a decreased adiponectin:leptin ratio [62]. There is weaker epidemiologic evidence of etiologic roles for IL-6 [65], TNF-α [63, 66], and the inflammatory marker C-reactive protein (CRP) [62, 65], produced in response to TNF-α and IL-6.
RCT findings overall imply that exercise in conjunction with sufficient weight loss can decrease circulating leptin [50 , 51, 67•] and perhaps IL-6 [43•, 68•] in postmenopausal women. Exercise-related decreases in CRP have been found in some RCTs of postmenopausal women [69–71], but not all [26], and in others, only with concurrent weight loss [68•, 72]. The Dose–response to Exercise in postmenopausal Women (DREW) study compared three doses of aerobic exercise (4, 8, or 12 kcal/kg/week at 50 % VO2max), but revealed no difference with respect to CRP change in exercisers versus controls over 6 months [72]. Six RCTs of postmenopausal women consistently showed no change in adiponectin levels, on average, with exercise alone [50, 53, 57, 67•, 69, 73]. Furthermore, in primary analyses from multiple RCTs there was no reported effect of exercise-only on TNF-α [53, 69, 70] or IL-6 [53, 68•, 69–71, 73] levels in postmenopausal women.
Weight loss is a plausible mediator of exercise-induced adipokine changes given strong biologic rationale and a growing body of RCT evidence, particularly for leptin. The NEW trial, for example, demonstrated up to a 53 % average decrease in leptin concentrations in subgroups with ≥10 % weight loss, which far exceeded changes in subgroups with <5 % weight loss (e.g., 5.5 % average decrease, exercise-only) [67•]. The mechanisms driving exercise-related inflammatory marker changes (CRP, TNF-α, IL-6) are probably more complex, relating not only to weight loss but potentially also to effects on muscle tissue, endothelial cells, and immune cells [59].
Immune function
Evading immunological destruction is an emerging hallmark of cancer and a diminished competence of the immune response is a recognized predictor of cancer risk; however, there is currently no consensus on which immune biomarkers are causally related to cancer risk [74, 75].
Physical activity might impede carcinogenesis by moderating the innate and adaptive immune systems [76] and, thus, enhance immunosurveillance and the tumor suppression capacity of the immune system. Furthermore, exercise could help to modulate obesity-related, proinflammatory immune responses [59] and prevent age-related immunosenescence [77]. To date, the acute, transient effects of exercise on immune function have been studied extensively, supporting beneficial effects with moderate-intensity but detrimental effects with high-intensity activity [76]. Although some studies have demonstrated altered number and function of circulating immune cells (e.g., enhanced natural killer cell cytotoxicity and T-lymphocyte proliferation capacity) with long-term exercise, there is a lack of supportive evidence from RCTs [76–78]. For example, the 12-month Physical Activity for Total Health study found no effect of aerobic exercise on in vitro natural killer cell cytotoxicity, T-lymphocyte proliferative response to stimulation, or the relative proportion of immune cell counts (e.g., T-cells, helper T-cells, cytotoxic T-cells, B cells, natural killer cells) [79].
Oxidative stress and telomere length
Oxidative stress results from an imbalance of increased systemic reactive oxygen species (ROS) production and/or reduced antioxidant capacity, including the ability to neutralize reactive intermediates and repair subsequent damage. It is hypothesized to play a central role in breast carcinogenesis [80] and in carcinogenic causal pathways linked to obesity [81]. ROS-induced damage to macromolecules leads to genetic alterations [82]. Because telomeres, nucleoprotein repeats at the ends of chromosomes that protect cells from chromosomal instability, suffer disproportionately from oxidative damage, an important etiologic pathway through which oxidative stress might affect breast cancer risk is through telomere attrition [83].
As part of a favourable biological adaptive response, regular exercise enhances antioxidant and oxidative damage repairing enzyme capacity and may subsequently reduce oxidative damage [84]. The epidemiologic evidence supporting that regular physical activity reduces oxidative damage to macromolecules or telomere attrition is, thus far, limited but suggestive. In the Physical Activity for Total Health study, there was decreased oxidative damage to lipids as measured by F2-isoprostane levels in exercisers compared to controls, with a more pronounced statistically significant reduction in exercisers who increased their physical fitness by >15 %; this effect occurred independent of obesity status [85]. RCTs of Tai Chi exercise [86] and resistance training [87] also showed reductions in oxidative damage. Yet with respect to telomere length, the NEW trial found no effect of dietary weight loss and/or aerobic exercise [88•], nor did a diet-physical activity lifestyle intervention RCT for diabetes prevention in high-risk adults [89].
Global DNA hypomethylation
The methylation of DNA is recognized as a key epigenetic mechanism in the regulation of gene expression and chromosomal stability. Global DNA hypomethylation in peripheral blood leukocytes represents a postulated biomarker for cancer risk [90] and epidemiologic evidence of an association with increased breast cancer risk is accumulating [91, 92]. Potential epigenetic modifications induced by exercise have been described [93•], and, to-date, two observational studies showed positive associations between physical activity and prevalent repetitive sequences (LINE-1) methylation, a surrogate measure of global methylation [94]. In middle-aged, white women with a family history of breast cancer, higher self-reported physical activity (≥9.8, 5.9, and 12.5 hours per week for childhood, teenage years, and past 12 months, respectively) was associated with a favorable 33 % increase of LINE-1 methylation [95]. Similarly, in another study, cancer-free adults with 26–30 minutes per day of recent physical activity (versus ≤10 minutes per day) as measured via accelerometer, had higher LINE-1 methylation [96].
25-hydroxyvitamin D
A protective, inverse association between vitamin D exposure and postmenopausal breast cancer risk is becoming increasingly clear [97, 98]. For some individuals, outdoor physical activity could improve vitamin D status by increasing cutaneous production of vitamin D3 when UV-B exposure is sufficiently high. Another mechanism involves body composition, because the metabolite 25-hydroxyvitamin D (25(OH)D), the most common serum indicator of vitamin D status, might sequester in body fat [99]. Evidence from the NEW trial supports this hypothesis; in overweight/obese postmenopausal women, sufficient weight loss (≥15 % body weight) over 12 months, whether induced with aerobic exercise or caloric restriction, increased serum 25(OH)D concentrations significantly relative to controls (p-trend = 0.002) [100•]. Furthermore a 2-year weight loss trial of 383 overweight/obese women revealed a clear dose–response relation between increasing weight loss and increasing serum 25(OH)D concentrations (p-trend = 0.005), and in multivariable models, weight loss >10 % was identified as a significant predictor of 25(OH)D change [101].
Proposed Biologic Model
An updated model
Figure 1 depicts a proposed, updated [8•, 11], biologic model relating physical activity to postmenopausal breast cancer risk via interrelated pathways with common linkages to adiposity. Notable exclusions from our model include mammographic density, which is a strong risk factor for breast cancer [102]; however, evidence from observational studies and RCTs overall do not support an association between long-term exercise and breast density [103]. Similarly, while elevated circulating IGF-1 might signify an increased risk for postmenopausal breast cancer [104], it has not been shown to be decreased with physical activity [50, 105, 106•]. Furthermore, we acknowledge that DNA damage, e.g., resulting from oxidative stress or genotoxic estrogen metabolites, could initiate breast cancer and that DNA repair mechanisms might be enhanced with physical activity [10]; however, this topic was beyond the scope of our review.
Fig. 1.
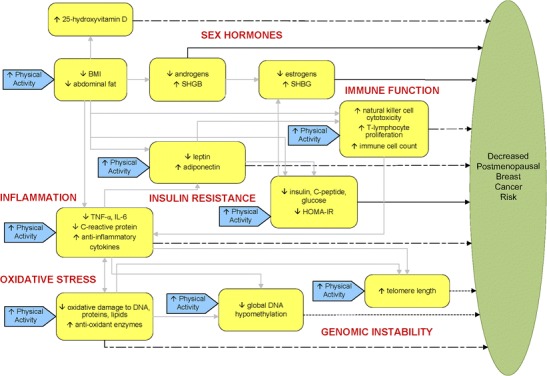
Hypothesized biological model relating physical activity to postmenopausal breast cancer risk. Strong epidemiologic evidence of an association with breast cancer risk (solid black arrows); limited epidemiologic evidence (irregular dashed arrows ); emerging epidemiologic evidence (short dashed arrows). Grey arrows relating biomarkers to each other are proposed in the literature; some of these relations are hypothesized, whereas others are well-established. Adapted from [11]
Role of body fat
Adiposity change could play a mediating role for any of the biomarkers proposed in our model, or for some biomarkers, physical activity might act independently. The state of being overweight or obese also could modify the effects of physical activity on other biomarkers. Distinguishing the effects of “fat and fit” on breast cancer biomarkers is methodologically challenging and of interest [44, 54•, 107]. Four-armed randomized trials, such as the NEW trial and the recent SHAPE-2 study [108••], were designed to address the fit-versus-fat controversy directly and provide some of the clearest distinctions between exercise and weight loss. Published findings from the NEW trial and other RCTs in this review imply particularly that exercise-related changes in estrogens, SHBG, leptin, and 25-hydroxyvitamin D are mediated largely by weight loss in healthy postmenopausal women.
Research Opportunities
While considerable advances have been made in our understanding of the biologic mechanisms relating physical activity to postmenopausal breast cancer risk, opportunity remains for future research. To begin, several newly hypothesized biologic pathways require further study to better understand their etiologic roles in postmenopausal breast cancer and hence, their suitability as biomarkers. Second, although our list of proposed biomarkers is fairly comprehensive, encompassing our own areas of research, additional biomarkers can be considered for our model, including, for example, those related to DNA repair mechanisms, additional epigenetic indicators, other adipokines, and anti-inflammatory cytokines. Several of these mechanisms may be more intensive to measure than what has been done previously, such as genomic alterations, and may require a tissue-specific approach. Hypothesized biomarkers also could be removed from the model as data becomes available; e.g., exercise-only trials in older women thus far have not produced changes in circulating adiponectin, TNF-α, or IL-6 levels. Third, biomarker-exercise RCTs could be analyzed using a systems epidemiology approach [109], quantifying the direct and indirect causal effects of exercise on multiple biomarkers simultaneously rather than on single biomarkers as in previous analyses. Results would identify pivotal exercise-induced biomarker changes and important mediators of those changes as targets for future intervention research. Fourth, new RCTs in healthy postmenopausal women are needed to compare different types of physical activity (e.g., aerobic versus resistance) and different doses (i.e., frequency, duration and intensity) to clarify the optimal prescription for breast cancer risk reduction. Studies exploring interindividual variability in exercise-induced biomarker changes, e.g., due to common genetic polymorphisms, would help tailor exercise prescriptions.
One recently completed, noteworthy study is the Breast Cancer and Exercise Trial in Alberta (BETA Trial). This trial, comprising 400 postmenopausal, previously inactive women who were randomized to undertake either 150 or 300 minutes per week of aerobic exercise for 1 year, was designed specifically to determine the optimal activity dose for lowering postmenopausal breast cancer risk. In secondary analyses of the ALPHA trial data, higher exercise duration was associated with desirable changes in adiposity and circulating sex steroid hormone levels, HOMA-IR, leptin, and CRP levels, with the greatest benefit observed for women undertaking >150 minutes per week [32] or >225 minutes per week [50, 70, 110] of aerobic exercise. Therefore, previous biomarker-exercise RCTs that generated null findings in primary analyses may have been limited by the relatively low dose of exercise that was prescribed or attained by study participants. Furthermore, it is possible that the current physical activity guidelines recommended for cancer prevention are insufficient for postmenopausal breast cancer. A recent prospective investigation of 30,797 postmenopausal women [111•] found no significant association between invasive breast cancer incidence and near-achievement of the WCRF/AICR 2007 minimum physical activity recommendations [1] for cancer prevention.
Conclusions
Evidence from randomized exercise trials in healthy, overweight and obese postmenopausal women implies that moderate-vigorous aerobic exercise prescriptions of 150–225 minutes per week over 12 months can lower estradiol levels by approximately 5-10 % on average, primarily through total body weight loss. Yet, there is biologic plausibility that some exercise-induced biomarker changes do not require loss of body fat, whereas others depend on abdominal fat loss. The preventive effect of exercise is probably the culmination of numerous interrelated biomarker changes that, when combined, act additively or synergistically to impede carcinogenesis in the breast. The level of physical activity required to induce these changes could be higher than the minimum level currently advised for cancer prevention and might depend on individual factors, e.g., genetic constitution. Identifying a physical activity prescription that produces clinically meaningful changes in key biomarkers and subgroups of women who would benefit the most from physical activity is a priority for future research. These recommendations will be used to inform prevention strategies for breast cancer after menopause.
Acknowledgments
The authors thank Darren Brenner for reviewing our manuscript. Christine Friedenreich was funded by a Health Senior Scholar Award from Alberta Innovates-Health Solutions (AI-HS) and through the Alberta Cancer Foundation’s Weekend to End Women’s Cancers Breast Cancer Chair. Shannon Conroy was funded by Postdoctoral Fellowships from the Canadian Institutes for Health Research and AI-HS.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Heather K. Neilson, Shannon M. Conroy, and Christine M. Friedenreich declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Heather K. Neilson, Email: Heather.Neilson@albertahealthservices.ca
Shannon M. Conroy, Email: Shannon.Conroy@albertahealthservices.ca
Christine M. Friedenreich, Email: Christine.Friedenreich@albertahealthservices.ca
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: World Cancer Research Fund and the American Institute for Cancer Research; 2007.
- 2.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki R, Orsini N, Saji S, et al. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status: a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 4.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134(2):769–781. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 5.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101(9):630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. CA Cancer J Clin. 2006;56(5):254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 8.•.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137(3):869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 10.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(1):227–236. [PubMed] [Google Scholar]
- 11.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18(1):11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 12.•.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lof M, Bergstrom K, Weiderpass E. Physical activity and biomarkers in breast cancer survivors: A systematic review. Maturitas. 2012. [DOI] [PubMed]
- 14.•.Patterson RE, Rock CL, Kerr J, et al. Metabolism and breast cancer risk: frontiers in research and practice. J Acad Nutr Diet. 2013;113(2):288–296. doi: 10.1016/j.jand.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose DP, Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic Inflammation, and breast cancer. Biofactors. 2013 doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- 16.John EM, Phipps AI, Sangaramoorthy M. Body size, modifying factors, and postmenopausal breast cancer risk in a multiethnic population: the San Francisco Bay Area Breast Cancer Study. SpringerPlus. 2013;2(1):239. doi: 10.1186/2193-1801-2-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.•.Liedtke S, Schmidt ME, Vrieling A, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring) 2012;20(5):1088–1095. doi: 10.1038/oby.2011.383. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 19.Lau DC, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;176(8):S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims ST, Larson JC, Lamonte MJ, et al. Physical activity and body mass: changes in younger versus older postmenopausal women. Med Sci Sports Exerc. 2012;44(1):89–97. doi: 10.1249/MSS.0b013e318227f906. [DOI] [PubMed] [Google Scholar]
- 21.•.Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033–E1039. doi: 10.1152/ajpendo.00291.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond) 2010;35:427–435. doi: 10.1038/ijo.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 24.•.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. 2012;20(8):1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velthuis MJ, Schuit AJ, Peeters PH, Monninkhof EM. Exercise program affects body composition but not weight in postmenopausal women. Menopause. 2009;16(4):777–784. doi: 10.1097/gme.0b013e318197122a. [DOI] [PubMed] [Google Scholar]
- 26.Bergstrom I, Lombardo C, Brinck J. Physical training decreases waist circumference in postmenopausal borderline overweight women. Acta Obstet Gynecol Scand. 2009;88(3):308–313. doi: 10.1080/00016340802695942. [DOI] [PubMed] [Google Scholar]
- 27.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 28.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uray IP, Brown PH. Prevention of breast cancer: current state of the science and future opportunities. Expert Opin Investig Drugs. 2006;15(12):1583–1600. doi: 10.1517/13543784.15.12.1583. [DOI] [PubMed] [Google Scholar]
- 30.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 31.Copeland JL, Tremblay MS. Effect of HRT on hormone responses to resistance exercise in post-menopausal women. Maturitas. 2004;48(4):360–371. doi: 10.1016/j.maturitas.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo EJ, Jun TW, Hawkins S. The effects of a walking exercise program on fall-related fitness, bone metabolism, and fall-related psychological factors in elderly women. Res Sports Med. 2010;18(4):236–250. doi: 10.1080/15438627.2010.510098. [DOI] [PubMed] [Google Scholar]
- 34.Monninkhof EM, Velthuis MJ, Peeters PH, et al. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol. 2009;27(27):4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 35.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64(8):2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 36.••.Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figueroa A, Going SB, Milliken LA, et al. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2003;58(3):M266–M270. doi: 10.1093/gerona/58.3.m266. [DOI] [PubMed] [Google Scholar]
- 38.Kim JW, Kim DY. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord. 2012;10(6):452–457. doi: 10.1089/met.2012.0036. [DOI] [PubMed] [Google Scholar]
- 39.Orsatti FL, Nahas EA, Maesta N, et al. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas. 2008;59(4):394–404. doi: 10.1016/j.maturitas.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson C, Lampe JW, Tworoger SS, et al. Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(5):868–874. [PubMed] [Google Scholar]
- 41.Campbell KL, Foster-Schubert KE, Makar KW, et al. Gene Expression Changes in Adipose Tissue with Diet- and/or Exercise-Induced Weight Loss. Cancer Prev Res. 2013;6(3):217–231. doi: 10.1158/1940-6207.CAPR-12-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McTiernan A, Tworoger SS, Rajan KB, et al. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1099–1105. [PubMed] [Google Scholar]
- 43.•.Carpenter CL, Duvall K, Jardack P, et al. Weight loss reduces breast ductal fluid estrogens in obese postmenopausal women: a single arm intervention pilot study. Nutr J. 2012;11:102. doi: 10.1186/1475-2891-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones ME, Schoemaker M, Rae M, et al. Changes in estradiol and testosterone levels in postmenopausal women after changes in body mass index. J Clin Endocrinol Metab. 2013;98(7):2967–2974. doi: 10.1210/jc.2013-1588. [DOI] [PubMed] [Google Scholar]
- 45.Autier P, Koechlin A, Boniol M, et al. Serum insulin and C-peptide concentration and breast cancer: a meta-analysis. Cancer Causes Control. 2013;24(5):873–883. doi: 10.1007/s10552-013-0164-6. [DOI] [PubMed] [Google Scholar]
- 46.Ahern TP, Hankinson SE, Willett WC, et al. Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1786–1796. doi: 10.1158/1055-9965.EPI-13-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19(6):R225–R241. doi: 10.1530/ERC-12-0203. [DOI] [PubMed] [Google Scholar]
- 48.Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer–chemoprevention strategies with a focus on metformin. Breast. 2011;20(Suppl 3):S31–S35. doi: 10.1016/S0960-9776(11)70291-0. [DOI] [PubMed] [Google Scholar]
- 49.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33(12):2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedenreich CM, Neilson HK, Woolcott CG, et al. Changes in insulin resistance indicators, insulin-like growth factors, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18(3):357–369. doi: 10.1530/ERC-10-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank LL, Sorensen BE, Yasui Y, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13(3):615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 52.•.Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arsenault BJ, Cote M, Cartier A, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207(2):530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.•.Friedenreich CM, Neilson HK, Woolcott CG, et al. Mediators and moderators of the effects of a year-long exercise intervention on endogenous sex hormones in postmenopausal women. Cancer Causes Control. 2011;22(10):1365–1373. doi: 10.1007/s10552-011-9809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86(3):1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 56.Tresierras MA, Balady GJ. Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev. 2009;29(2):67–75. doi: 10.1097/HCR.0b013e318199ff69. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa A, Vicil F, Sanchez-Gonzalez MA, et al. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens. 2013;26(3):416–423. doi: 10.1093/ajh/hps050. [DOI] [PubMed] [Google Scholar]
- 58.Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- 59.You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013;43(4):243–256. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 60.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47(1):33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 61.He B, Niu J, Jiang L, et al. The association between leptin level and breast cancer: a meta-analysis. PLoS ONE. 2013;8(6):e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic Leptin, Adiponectin, C-Reactive Protein, and the Risk of Postmenopausal Breast Cancer. Cancer Prev Res. 2013;6(3):188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gross AL, Newschaffer CJ, Hoffman-Bolton J, et al. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1319–1324. doi: 10.1158/1055-9965.EPI-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh S, Liu L-Y, Wang M, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS ONE. 2013;8(8):e73183. doi: 10.1371/journal.pone.0073183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 66.Shen C, Sun H, Sun D, et al. Polymorphisms of tumor necrosis factor-alpha and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;126(3):763–770. doi: 10.1007/s10549-010-1184-5. [DOI] [PubMed] [Google Scholar]
- 67.•.Abbenhardt C, McTiernan A, Alfano CM, et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J Intern Med. 2013;274(2):163–175. doi: 10.1111/joim.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.•.Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72(9):2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Friedenreich CM, Neilson HK, Woolcott CG, et al. Inflammatory marker changes in a year-long randomized exercise intervention trial among postmenopausal women. Cancer Prev Res. 2012;5(1):98–108. doi: 10.1158/1940-6207.CAPR-11-0369. [DOI] [PubMed] [Google Scholar]
- 71.Campbell PT, Campbell KL, Wener MH, et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41(8):1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart LK, Earnest CP, Blair SN, Church TS. Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc. 2010;42(4):701–707. doi: 10.1249/MSS.0b013e3181c03a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips MD, Patrizi RM, Cheek DJ, et al. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44(11):2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- 74.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Standish LJ, Sweet ES, Novack J, et al. Breast cancer and the immune system. J Soc Integr Oncol. 2008;6(4):158–168. [PMC free article] [PubMed] [Google Scholar]
- 76.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement Part one: Immune function and exercise. ExercImmunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 77.Simpson RJ, Lowder TW, Spielmann G, et al. Exercise and the aging immune system. Ageing Res Rev. 2012;11(3):404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Haaland DA, Sabljic TF, Baribeau DA, et al. Is regular exercise a friend or foe of the aging immune system? A systematic review. Clin J Sport Med. 2008;18(6):539–548. doi: 10.1097/JSM.0b013e3181865eec. [DOI] [PubMed] [Google Scholar]
- 79.Campbell PT, Wener MH, Sorensen B, et al. Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J Appl Physiol. 2008;104(6):1648–1655. doi: 10.1152/japplphysiol.01349.2007. [DOI] [PubMed] [Google Scholar]
- 80.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, et al. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells Biological bases to develop oxidative-based therapies. Crit Rev Oncol Hematol. 2011;80(3):347–368. doi: 10.1016/j.critrevonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 81.Crujeiras AB, Diaz-Lagares A, Carreira MC, et al. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res. 2013;47(4):243–256. doi: 10.3109/10715762.2013.772604. [DOI] [PubMed] [Google Scholar]
- 82.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711(1–2):167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Prescott J, Wentzensen IM, Savage SA, De Vivo I. Epidemiologic evidence for a role of telomere dysfunction in cancer etiology. Mutat Res. 2012;730(1–2):75–84. doi: 10.1016/j.mrfmmm.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 85.Campbell PT, Gross MD, Potter JD, et al. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010;42(8):1448–1453. doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qian G, Xue K, Tang L, et al. Mitigation of oxidative damage by green tea polyphenols and Tai Chi exercise in postmenopausal women with osteopenia. PLoS One. 2012;7(10):e48090. doi: 10.1371/journal.pone.0048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring) 2006;14(11):1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 88.•.Mason C, Risques R-A, Xiao L, et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity. 2013 doi: 10.1002/oby.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hovatta I, de Mello VD, Kananen L, et al. Leukocyte telomere length in the Finnish Diabetes Prevention Study. PLoS ONE. 2012;7(4):e34948. doi: 10.1371/journal.pone.0034948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woo HD, Kim J. Global DNA hypomethylation in peripheral blood leukocytes as a biomarker for cancer risk: a meta-analysis. PLoS One. 2012;7(4):e34615. doi: 10.1371/journal.pone.0034615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delgado-Cruzata L, Wu HC, Perrin M, et al. Global DNA methylation levels in white blood cell DNA from sisters discordant for breast cancer from the New York site of the Breast Cancer Family Registry. Epigenetics. 2012;7(8):868–874. doi: 10.4161/epi.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deroo LA, Bolick SC, Xu Z et al. Global DNA methylation and one-carbon metabolism gene polymorphisms and the risk of breast cancer in the Sister Study. Carcinogenesis. 2013 [DOI] [PMC free article] [PubMed]
- 93.•.Ntanasis-Stathopoulos J, Tzanninis JG, Philippou A, Koutsilieris M. Epigenetic regulation on gene expression induced by physical exercise. J Musculoskelet Neuronal Interact. 2013;13(2):133–146. [PubMed] [Google Scholar]
- 94.Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White AJ, Sandler DP, Bolick SCE, et al. Recreational and household physical activity at different time points and DNA global methylation. Eur J Cancer. 2013;49(9):2199–2206. doi: 10.1016/j.ejca.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang FF, Cardarelli R, Carroll J, et al. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6(3):293–299. doi: 10.4161/epi.6.3.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose–response meta-analysis of prospective studies. Medicine (Baltimore) 2013;92(3):123–131. doi: 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumour Biol. 2013. [DOI] [PubMed]
- 99.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 100.•.Mason C, Xiao L, Imayama I, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr. 2011;94(1):95–103. doi: 10.3945/ajcn.111.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rock CL, Emond JA, Flatt SW, et al. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity. 2012;20(11):2296–2301. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 103.Yaghjyan L, Colditz GA, Wolin K. Physical activity and mammographic breast density: a systematic review. Breast Cancer Res Treat. 2012;135(2):367–380. doi: 10.1007/s10549-012-2152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McTiernan A, Sorensen B, Yasui Y, et al. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2005;14(4):1020–1021. doi: 10.1158/1055-9965.EPI-04-0834. [DOI] [PubMed] [Google Scholar]
- 106.•.Mason C, Xiao L, Duggan C, et al. Effects of Dietary Weight Loss and Exercise on Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-Binding Protein-3 in Postmenopausal Women: A Randomized Controlled Trial. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1457–1463. doi: 10.1158/1055-9965.EPI-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liedtke S, Schmidt ME, Becker S, et al. Physical activity and endogenous sex hormones in postmenopausal women: to what extent are observed associations confounded or modified by BMI? Cancer Causes Control. 2011;22(1):81–89. doi: 10.1007/s10552-010-9677-4. [DOI] [PubMed] [Google Scholar]
- 108.••.van Gemert WAM, Iestra JI, Schuit AJ, et al. Design of the SHAPE-2 study: the effect of physical activity, in addition to weight loss, on biomarkers of postmenopausal breast cancer risk. BMC Cancer. 2013;13(1):395. doi: 10.1186/1471-2407-13-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joffe M, Gambhir M, Chadeau-Hyam M, Vineis P. Causal diagrams in systems epidemiology. Emerg Themes Epidemiol. 2012;9(1):1. doi: 10.1186/1742-7622-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond) 2011;35(3):427–435. doi: 10.1038/ijo.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.•.Hastert TA, Beresford SAA, Patterson RE, et al. Adherence to WCRF/AICR Cancer Prevention Recommendations and Risk of Postmenopausal Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1498–1508. doi: 10.1158/1055-9965.EPI-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]


