Abstract
Ghrelin, the ligand of growth hormone secretagogue receptor 1a, takes part in several functions of the digestive system, including regulation of appetite, energy homeostasis, gastric acid secretion and motility. Ghrelin has also immunoregulatory properties and is supposed to inhibit some inflammatory pathways that can mediate gastric damage. Interestingly, ghrelin synthesis is reduced in the gastric mucosa of patients with Helicobacter pylori (H. pylori) infection, a worldwide condition inducing a T helper (Th)1/Th17 cell response-driven gastritis, which may evolve towards gastric atrophy and cancer. In this article, we review the available data on the expression of ghrelin in H. pylori infection and discuss how the defective ghrelin synthesis may contribute to sustain the ongoing inflammatory response in this disease.
Keywords: Gastritis, Ghrelin, Helicobacter pylori, T helper 1 cells
Core tip: The review reports current statements about relationship between gastric ghrelin expression and Helicobacter pylori infection. Data present in the literature and emerging from a very recent our study on the anti-inflammatory role of ghrelin and T helper 1 cell response in the stomach during Helicobacter pylori infection are included.
GHRELIN, A HORMONE WITH IMMUNOREGULATORY FUNCTIONS
Initially described as a ligand of growth hormone secretagogue receptor 1a (GHS-R1a) expressed by growth hormone-secreting pituitary cells[1], ghrelin is a potent stimulator of growth hormone secretion[2]. Ghrelin is largely produced in the alimentary tract, mainly in the stomach, by gastric X/A-like endocrine cells in rodents and fundic P/D1 cells in humans, while its synthesis gradually diminishes from duodenum to the colon[3]. The large synthesis of ghrelin and expression of GHS-R in the stomach and in other organs and tissues suggested additional effects other than stimulation of growth hormone in the pituitary. Indeed, it is now known that ghrelin takes part in several functions (Figure 1), including the regulation of appetite and energy homeostasis, which could favour adiposity and obesity[4-6]. Ghrelin is also produced in pancreas, lung, kidney, testis, placenta and by immune cells[7]. Ghrelin circulates in two major forms, acyl and desacyl ghrelin[8]. Acyl ghrelin has an octanoyl group essential to activate GHS-R1a[9,10-13]. Desacyl ghrelin lacks this octanoyl group and it was early thought to be an inactive form of ghrelin since it does not activate GHS-R1a. Indeed, desacyl ghrelin has been demonstrated to counteract acyl ghrelin and inhibit the stimulation of food intake, gastric and bowel emptying[14,15] and to be involved in several other biological functions (e.g., in the reproductive system, bone metabolism, cardiovascular protection)[16-18]. Ghrelin has been also found to stimulate neurogenesis[19], improve central memory[20], influence sleep-wake cycle[21].
Figure 1.
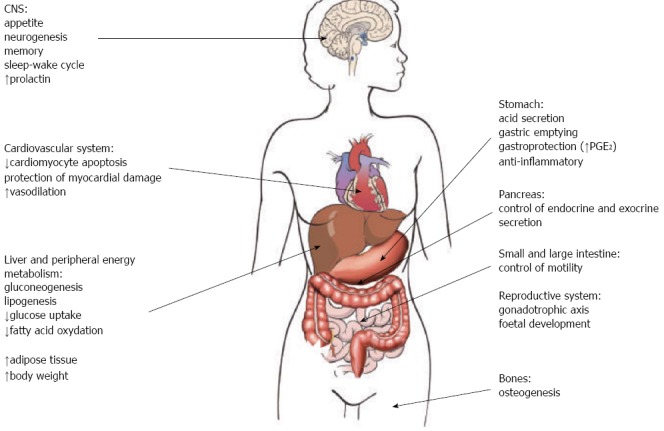
Main functions of ghrelin in the human body. INF: Interferon; IL: Interleukin; PGE2: Prostaglandin E2; Th1: T helper 1; APC: Antigen presenting cell.
Acylation of ghrelin is mediated by ghrelin-O-acyltransferase (GOAT) in both mice and humans[22,23], an enzyme expressed by several tissues, including the stomach and pancreas[15]. Although GOAT expression is high in the stomach, a direct quantitative correlation with the expression of ghrelin mRNA has not been demonstrated[24]. GOAT is also present in the plasma and varies in relationship with the fasting or feeding status[25]. As acylated ghrelin has a short in vivo half-life (about 9-13 min)[26], desacyl ghrelin accounts for > 90% of the circulating ghrelin[8] with a ratio of acyl/desacyl ghrelin varying from 1:15 to 1:55[8,27].
Besides physiologic activities, ghrelin exerts a gastro-protective effect during pathological conditions. Indeed, in vivo in rats administration of ghrelin attenuates the gastric mucosal lesions induced by detrimental agents, such as ethanol and indomethacin, through an increase of mucosal generation of prostaglandins prostaglandin E2 (PGE2)[28,29]. Ghrelin is also an important regulator of NOS and cyclooxygenase (COX) enzyme systems[1,30-33]. Moreover, studies in different animal models revealed that ghrelin reduces the release of pro-inflammatory cytokines, such as interleukin (IL)-1β, TNF-α, IL-6[34-37] and stimulates the expression of the anti-inflammatory cytokine IL-10[38,39] by T lymphocytes and macrophages in different mechanical or chemical-induced inflammatory conditions. Treatment of human T lymphocytes and monocytes with exogenous ghrelin inhibits the release of pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6[40,41].
Altogether these data underline the gastroprotective functions and the anti-inflammatory role of ghrelin.
HELICOBACTER PYLORI INFECTION ASSOCIATES WITH DECREASED GASTRIC PRODUCTION OF GHRELIN
Helicobacter pylori (H. pylori) is a Gram negative microorganism, which colonizes the stomach and causes a chronic gastritis, with the downstream effect of promoting peptic ulcer and cancer[42]. H. pylori-related gastritis may progress to atrophy with loss of pyloric and oxyntic glands which, in turn, may negatively affect secretory functions in the stomach. This gastritis related-damage may interfere with ghrelin expression through a loss of P/D1 cells in the fundus and body of the stomach. On the other hand, H. pylori could directly act on mechanisms controlling ghrelin production through the release of cytotoxins, lipopolysaccharide (LPS) and other noxious agents[43]. Indeed, ghrelin expression is reduced in gastric biopsies of H. pylori uninfected subjects following a 24 h incubation with H. pylori-derived culture broth[44]. This evidence is in keeping with the observation[45] that LPS originating from Gram negative bacteria wall, such as H. pylori, intraperitoneally administered in rats reduces plasma ghrelin levels during the first three hours post-injection, probably through an interleukin-1-stimulated release of prostacyclin, which acts directly on PGI2 receptor-expressing ghrelin-producing cells of the gastric oxyntic mucosa.
Many researchers compared circulating values of ghrelin in infected and non-infected patients with inconsistent results. The majority of these studies[46-66] found lower levels of circulating ghrelin in H. pylori positive subjects in Asia and Europe but not in United States. Conflicting results were also obtained when the effect of H. pylori eradication on ghrelin plasma levels was evaluated[46,56-58,67-72]. A metanalysis by Nweneka and Prentice concluded that circulating ghrelin is significantly lower in H. pylori-positive than negative subjects but H. pylori eradication does not significantly modify plasma ghrelin levels[73]. Several factors could explain discrepancy in the results, such as gender[53,74], age[75,76], gastric-related diseases (higher levels in gastritis and peptic ulcer[77] than in gastric cancer[78]), H. pylori strain differences (different expression of cytotoxyns)[43], extent and severity of gastritis (presence or not of atrophy)[60,79,80] and different immunoassays used to measure ghrelin.
Ghrelin expression in the stomach was also assessed by quantification of the gastric ghrelin peptide content or ghrelin mRNA expression in endoscopic biopsies[44,51,52,58,59,62,65,70,71,80-83]. In all studies but three[51,59,65] lower amounts of ghrelin peptide were found in H. pylori infected in respect to non-infected subjects. In contrast to circulating ghrelin, ghrelin mRNA[61,71] and ghrelin immunoreactive cells[83] increased after H. pylori eradication.
H. pylori infection has been reported to influence body mass index (BMI), as it seems to be higher in infected patients than non-infected subjects[84]. Several studies[85-88] also reported an increase of BMI following H. pylori eradication in Asia and in Europe. How H. pylori may influence BMI has not yet been fully understood but the reduction of dyspepsia following H. pylori eradication could increase the appetite and consequently body weight[88]. The restoration of gastric ghrelin expression and an increase of circulating ghrelin levels have been thought to be responsible of the weight gain process following H. pylori eradication[67]. This hypothesis, however, has not been confirmed in other studies[68,81] in which an increase of gastric ghrelin synthesis following H. pylori eradication was not correlated to the raise of BMI. Therefore, the exact contribution of ghrelin in the increase of BMI following H. pylori eradication remains to be elucidated.
IMPACT OF GHRELIN DOWN-REGULATION ON THE GASTRIC INFLAMMATORY RESPONSE TO H. PYLORI
The mechanisms by which H. pylori drives the tissue damaging inflammatory response in the stomach have been largely investigated. It is known that the response to H. pylori infection and the variable mucosal damage are probably influenced by bacterial and host factors[89,90]. Gastric epithelial cells infected by H. pylori exhibit hyperactivation of nuclear factor κB (NF-κB) and produce elevated levels of chemokines, such as IL-8, which contribute to recruit neutrophils into the inflamed tissue[91,92]. H. pylori-driven gastritis is associated with a strong activation of T helper (Th)-type 1 cells, which release large amounts of interferon (IFN)-γ[93,94] and express high T-bet, a Th1-inducing transcription factor[95]. Consistently, H. pylori-infected biopsies contain elevated levels of IL-12, the main Th1 inducing factor in human beings[96]. The large release of INF-γ leads to several inflammatory responses, including the induction of Smad7[97], a strong inhibitor of transforming growth factor (TGF)-β1 activity[98,99]. The defective TGF-β1 activity documented in H. pylori-infected biopsies fits with the demonstration that loss of TGF-β1 in mice associates with a severe gastric inflammation and mucosal damage[100]. Restoring TGF-β1/Smad3 activity with a specific SMAD7 antisense oligonucleotide markedly inhibits Th1 inflammatory cytokine response[97], thus highlighting the importance of the impaired TGF-β1 activity in maintaining the H. pylori-driven pathological response. Recently, H. pylori-associated gastritis has been demonstrated to be characterised by elevated levels of another subset of Th cells, termed Th17 and producing IL-17A, IL-17F, IL-21, IL-22 and IL-26[101-105]. Collectively these data, together with the demonstration that both Th1 and Th17 cells can be pathogenic in mice infected with Helicobacter species, suggest that H. pylori infection may elicit Th1- and Th17-cell immune response in the gastric mucosa thereby contributing to amplify the ongoing mucosal inflammation and favouring the development of gastric lesions. How H. pylori infection induces Th1 and TH17 cell response is not, however, fully understood. It is possible that factors released by H. pylori stimulate macrophages and dendritic cells which, in turn, produce factors driving Th1 and Th17 responses. This is supported by the evidence that the H. pylori neutrophil-activating protein (H. pylori-NAP) is able in vitro to stimulate IL-12 production via agonistic interaction with toll-like receptor 2 and promote Th1 cell polarization[105]. H. pylori-NAP enhances also IL-23, a cytokine involved in the expansion/maintenance of Th17 cell responses. H. pylori infection may also down-regulate factors involved in the negative regulation of Th1 and Th17 cell responses.
The H. pylori-related impairment of ghrelin synthesis in the stomach could represent another step in the damaging process caused by the microorganism (Figure 2). We have recently shown that treatment of H. pylori-infected human gastric biopsies and lamina propria mononuclear cells isolated from H. pylori-colonized gastric biopsies with exogenous ghrelin down-regulated the expression of IFN-γ and IL-12[44]. In contrast, no change in IL-4 was seen following ghrelin treatment, thus indicating that action of ghrelin is confined to Th1 cell immune response. These finding are in line with previous studies showing that ghrelin counteracts other H. pylori-induced pathogenic signals, such apoptosis of gastric epithelial cells[33] and activation of important transcription factors, such NF-κB and MAP kinases[106].
Figure 2.
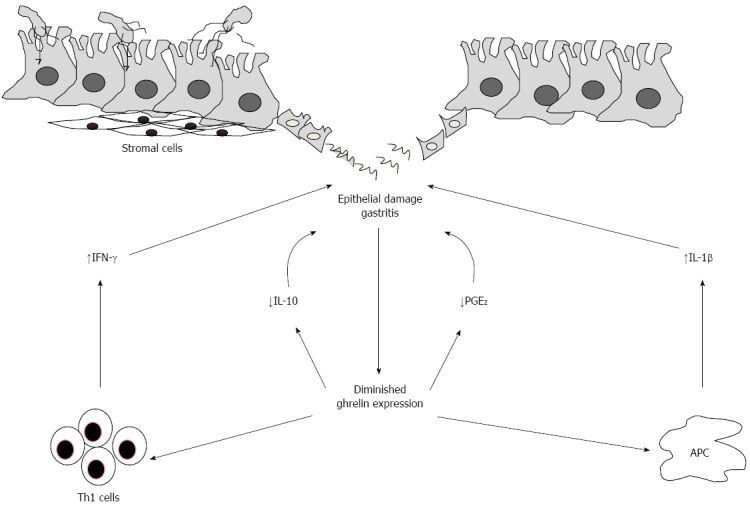
Downregulation of ghrelin expression in the stomach during Helicobacter pylori infection. Epithelial damage and gastritis induced by Helicobacter pylori determine a diminished expression of ghrelin which, in turn, sustains the ongoing T helper (Th) 1 cells response. Down regulation of ghrelin is also followed by a reduced release of Prostaglandin E2 (PGE2) and interleukin (IL)-10 which, together with pro-inflammatory factors as IL-1β, contribute to the detrimental immune response and damage in the stomach. APC: Antigen presenting cell.
The exact role of ghrelin in the development and progression of gastric cancer cells remains to be ascertained. There is evidence that circulating ghrelin levels are deeply diminished in patients with gastric cancer as compared to healthy subjects[78,107], raising the possibility that the diminished ghrelin expression seen in H. pylori-positive patients may be involved in the progression of gastric cancer. On the other hand, studies[108,109] with cultured gastric cancer cells have shown that ghrelin may be mitogenic and, therefore, have a promoting effect on neoplastic cell growth.
CONCLUSION
Ghrelin, a hormone with anti-inflammatory and anti-apoptotic properties, is supposed to play an important gastroprotective role. The findings described in this article indicate that H. pylori infection associates with a marked down-regulation of ghrelin synthesis, thus delineating a scenario in which such a defect contributes to sustain the H. pylori-driven pathogenic response. Further experimentation would be, however, necessary to ascertain the basic mechanism underlying the negative regulation of ghrelin synthesis by H. pylori as well as to evaluate whether there are inflammatory pathways which rely strongly on ghrelin down-regulation. Studies are also needed to determine the contribution of the diminished ghrelin production in the evolution of H. pylori-associated pathology, as ghrelin can increase the production of PGE2, a protective factor for the gastric mucosa, which has been also implicated in the pathogenesis of cancer[80,109].
Footnotes
P- Reviewers: Lai CH, Lee YC, Koutsilieris M S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Malagón MM, Luque RM, Ruiz-Guerrero E, Rodríguez-Pacheco F, García-Navarro S, Casanueva FF, Gracia-Navarro F, Castaño JP. Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology. 2003;144:5372–5380. doi: 10.1210/en.2003-0723. [DOI] [PubMed] [Google Scholar]
- 3.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, Mckee KK, Pong SS, Griffin P, Howard A. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14:9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- 5.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 7.Peeters TL. Ghrelin: a new player in the control of gastrointestinal functions. Gut. 2005;54:1638–1649. doi: 10.1136/gut.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 9.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 10.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–369. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 13.Yakabi K, Kawashima J, Kato S. Ghrelin and gastric acid secretion. World J Gastroenterol. 2008;14:6334–6338. doi: 10.3748/wjg.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, Chao Y, Chang FY, Chien EJ, Lee SD, Doong ML. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int J Mol Med. 2005;16:695–699. [PubMed] [Google Scholar]
- 16.Navarro VM, Kaiser UB. Metabolic influences on neuroendocrine regulation of reproduction. Curr Opin Endocrinol Diabetes Obes. 2013;20:335–341. doi: 10.1097/MED.0b013e32836318ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amini P, Cahill F, Wadden D, Ji Y, Pedram P, Vidyasankar S, Yi Y, Gulliver W, Paterno G, Zhang H, et al. Beneficial association of serum ghrelin and peptide YY with bone mineral density in the Newfoundland population. BMC Endocr Disord. 2013;13:35. doi: 10.1186/1472-6823-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, Mulholland MW. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 21.García-García F, Juárez-Aguilar E, Santiago-García J, Cardinali DP. Ghrelin and its interactions with growth hormone, leptin and orexins: Implications for the sleep-wake cycle and metabolism. Sleep Med Rev. 2014;18:89–97. doi: 10.1016/j.smrv.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Lim CT, Kola B, Korbonits M. The ghrelin/GOAT/GHS-R system and energy metabolism. Rev Endocr Metab Disord. 2011;12:173–186. doi: 10.1007/s11154-011-9169-1. [DOI] [PubMed] [Google Scholar]
- 25.Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun. 2010;392:67–71. doi: 10.1016/j.bbrc.2009.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447–455. doi: 10.1530/eje.0.1500447. [DOI] [PubMed] [Google Scholar]
- 27.Raff H. Total and active ghrelin in developing rats during hypoxia. Endocrine. 2003;21:159–161. doi: 10.1385/ENDO:21:2:159. [DOI] [PubMed] [Google Scholar]
- 28.Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 29.Konturek PC, Brzozowski T, Pajdo R, Nikiforuk A, Kwiecien S, Harsch I, Drozdowicz D, Hahn EG, Konturek SJ. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325–336. [PubMed] [Google Scholar]
- 30.Sibilia V, Pagani F, Rindi G, Lattuada N, Rapetti D, De Luca V, Campanini N, Bulgarelli I, Locatelli V, Guidobono F, et al. Central ghrelin gastroprotection involves nitric oxide/prostaglandin cross-talk. Br J Pharmacol. 2008;154:688–697. doi: 10.1038/bjp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Jhun BS, Ha CH, Jin ZG. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slomiany BL, Slomiany A. Involvement of constitutive nitric oxide synthase in ghrelin-induced cytosolic phospholipase A(2) activation in gastric mucosal cell protection against ethanol cytotoxicity. Inflammopharmacology. 2009;17:245–253. doi: 10.1007/s10787-009-0013-0. [DOI] [PubMed] [Google Scholar]
- 33.Slomiany BL, Slomiany A. Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediators Inflamm. 2010;2010:280464. doi: 10.1155/2010/280464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YT, Tsai SH, Sheu SY, Tsai LH. Ghrelin improves LPS-induced gastrointestinal motility disturbances: roles of NO and prostaglandin E2. Shock. 2010;33:205–212. doi: 10.1097/SHK.0b013e3181ae841b. [DOI] [PubMed] [Google Scholar]
- 35.Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Stachura J, Konturek SJ, Konturek PC. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561–573. [PubMed] [Google Scholar]
- 36.Kasımay O, Işeri SO, Barlas A, Bangir D, Yeğen C, Arbak S, Yeğen BC. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res. 2006;36:11–19. doi: 10.1016/j.hepres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Işeri SO, Sener G, Saglam B, Ercan F, Gedik N, Yeğen BC. Ghrelin alleviates biliary obstruction-induced chronic hepatic injury in rats. Regul Pept. 2008;146:73–79. doi: 10.1016/j.regpep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Warzecha Z, Ceranowicz P, Dembinski A, Cieszkowski J, Kusnierz-Cabala B, Tomaszewska R, Kuwahara A, Kato I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol. 2010;61:419–427. [PubMed] [Google Scholar]
- 39.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konturek PC, Bielański W, Konturek SJ, Hahn EG. Helicobacter pylori associated gastric pathology. J Physiol Pharmacol. 1999;50:695–710. [PubMed] [Google Scholar]
- 43.Isomoto H, Nishi Y, Ohnita K, Mizuta Y, Kohno S, Ueno H, Nakazato M. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am J Gastroenterol. 2005;100:1425–1427. doi: 10.1111/j.1572-0241.2005.41929_7.x. [DOI] [PubMed] [Google Scholar]
- 44.Paoluzi OA, Del Vecchio Blanco G, Caruso R, Monteleone I, Caprioli F, Tesauro M, Turriziani M, Monteleone G, Pallone F. Helicobacter pylori infection associates with a mucosal downregulation of ghrelin, negative regulator of Th1-cell responses. Helicobacter. 2013;18:406–412. doi: 10.1111/hel.12065. [DOI] [PubMed] [Google Scholar]
- 45.Stengel A, Goebel M, Wang L, Reeve JR, Taché Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol. 2007;13:1595–1598. doi: 10.3748/wjg.v13.i10.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czesnikiewicz-Guzik M, Bielanski W, Guzik TJ, Loster B, Konturek SJ. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J Physiol Pharmacol. 2005;56 Suppl 6:77–89. [PubMed] [Google Scholar]
- 48.de Martel C, Haggerty TD, Corley DA, Vogelman JH, Orentreich N, Parsonnet J. Serum ghrelin levels and risk of subsequent adenocarcinoma of the esophagus. Am J Gastroenterol. 2007;102:1166–1172. doi: 10.1111/j.1572-0241.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 49.Gao XY, Kuang HY, Liu XM, Duan P, Yang Y, Ma ZB. Circulating ghrelin/obestatin ratio in subjects with Helicobacter pylori infection. Nutrition. 2009;25:506–511. doi: 10.1016/j.nut.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Konturek PC, Cześnikiewicz-Guzik M, Bielanski W, Konturek SJ. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol. 2006;57 Suppl 5:67–81. [PubMed] [Google Scholar]
- 51.Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008;93:2350–2357. doi: 10.1210/jc.2007-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salles N, Ménard A, Georges A, Salzmann M, de Ledinghen V, de Mascarel A, Emeriau JP, Lamouliatte H, Mégraud F. Effects of Helicobacter pylori infection on gut appetite peptide (leptin, ghrelin) expression in elderly inpatients. J Gerontol A Biol Sci Med Sci. 2006;61:1144–1150. doi: 10.1093/gerona/61.11.1144. [DOI] [PubMed] [Google Scholar]
- 53.Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, Chang WL, Yao WJ. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54–60. doi: 10.1111/j.1523-5378.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 54.D’Onghia V, Leoncini R, Carli R, Santoro A, Giglioni S, Sorbellini F, Marzocca G, Bernini A, Campagna S, Marinello E, et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed Pharmacother. 2007;61:137–141. doi: 10.1016/j.biopha.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Plonka M, Konturek PC, Bielanski W, Pawlik T, Brzozowski T, Konturek SJ. Relationship between ghrelin and Helicobacter pylori infection in Polish adult shepherds and their children. Aliment Pharmacol Ther. 2006;24:160–168. [Google Scholar]
- 56.Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429–432. doi: 10.1016/j.amjmed.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 57.Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol. 2005;11:1644–1648. doi: 10.3748/wjg.v11.i11.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S, et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711–1720. doi: 10.1111/j.1572-0241.2005.41492.x. [DOI] [PubMed] [Google Scholar]
- 59.Jun DW, Lee OY, Lee YY, Choi HS, Kim TH, Yoon BC. Correlation between gastrointestinal symptoms and gastric leptin and ghrelin expression in patients with gastritis. Dig Dis Sci. 2007;52:2866–2872. doi: 10.1007/s10620-006-9651-x. [DOI] [PubMed] [Google Scholar]
- 60.Kawashima J, Ohno S, Sakurada T, Takabayashi H, Kudo M, Ro S, Kato S, Yakabi K. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol. 2009;44:1046–1054. doi: 10.1007/s00535-009-0120-0. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki H, Nishizawa T, Tsuchimoto K, Hibi T. [Helicobacter pylori infected gastric mucosa--inflammation, atrophy and carcinogenesis] Nihon Saikingaku Zasshi. 2005;60:453–457. doi: 10.3412/jsb.60.453. [DOI] [PubMed] [Google Scholar]
- 62.Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10–16. doi: 10.1210/jc.2004-1330. [DOI] [PubMed] [Google Scholar]
- 63.Alonso N, Granada ML, Salinas I, Reverter JL, Flores L, Ojanguren I, Martínez-Cáceres EM, Sanmartí A. Plasma ghrelin concentrations in type 1 diabetic patients with autoimmune atrophic gastritis. Eur J Endocrinol. 2007;157:763–769. doi: 10.1530/EJE-07-0300. [DOI] [PubMed] [Google Scholar]
- 64.Plonka M, Bielanski W, Konturek SJ, Targosz A, Sliwowski Z, Dobrzanska M, Kaminska A, Sito E, Konturek PC, Brzozowski T. Helicobacter pylori infection and serum gastrin, ghrelin and leptin in children of Polish shepherds. Dig Liver Dis. 2006;38:91–97. doi: 10.1016/j.dld.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Uzzan B, Catheline JM, Lagorce C, Airinei G, Bon C, Cohen R, Perret GY, Aparicio T, Benamouzig R. Expression of ghrelin in fundus is increased after gastric banding in morbidly obese patients. Obes Surg. 2007;17:1159–1164. doi: 10.1007/s11695-007-9197-9. [DOI] [PubMed] [Google Scholar]
- 66.Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, Patterson C, Weinshel E, Fielding GA, Ren C, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089–1096. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–640. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang EJ, Park SW, Park JS, Park SJ, Hahm KB, Paik SY, Sin MK, Lee ES, Oh SW, Park CY, et al. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008;23 Suppl 2:S278–S285. doi: 10.1111/j.1440-1746.2008.05415.x. [DOI] [PubMed] [Google Scholar]
- 69.Czesnikiewicz-Guzik M, Loster B, Bielanski W, Guzik TJ, Konturek PC, Zapala J, Konturek SJ. Implications of oral Helicobacter pylori for the outcome of its gastric eradication therapy. J Clin Gastroenterol. 2007;41:145–151. doi: 10.1097/01.mcg.0000225654.85060.3d. [DOI] [PubMed] [Google Scholar]
- 70.Choe YH, Lee JH, Lee HJ, Paik KH, Jin DK, Song SY, Lee JH. Ghrelin Levels in Gastric Mucosa before and after Eradication of Helicobacter pylori. Gut Liver. 2007;1:132–137. doi: 10.5009/gnl.2007.1.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee ES, Yoon YS, Park CY, Kim HS, Um TH, Baik HW, Jang EJ, Lee S, Park HS, Oh SW. Eradication of Helicobacter pylori increases ghrelin mRNA expression in the gastric mucosa. J Korean Med Sci. 2010;25:265–271. doi: 10.3346/jkms.2010.25.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacifico L, Anania C, Osborn JF, Ferrara E, Schiavo E, Bonamico M, Chiesa C. Long-term effects of Helicobacter pylori eradication on circulating ghrelin and leptin concentrations and body composition in prepubertal children. Eur J Endocrinol. 2008;158:323–332. doi: 10.1530/EJE-07-0438. [DOI] [PubMed] [Google Scholar]
- 73.Nweneka CV, Prentice AM. Helicobacter pylori infection and circulating ghrelin levels - a systematic review. BMC Gastroenterol. 2011;11:7. doi: 10.1186/1471-230X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greenman Y, Rouach V, Limor R, Gilad S, Stern N. Testosterone is a strong correlate of ghrelin levels in men and postmenopausal women. Neuroendocrinology. 2009;89:79–85. doi: 10.1159/000151768. [DOI] [PubMed] [Google Scholar]
- 75.Bellone S, Rapa A, Vivenza D, Castellino N, Petri A, Bellone J, Me E, Broglio F, Prodam F, Ghigo E, et al. Circulating ghrelin levels as function of gender, pubertal status and adiposity in childhood. J Endocrinol Invest. 2002;25:RC13–RC15. doi: 10.1007/BF03344026. [DOI] [PubMed] [Google Scholar]
- 76.Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–1542. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 77.Fukuhara S, Suzuki H, Masaoka T, Arakawa M, Hosoda H, Minegishi Y, Kangawa K, Ishii H, Kitajima M, Hibi T. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G138–G145. doi: 10.1152/ajpgi.00298.2004. [DOI] [PubMed] [Google Scholar]
- 78.Zub-Pokrowiecka A, Rembiasz K, Konturek SJ, Budzynski A, Konturek PC, Budzynski P. Ghrelin in diseases of the gastric mucosa associated with Helicobacter pylori infection. Med Sci Monit. 2010;16:CR493–CR500. [PubMed] [Google Scholar]
- 79.Campana D, Nori F, Pagotto U, De Iasio R, Morselli-Labate AM, Pasquali R, Corinaldesi R, Tomassetti P. Plasma acylated ghrelin levels are higher in patients with chronic atrophic gastritis. Clin Endocrinol (Oxf) 2007;67:761–766. doi: 10.1111/j.1365-2265.2007.02959.x. [DOI] [PubMed] [Google Scholar]
- 80.Stec-Michalska K, Malicki S, Michalski B, Peczek L, Wisniewska-Jarosinska M, Nawrot B. Gastric ghrelin in relation to gender, stomach topography and Helicobacter pylori in dyspeptic patients. World J Gastroenterol. 2009;15:5409–5417. doi: 10.3748/wjg.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H, et al. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954–961. doi: 10.1007/s00535-006-1880-4. [DOI] [PubMed] [Google Scholar]
- 82.Liew PL, Lee WJ, Lee YC, Chen WY. Gastric ghrelin expression associated with Helicobacter pylori infection and chronic gastritis in obese patients. Obes Surg. 2006;16:612–619. doi: 10.1381/096089206776945002. [DOI] [PubMed] [Google Scholar]
- 83.Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121–2127. doi: 10.1111/j.1572-0241.2004.30291.x. [DOI] [PubMed] [Google Scholar]
- 84.Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ. 1998;316:1130–1132. doi: 10.1136/bmj.316.7138.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002;16:799–806. doi: 10.1046/j.1365-2036.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 86.Fujiwara Y, Higuchi K, Arafa UA, Uchida T, Tominaga K, Watanabe T, Arakawa T. Long-term effect of Helicobacter pylori eradication on quality of life, body mass index, and newly developed diseases in Japanese patients with peptic ulcer disease. Hepatogastroenterology. 2002;49:1298–1302. [PubMed] [Google Scholar]
- 87.Azuma T, Suto H, Ito Y, Muramatsu A, Ohtani M, Dojo M, Yamazaki Y, Kuriyama M, Kato T. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther. 2002;16 Suppl 2:240–244. doi: 10.1046/j.1365-2036.16.s2.31.x. [DOI] [PubMed] [Google Scholar]
- 88.Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:922–929. doi: 10.1111/j.1365-2036.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 89.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 90.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 91.Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003;48:257–265. doi: 10.1023/a:1021963007225. [DOI] [PubMed] [Google Scholar]
- 92.Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2002;368:121–129. doi: 10.1042/BJ20020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehmann FS, Terracciano L, Carena I, Baeriswyl C, Drewe J, Tornillo L, De Libero G, Beglinger C. In situ correlation of cytokine secretion and apoptosis in Helicobacter pylori-associated gastritis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G481–G488. doi: 10.1152/ajpgi.00422.2001. [DOI] [PubMed] [Google Scholar]
- 95.Eaton KA, Benson LH, Haeger J, Gray BM. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect Immun. 2006;74:4673–4684. doi: 10.1128/IAI.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellicanò A, Sebkova L, Monteleone G, Guarnieri G, Imeneo M, Pallone F, Luzza F. Interleukin-12 drives the Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa. Infect Immun. 2007;75:1738–1744. doi: 10.1128/IAI.01446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monteleone G, Del Vecchio Blanco G, Palmieri G, Vavassori P, Monteleone I, Colantoni A, Battista S, Spagnoli LG, Romano M, Borrelli M, et al. Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology. 2004;126:674–682. doi: 10.1053/j.gastro.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 98.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 99.Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hahm KB, Lee KM, Kim YB, Hong WS, Lee WH, Han SU, Kim MW, Ahn BO, Oh TY, Lee MH, et al. Conditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002;16 Suppl 2:115–127. doi: 10.1046/j.1365-2036.16.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 101.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 102.Mizuno T, Ando T, Nobata K, Tsuzuki T, Maeda O, Watanabe O, Minami M, Ina K, Kusugami K, Peek RM, et al. Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J Gastroenterol. 2005;11:6305–6311. doi: 10.3748/wjg.v11.i40.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caruso R, Fina D, Paoluzi OA, Del Vecchio Blanco G, Stolfi C, Rizzo A, Caprioli F, Sarra M, Andrei F, Fantini MC, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur J Immunol. 2008;38:470–478. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- 104.Hitzler I, Kohler E, Engler DB, Yazgan AS, Müller A. The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front Immunol. 2012;3:142. doi: 10.3389/fimmu.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D’Elios MM, Del Prete G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slomiany BL, Slomiany A. Involvement of p38 MAPK-dependent activator protein (AP-1) activation in modulation of gastric mucosal inflammatory responses to Helicobacter pylori by ghrelin. Inflammopharmacology. 2013;21:67–78. doi: 10.1007/s10787-012-0141-9. [DOI] [PubMed] [Google Scholar]
- 107.Murphy G, Kamangar F, Dawsey SM, Stanczyk FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Albanes D, Freedman ND. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011;103:1123–1129. doi: 10.1093/jnci/djr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tian PY, Fan XM. The proliferative effects of ghrelin on human gastric cancer AGS cells. J Dig Dis. 2012;13:453–458. doi: 10.1111/j.1751-2980.2012.00616.x. [DOI] [PubMed] [Google Scholar]
- 109.Tian C, Zhang L, Hu D, Ji J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol Cell Biochem. 2013;382:163–172. doi: 10.1007/s11010-013-1731-6. [DOI] [PubMed] [Google Scholar]


