Abstract
Immune thrombocytopenia (ITP) is an autoimmune disease mediated by anti-platelet autoantibodies. There is growing evidence that the eradication of Helicobacter pylori (H. pylori) effectively increases platelet count in a considerable proportion of ITP patients infected with this bacterium. In the majority of ITP patients responding to H. pylori eradication therapy, the anti-platelet autoantibody response is completely resolved with no relapse for more than 7 years, indicating that the disease is cured. Therefore, adult patients with suspected ITP should be examined for H. pylori infection, and eradication therapy is recommended if the infection is present. Notably, however, the efficacy of H. pylori eradication therapy in ITP patients varies widely among countries, with a higher response rate in Japan compared with the United States and European countries other than Italy. The pathogenesis of H. pylori-associated ITP is still uncertain, although the mechanisms are known to involve multiple factors. H. pylori may modulate the Fcγ-receptor balance of monocytes/macrophages in favor of activating Fcγ receptors, and H. pylori components may mimic the molecular makeup of platelet antigens. Further studies of the pathogenic process of H. pylori-associated ITP may be useful for the development of new therapeutic strategies for ITP.
Keywords: Autoantibody, Childhood, Helicobacter pylori, Fcγ receptor, Immune thrombocytopenia, Idiopathic thrombocytopenic purpura, Systemic lupus erythematosus
Core tip: In this review, we summarize recent updates on basic and clinical aspects of Helicobacter pylori (H. pylori)-associated immune thrombocytopenia (ITP). We highlight the efficacy of H. pylori eradication in adult and childhood ITP as well as in secondary ITP, variability in the efficacy of eradication in various countries, factors predicting the eradication-related platelet response, and the mechanisms responsible for the development of ITP in association with H. pylori infection. It is apparent that in a distinct subgroup of H. pylori-associated ITP, this bacterial infection is central to the ITP pathogenesis.
INTRODUCTION
Helicobacter pylori (H. pylori), a gram-negative spiral bacterium, is the causative agent in chronic gastritis, gastric and duodenal ulcer disease, and gastric cancer. H. pylori has also been implicated in the pathogenesis of extra-digestive disorders, including cardiovascular, hematologic, and autoimmune diseases[1]. The strongest evidence has been reported for immune thrombocytopenia (ITP), with high-quality studies showing that the disease improved after H. pylori was successfully eradicated.
ITP is a typical organ-specific autoimmune disease; it is mediated by anti-platelet autoantibodies that bind to platelets and megakaryocytes, accelerating platelet destruction by the reticuloendothelial system and suppressing platelet production[2]. The autoantibody response primarily targets platelet-surface glycoproteins such as GPIIb/IIIa and GPIb. This condition is known as primary ITP when it occurs without an underlying disease, but it is also seen in patients with various diseases, including systemic lupus erythematosus (SLE). Although the etiology of ITP is obscure, microorganisms such as human immunodeficiency virus and hepatitis C virus are known to contribute to its development[3], indicating that in a particular subset of ITP, infectious agents play a significant role in the pathogenesis of the autoimmune response.
First observed in 1988[4], the increase in platelet counts in ITP patients after eradicating H. pylori has since been confirmed by several studies. Consequently, H. pylori eradication therapy is now a treatment option for ITP[5]. However, a number of questions regarding the relationship between H. pylori infection and ITP remained unsolved, including the great variability in the efficacy of H. pylori eradication therapy among countries, factors predicting the platelet response after H. pylori eradication, and mechanisms responsible for the platelet response associated with H. pylori eradication[6]. Some of these questions have been answered in the past few years. This review summarizes recent updates on clinical and therapeutic aspects of H. pylori-associated ITP, as well as the pathogenesis of this disease.
EFFICACY OF H. PYLORI ERADICATION IN ITP
Adult ITP
In 1988, Gasbarrini et al[4] reported that platelet counts increased in all of 8 H. pylori-infected patients with ITP who were treated with a regimen to eradicate H. pylori, while the platelet counts were unchanged in 3 H. pylori-infected patients who did not receive the regimen. Reports followed of partial or complete platelet responses observed in a large proportion of ITP patients treated with an H. pylori eradication regimen consisting of a 1- to 2-wk standard triple therapy with a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole[6-8]. A nation-wide survey in Japan involved 207 H. pylori-infected adult patients with ITP, making it the largest study on the efficacy of eradicating H. pylori in ITP patients[9]. In that study, after the successful eradication of H. pylori, 63% of the patients achieved some degree of platelet recovery, and within this group, 23% showed complete remission at 12 mo after the eradication. Although most early studies excluded patients with severe thrombocytopenia, who were at high risk of bleeding, several case series have reported the efficacy of eradicating H. pylori even in patients with refractory ITP, including patients with severe thrombocytopenia that resisted multiple therapeutic regimens including splenectomy[9-11]. Although most studies began assessing the platelet counts one month after starting eradication therapy, we observed platelet recovery after just a week in almost half of those responding[12]. Long-term follow-up studies showed that this platelet response lasted 7 or more years after H. pylori was eradicated, with very few cases of relapse[13,14]. An assessment of circulating B cells producing anti-GPIIb/IIIa antibodies before and after eradication treatment indicated that the anti-platelet autoantibodies disappeared after platelet recovery when H. pylori had been successfully eradicated[12]. Thus, in certain patients, ITP appears to be clinically and immunologically cured by eradicating H. pylori.
In the first systematic review with meta-analysis, Franchini et al[15] reviewed 788 patients with ITP, including 494 H pylori-infected patients collected from 17 studies, in 2007. Platelet counts increased in ITP patients who received eradication treatment, compared with untreated patients, and the weighted mean difference (WMD) in platelet count was 34.0 × 109/L regardless of the outcome of H. pylori eradication. The platelet counts increased significantly in H. pylori-infected patients after successful H. pylori eradication, compared with the following groups: untreated H. pylori-infected patients (WMD of 40.8 × 109/L), H. pylori-infected patients who failed eradication (WMD of 52.2 × 109/L), and H. pylori-uninfected patients (WMD of 46.4 × 109/L). Another systematic literature review involving 1555 patients revealed a weighted mean complete response (platelet count ≥ 100 × 109/L) after successful H. pylori eradication of 42.7%, and an overall response (platelet count ≥ 30 × 109/L, and at least doubling of the basal count) of 50.3%[16]. Even in patients with a low baseline platelet count (< 30 × 109/L), the overall response rate was 35.2%, including 20.1% with a complete response. These findings indicate that H. pylori eradication is closely related to platelet recovery in adult ITP patients. Finally, another systematic review evaluated the efficacy of the H. pylori eradication regimen in patients with ITP by comparing the platelet response in patients with or without an H. pylori infection[17]. The odds of achieving platelet recovery following the eradication regimen were 14.5 times higher in 205 H. pylori-infected patients than in 77 H. pylori-uninfected patients. This clearly indicates that platelet recovery after the eradication regimen results from the eradication of H. pylori itself, rather than from H. pylori-independent mechanisms such as immune-modulatory effects of the drugs themselves, or the eradication of bacteria other than H. pylori.
The clear linkage between platelet recovery and the disappearance of H. pylori suggests a direct role for H. pylori infection in the ITP pathogenesis. In addition, in the majority of ITP patients who achieved a complete platelet response after H. pylori eradication, the anti-platelet autoantibody response was also eliminated. Thus, patients who are infected with H. pylori and who respond to the eradication therapy fall into a distinct, widely recognized ITP subgroup, termed H. pylori-associated ITP[18,19], that is considered a type of secondary ITP[5]. Given the relatively high efficacy, safety, and economy of H. pylori eradication therapy, H. pylori detection should be considered when examining adult patients who are suspected to have ITP, and eradication therapy is recommended if H. pylori infection is present[5,8].
Variability among countries
Although the efficacy of H. pylori eradication therapy in infected adults with ITP has been confirmed by high-quality systematic reviews, some studies have reported little to no platelet response after H. pylori eradication therapy. For example, Jarque et al[20] and Ahn et al[21] observed platelet recovery was in only 13% and 7%, respectively, of adult patients with ITP after successfully eradicating H. pylori. Michel et al[22] found no platelet response in 14 H. pylori-infected ITP patients even after successfully eradicating the H. pylori. These widely varying reports of the efficacy of H. pylori eradication therapy in ITP patients are explained, in part, by different eligibility criteria and different definitions of platelet response among the various studies. However, response rates differed between the 57.9% reported by studies in Japan, and the 38.3% rate reported by studies from other countries[16]. Studies from Japan and Italy tend toward better response rates, ranging from 28% to 100%, than studies from the United States and Spain (< 13).
Thus, variability in the efficacy of H. pylori eradication in different populations should be considered to establish recommendations for screening and eradicating H. pylori. For example, H. pylori screening is certainly worthwhile in Japan, a country with a high prevalence of infection and a high response rate to eradication treatment. In fact, in a recently developed reference guide for managing adult ITP in Japan, H. pylori eradication is a prominent strategy for managing ITP in adult patients[23]. Namely, the guide states that all patients diagnosed with ITP should be screened for H. pylori infection, and eradication therapy is recommended as a first line of treatment, regardless of platelet count, if H. pylori infection is present. These recommendations may not be appropriate in the United States or in European countries (other than Italy), in which both the prevalence of infection and the response rates to eradication therapy are low. Indeed, H. pylori eradication is not mentioned in the international consensus report on the management of primary ITP[24], while the eradication therapy is recommended in patients who are found to have H. pylori infection in the American Society of Hematology 2011 evidence-based practice guideline for ITP[5].
A recent systematic review demonstrated a correlation in ITP patients between the prevalence of H. pylori infection and the platelet response rates to eradication therapy[16]. The reason for such variability among countries is not clear, but differences in the epidemic H. pylori strains according to geographical area could account for differences in the clinical response. In this regard, H. pylori strains that possess cytotoxin-associated gene A (CagA) have been proposed to have a role in the pathogenesis of H. pylori-associated ITP based on the potential cross-reactivity between CagA and platelet glycoproteins[25,26]. The frequency of CagA-positive strains varies by geographic location: the majority of the H. pylori strains found in Eastern Asia, including Japan, express CagA, whereas the proportion of CagA-positive strains in European countries and North America is much lower[27]. However, genetic and other environmental factors are also likely to contribute to the variability in platelet recovery after H. pylori eradication among different populations.
Childhood ITP
The clinical course of ITP is quite different in children than in adult patients. ITP in children is usually an acute form with spontaneous recovery within 6 mo, although thrombocytopenia lasts more than 6 mo in about 20% of children with ITP[28]. There are only a few pediatric studies evaluating the role of H. pylori infection in chronic ITP in children. Table 1 summarizes the studies assessing the prevalence of H. pylori infection and platelet response after eradicating H. pylori in children with ITP. Although a Finnish study failed to detect H. pylori infection in any of 17 children with chronic ITP[29], other studies detected H. pylori infection in a small proportion of children with chronic ITP[30-42]. The highest prevalence was reported in a study conducted in Taiwan, which showed an infection rate of 41%[29]. A recent multicenter study in Italy revealed that 50 (20) of 244 children with ITP were infected with H. pylori[42]. In general, the prevalence of H. pylori infection is lower in children than in adults with ITP in a given population.
Table 1.
Prevalence of Helicobacter pylori infection and platelet response after Helicobacter pylori eradication in children with immune thrombocytopenia n (%)
| Ref. | Country | Prevalence of Helicobacter pylori infection | Platelet response |
| Rajantie et al[29] | Finland | 0/17 (0) | ND |
| Jaing et al[30] | Taiwan | 9/22 (41) | 5/9 (50) |
| Hayashi et al[31] | Japan | 2/10 (20) | 1/1 (100) |
| Yetgin et al[32] | Turkey | 11/35 (31) | 0/9 (0) |
| Jaing et al[33] | Taiwan | 10/63 (16) | ND |
| Loffredo et al[34] | Italy | 8/39 (21) | 0/8 (0) |
| Neefjes et al[35] | Netherlands | 3/47 (6) | 3/3 (100) |
| Wu et al[36] | Taiwan | 6/32 (19) | ND |
| Bisogno et al[37] | Italy | 8/24 (33) | 1/8 (13) |
| Hamidieh et al[38] | Iran | 4/31 (13) | 0/4 (0) |
| Treepongkaruna et al[39] | Thailand | 16/55 (29) | 0/7 (0) |
| Ferrara et al[40] | Italy | 8/24 (33) | 8/8 (100) |
| Maghbool et al[41] | Iran | 5/30 (17) | 5/5 (100) |
| Russo et al[42] | Italy | 50/244 (20) | 13/33 (39) |
ND: Not described.
Reports of platelet recovery after H. pylori eradication therapy in children are highly variable and inconsistent. In the first study in Taiwan, platelet counts increased in 5 (56) of 9 H. pylori-infected children with ITP after eradication therapy[29]. In studies from the Netherland, Italy, and Iran, platelet counts increased measurably in all H. pylori-infected children after eradication treatment, although the number of subjects analyzed was very small (3, 8, and 5, respectively)[35,40,41]. However, other studies from Turkey, Italy, Iran, and Thailand showed that none of the patients who underwent treatment to eradicate H. pylori infection responded to it[32,34,38,39]. Even studies from the same country gave diametrically opposite accounts of the efficacy of H. pylori eradication. These contradictory results may be partly due to the small number of H. pylori-infected children with ITP. An Italian study, which had the largest number of patients, showed that 13 (39) of 33 children with H. pylori infection responded to the eradication treatment[42]. The prevalence of H. pylori infection in children with chronic ITP is generally low, suggesting that H. pylori infection plays only a minor role in the development of childhood ITP. However, we should recognize that some children infected with H. pylori can recover if the H. pylori is successfully eliminated. A large-scale study is necessary to confirm the relationship between H. pylori infection and childhood ITP.
Other forms of secondary ITP
ITP may develop in the context of other disorders or conditions, including lymphoproliferative, autoimmune, and infectious diseases[43]. Although H. pylori-associated ITP is now categorized as a secondary ITP condition, the efficacy of H. pylori eradication therapy has not been deeply examined in patients with other types of secondary ITP. To evaluate the role of H. pylori infection in the pathogenesis of secondary ITP, we conducted an open-label prospective study involving 34 consecutive H. pylori-infected patients with ITP, including 16 with primary ITP, 8 with secondary ITP associated with SLE, and 10 with secondary ITP associated with liver cirrhosis. All the patients received a standard H. pylori eradication regimen consisting of amoxicillin, clarithromycin, and lansoprazole, and the H. pylori was successfully eradicated in all except one patient with primary ITP, and another with liver cirrhosis. As shown in Figure 1, the platelet count had increased 3 mo after eradication treatment in nearly all the patients with primary ITP, but was virtually unchanged in patients with SLE or liver cirrhosis. In addition, a decrease in circulating anti-GPIIb/IIIa antibody-producing B cells was observed in patients with primary ITP, but not in ITP patients with SLE or liver cirrhosis. These findings clearly indicate that the eradication of H. pylori fails to improve the pathogenic process in patients with some forms of secondary ITP. Thus, the efficacy of H. pylori eradication therapy is likely to be restricted to patients in a subgroup of seemingly primary ITP (H. pylori-associated ITP), and H. pylori infection appears to be uninvolved in the pathogenic process of other secondary ITPs.
Figure 1.
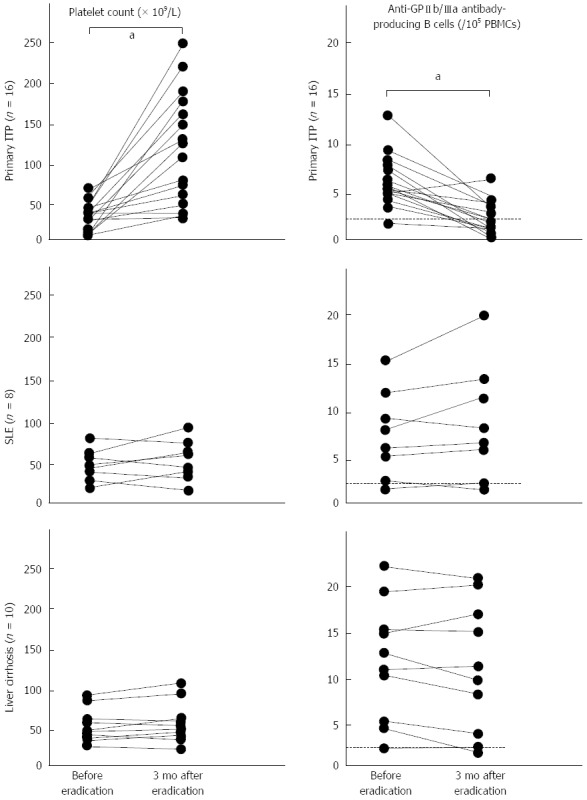
Changes in platelet count and in anti-GPIIb/IIIa antibody-producing circulating B cells before and 3 mo after an Helicobacter pylori eradication regimen in Helicobacter pylori-positive immune thrombocytopenia patients with no additional disease, or with systemic lupus erythematosus or liver cirrhosis. Changes in absolute values were compared by paired t test. aP < 0.05. A dotted line indicates the cut-off for circulating anti-GPIIb/IIIa antibody-producing B cells, which was 2/105 peripheral blood mononuclear cells (PBMCs). ITP: Immune thrombocytopenia; SLE: Systemic lupus erythematosus.
CHARACTERISTICS OF H. PYLORI-ASSOCIATED ITP
Features of ITP patients infected with H. pylori
Several authors have tried to identify characteristics of H. pylori-associated ITP by comparing the clinical features of adult ITP patients with or without H. pylori infection. ITP patients infected with H. pylori were significantly older than uninfected patients[9], but this is predictable because the prevalence of H. pylori infection increases with age in the general population[44]. Multiple studies have failed to detect significant differences in any other demographic or clinical characteristic, including sex, platelet count, or response to therapy.
Several studies have looked for differences in genetic factors in ITP patients with or without H. pylori. Veneri et al[45] examined the human leukocyte antigen (HLA)-DRB1 and DQB1 alleles in Italian patients with ITP, and found that H. pylori-positive patients had a lower frequency of DRB1*03, and higher frequencies of DRB1*11, DRB1*14, and DQB1*03, compared with H. pylori-negative patients. However, we failed to detect any association between H. pylori infection and HLA-DRB1 or DQB1 alleles in Japanese patients with ITP[46]. Instead, we found that gene polymorphism within the loci for interleukin (IL)-1β was associated with H. pylori infection in patients diagnosed before age 50. While these observations suggest an involvement of genetic background in H. pylori-related ITP, the associations should be confirmed by replication studies enrolling patients from other populations.
On the other hand, there was no difference in the IL-2, IL-4, or IL-6 serum levels between patients with and without H. pylori infection[47]. The serum levels of chemokines, including monocyte chemoattractant protein-1, regulated upon activation normally T-cell expressed and secreted, and epithelial cell-derived neutrophil attractant-78, were significantly higher in patients with H. pylori infection than in those without[48], although increased levels of these chemokines were also observed in individuals that had H. pylori-related gastrointestinal disorders but did not have ITP. Thus, studies have failed to identify demographic, clinical, genetic, or immunologic characteristics unique to ITP patients infected with H. pylori. This is probably because there are at least two distinct subgroups of H. pylori-infected ITP patients: those with H. pylori-associated secondary ITP, who respond to eradication therapy, and those with primary ITP and a coincidental H. pylori infection.
Factors predicting a positive response to H. pylori eradication therapy
Parameters that predict the platelet response to H. pylori eradication therapy have been extensively analyzed in H. pylori-infected ITP patients. The most consistently reported feature that predicts a favorable response is a shorter duration of ITP[9,49,50], but other studies have not found this association[51-53]. Other clinical characteristics, including an age less than 65 when diagnosed with ITP[49], a higher baseline platelet count[49], no prior corticosteroid therapy[51], no concomitant corticosteroid therapy[54,55], and no prior therapy for ITP[49], have been reported as factors predicting the platelet response, but other studies have reported conflicting results.
Several studies have examined whether there is a genetic predisposition to the platelet response. An association was shown between the HLA-DQB1*03 haplotypes and a higher probability of the platelet response[45]. Moreover, single nucleotide polymorphisms within the genes for tumor necrosis factor-β and an inhibitory Fcγ receptor IIB (FcγRIIB) were found to be useful for predicting the response to the eradication treatment[55,56]. In terms of the anti-platelet autoantibody specificity, we found that the presence of an anti-GPIb autoantibody response predicts resistance to H. pylori eradication therapy. Interestingly, a study from Italy reported that ITP patients with antibodies to CagA were more likely to respond to eradication therapy than patients without these antibodies[57]; although a study conducted in Japan failed to confirm this observation[50], we should recognize that CagA-positive strains are more common in Japan than in Italy.
Finally, Sato et al[58] assessed potential associations of the platelet response to H. pylori eradication with upper gastrointestinal endoscopic findings and histologic features of stomach tissue obtained by biopsy. A severe degree of gastric atrophy on endoscopy and intense inflammation and atrophy in the gastric corpus detected by biopsy were predictors for a favorable response. These findings together indicate that both genetic background and bacterial factors, which collaboratively regulate the host inflammatory response to the bacterium, can account, at least in part, for the variable response to H. pylori eradication therapy.
MECHANISMS OF H. PYLORI-ASSOCIATED ITP
It has become clear that H. pylori-associated ITP is a subset of ITP in which H. pylori infection is actively involved in the pathogenic process. In patients with H. pylori-associated ITP, H. pylori eradication increases the platelet count in parallel with a suppression of anti-platelet autoantibody production, and results in the remission or even cure of the disease in many patients. Since eradicating H. pylori does not increase the platelet count in non-ITP subjects[59], the platelet recovery after successful H. pylori eradication is specific to ITP patients, and is likely to be mediated through the inhibition of an ongoing autoimmune response to platelets.
Molecular mimicry
Several hypotheses have been proposed regarding the mechanism by which H. pylori induces the development of ITP. One intriguing theory is that cross-reactive antibodies are produced that react with both H. pylori components and platelet surface antigens through molecular mimicry. Michel et al[22] investigated this molecular-mimicry hypothesis by testing platelet eluates derived from H. pylori-infected ITP patients, and found that platelet eluates with the capacity to react with GPIIb/IIIa or GPIb failed to recognize any H. pylori antigens. On the other hand, Takahashi et al[25] reported that platelet eluates from H. pylori-infected ITP patients recognized CagA in immunoblots, but those from H. pylori-infected non-thrombocytopenic individuals did not. Unfortunately, since the IgG concentrations in the eluates were not adjusted in these studies, the intensity of individual bands was not quantitative. In addition, since platelets are known to take up and concentrate circulating IgG in intracellular granules, it is not clear whether the anti-CagA antibodies detected truly cross-react with platelet-surface antigens. Although it was recently reported that monoclonal antibodies generated against recombinant H. pylori urease B react with GPIIb/IIIa expressed on the platelet surface, this study failed to show the presence of this cross-reactive antibody repertoire in patients with H. pylori-associated ITP[60]. While these findings suggest that cross-reacting antibodies against H. pylori may be present in patients with ITP, their pathogenic role remains obscure.
Non-specific activation of the immune system
In another potential mechanism, chronic H. pylori infection may act on the host’s immune system to stimulate acquired immune responses, causing autoreactive T and B cells to emerge. Yamanishi et al[61] showed that H. pylori components are able to initiate autoimmune responses via autoantibodies that are produced through the activation of B-1 cells. However, this non-specific mechanism alone does not explain how an autoimmune response specific to platelet glycoproteins, such as that observed in ITP patients, would develop. In fact, there is no difference in the production of non-specific autoantibodies, including anti-nuclear, anti-microsome, and anti-smooth muscle antibodies, in individuals with and without H. pylori[62].
Modulation of monocyte/macrophage function
We have been evaluating mechanisms that elicit and maintain autoantibody responses to platelet glycoproteins in patients with ITP[63-65]. After detailed analyses of the GPIIb/IIIa-reactive CD4+ T cells and B cells in ITP patients, we proposed a “pathogenic loop” model for the ongoing IgG anti-platelet autoantibody response in ITP patients[66]. Specifically, macrophages in the reticuloendothelial system capture opsonized platelets via Fcγ receptors, and present antigenic platelet glycoprotein-derived peptides to T cells. Autoreactive CD4+ T cells are then activated by their recognition of the antigenic peptides and exert helper activity to stimulate B cells to produce IgG anti-platelet autoantibodies, which in turn bind to circulating platelets. Theoretically, once this pathogenic loop is established, the production of IgG anti-platelet autoantibodies continues endlessly. Since anti-platelet autoantibodies are eliminated after eradicating H. pylori in patients with H. pylori-associated ITP[12], this pathogenic loop would consequently be disrupted and blocked. To elucidate the mechanism by which H. pylori eradication changes the ongoing pathogenic loop in ITP patients, we conducted a prospective study in which the phenotype and function of the autoreactive T and B cells and of the monocytes/macrophages involved in the pathogenic loop were serially measured in H. pylori-infected and -uninfected ITP patients treated with a standard eradication regimen[67]. At baseline, we found enhanced phagocytic capacity and low expression levels of inhibitory FcγRIIB in the circulating monocytes from H. pylori-infected patients, but not in those from uninfected patients. Suppression of this activated-monocyte phenotype was observed one week after starting the H. pylori eradication regimen, when eradication was successful. The anti-platelet autoantibody responses and platelet kinetic parameters subsequently improved, indicating that suppression of the activated-monocyte function precedes the improvement in the autoantibody response.
Interestingly, modulation of the Fcγ-receptor balance toward an activating phenotype has also been observed in H. pylori-infected mice, through a downregulation of inhibitory FcγRIIB in splenic and circulating monocytes/macrophages. A study in China recently confirmed that the FcγRIIB expression on circulating monocytes is down-regulated in H. pylori-infected ITP patients[68]. Therefore, H. pylori infection plays an important role in ITP pathogenesis by altering the Fcγ receptor balance of monocytes/macrophages in favor of activating Fcγ receptors, through downregulation of the inhibitory receptor FcγRIIB. These findings indicate that the platelet recovery after H. pylori eradication in ITP patients is mediated, at least in part, through a change in Fcγ receptor balance toward the inhibitory FcγRIIB. The molecular events that induce this change in monocytes/macrophage properties are unclear. Although H. pylori does not invade the gastric epithelium, it can induce both the secretion of soluble inflammatory mediators and cellular apoptosis in the host, leading to local inflammation in the epithelium and the subepithelial layers. Released H. pylori components have been reported to be responsible for activating dendritic cells and macrophages through toll-like receptor signaling[69,70].
Interestingly, a change in Fcγ receptor balance toward the inhibitory FcγRIIB in monocytes/macrophages has also reported in the therapeutic action of other established treatment regimens for ITP, such as intravenous immunoglobulin and high-dose dexamethasone. Samuelsson et al. demonstrated that intravenous immunoglobulin requires the presence of FcγRIIB to prevent antibody-induced thrombocytopenia in a murine model of passive ITP[71], in which the FcγRIIB expression on splenic macrophages was upregulated upon intravenous immunoglobulin treatment. The upregulation of inhibitory FcγRIIB expression on circulating monocytes was also reported in ITP patients successfully treated with high-dose dexamethasone[72]. These findings point to the Fcγ receptor balance of monocytes/macrophages as an attractive therapeutic target for ITP.
A multifactorial mechanism
The pathogenesis of H. pylori-associated ITP most likely involves several factors. The mechanism of Fcγ-receptor-balance modulation in monocytes/macrophages does not exclude other proposed mechanisms for platelet recovery in ITP after H. pylori eradication, such as molecular mimicry between CagA and platelet-surface antigens. Moreover, some H. pylori strains induce platelet aggregation that is dependent on the interaction of von Willebrand factor and IgG antibodies against H. pylori with their corresponding receptors, GPIb and FcγRIIA, on platelets[73]. In this model, anti-H. pylori antibodies are capable of opsonizing platelets by binding to H. pylori, von Willebrand factor, and GPIb, like anti-platelet autoantibodies. However, we should recognize that H. pylori infection is usually established in infants with an immature immune system, and that the prevalence of ITP among H. pylori-infected individuals is extremely low. Therefore, it is apparent that H. pylori infection alone is insufficient to induce the onset of ITP. Additional triggers would be necessary to elicit the anti-platelet autoimmune response observed in H. pylori-associated ITP.
CONCLUSION
The past few years have witnessed important advances in our understanding of the relationship between H. pylori infection and ITP. The sustained efficacy of the H. pylori eradication regimen in adults with ITP has affected decision-making in clinical practice. The importance of detecting and eradicating H. pylori in patients with seemingly typical ITP is now recognized, especially where H. pylori infection is prevalent, such as in Japan. A growing body of evidence indicates that H. pylori-associated ITP is a unique ITP subset in which the bacterium is central to the pathogenic process. The development of H. pylori-associated ITP appears to depend on multiple factors. Among these, modulation of the Fcγ receptor balance of monocytes/macrophages through inhibition of the immunosuppressive FcγRIIB signal, which is a host immune response associated with H. pylori infection, is a key mechanism for initiating and maintaining the anti-platelet autoantibody response. These insights provide valuable clues that may assist in the development of new therapeutic strategies for ITP.
ACKNOWLEDGMENTS
I am grateful to Yuka Okazaki for collecting data on the anti-GPIIb/IIIa autoantibody response.
Footnotes
Supported by A research grant for Research on Intractable Diseases from the Japanese Ministry of Health, Labor, and Welfare, No. H23-Nanchi-Ippan-002
P- Reviewers: Lee YC, Rabelo-Goncalves EMA S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
References
- 1.Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012;13:342–349. doi: 10.1111/j.1751-2980.2012.00599.x. [DOI] [PubMed] [Google Scholar]
- 2.Stasi R. Immune thrombocytopenia: pathophysiologic and clinical update. Semin Thromb Hemost. 2012;38:454–462. doi: 10.1055/s-0032-1305780. [DOI] [PubMed] [Google Scholar]
- 3.Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23:1275–1297. doi: 10.1016/j.hoc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 5.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 6.Kuwana M, Ikeda Y. Helicobacter pylori and immune thrombocytopenic purpura: unsolved questions and controversies. Int J Hematol. 2006;84:309–315. doi: 10.1532/IJH97.06188. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Veneri D. Helicobacter pylori-associated immune thrombocytopenia. Platelets. 2006;17:71–77. doi: 10.1080/09537100500438057. [DOI] [PubMed] [Google Scholar]
- 8.Stasi R, Provan D. Helicobacter pylori and Chronic ITP. Hematology Am Soc Hematol Educ Program. 2008:206–211. doi: 10.1182/asheducation-2008.1.206. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura K, Kuwana M, Kurata Y, Imamura M, Harada H, Sakamaki H, Teramura M, Koda K, Nomura S, Sugihara S, et al. Is eradication therapy useful as the first line of treatment in Helicobacter pylori-positive idiopathic thrombocytopenic purpura? Analysis of 207 eradicated chronic ITP cases in Japan. Int J Hematol. 2005;81:162–168. doi: 10.1532/ijh97.04146. [DOI] [PubMed] [Google Scholar]
- 10.Soldinger E, Pilia MC, Piubello W, Nadali G. Multi-resistant idiopathic thrombocytopenia successfully treated by eradication of Helicobacter pylori. Dig Liver Dis. 2001;33:732. doi: 10.1016/s1590-8658(01)80053-0. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldi CR, Camera A, Viscardi G, Pane F, Rotoli B. Complete remission in a case of severe multi-resistant idiopathic thrombocytopenic purpura after Helicobacter pylori eradication. Am J Hematol. 2008;83:683–684. doi: 10.1002/ajh.21200. [DOI] [PubMed] [Google Scholar]
- 12.Asahi A, Kuwana M, Suzuki H, Hibi T, Kawakami Y, Ikeda Y. Effects of a Helicobacter pylori eradication regimen on anti-platelet autoantibody response in infected and uninfected patients with idiopathic thrombocytopenic purpura. Haematologica. 2006;91:1436–1437. [PubMed] [Google Scholar]
- 13.Tsumoto C, Tominaga K, Okazaki H, Tanigawa T, Yamagami H, Watanabe K, Nakao T, Koh K, Watanabe T, Fujiwara Y, et al. Long-term efficacy of Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura: 7-year follow-up prospective study. Ann Hematol. 2009;88:789–793. doi: 10.1007/s00277-008-0667-5. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi T, Kobayashi T, Yamashita T, Ohashi K, Sakamaki H, Akiyama H. Eight-year follow-up of patients with immune thrombocytopenic purpura related toH. pyloriinfection. Platelets. 2011;22:61–64. doi: 10.3109/09537104.2010.515272. [DOI] [PubMed] [Google Scholar]
- 15.Franchini M, Cruciani M, Mengoli C, Pizzolo G, Veneri D. Effect of Helicobacter pylori eradication on platelet count in idiopathic thrombocytopenic purpura: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:237–246. doi: 10.1093/jac/dkm195. [DOI] [PubMed] [Google Scholar]
- 16.Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 17.Arnold DM, Bernotas A, Nazi I, Stasi R, Kuwana M, Liu Y, Kelton JG, Crowther MA. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica. 2009;94:850–856. doi: 10.3324/haematol.2008.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimura K. Helicobacter pylori infection and idiopathic thrombocytopenic purpura. Int J Hematol. 2005;81:113–118. doi: 10.1532/ijh97.04161. [DOI] [PubMed] [Google Scholar]
- 19.Franchini M, Vescovi PP, Garofano M, Veneri D. Helicobacter pylori-associated idiopathic thrombocytopenic purpura: a narrative review. Semin Thromb Hemost. 2012;38:463–468. doi: 10.1055/s-0032-1305781. [DOI] [PubMed] [Google Scholar]
- 20.Jarque I, Andreu R, Llopis I, De la Rubia J, Gomis F, Senent L, Jiménez C, Martín G, Martínez JA, Sanz GF, et al. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2001;115:1002–1003. doi: 10.1046/j.1365-2141.2001.03194.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahn ER, Tiede MP, Jy W, Bidot CJ, Fontana V, Ahn YS. Platelet activation in Helicobacter pylori-associated idiopathic thrombocytopenic purpura: eradication reduces platelet activation but seldom improves platelet counts. Acta Haematol. 2006;116:19–24. doi: 10.1159/000092343. [DOI] [PubMed] [Google Scholar]
- 22.Michel M, Khellaf M, Desforges L, Lee K, Schaeffer A, Godeau B, Bierling P. Autoimmune thrombocytopenic Purpura and Helicobacter pylori infection. Arch Intern Med. 2002;162:1033–1036. doi: 10.1001/archinte.162.9.1033. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura K, Miyakawa Y, Kurata Y, Kuwana M, Tomiyama Y, Murata M. Reference guide for management of adult idiopathic thrombocytopenic purpura (ITP) 2012 version. Rinsho Ketsueki. 2012;53:433–442. [PubMed] [Google Scholar]
- 24.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi F, Christodoulides N, Kroll MH, Genta RM. Helicobacter pylori and idiopathic thrombocytopenic purpura. Ann Intern Med. 2004;140:766–767. doi: 10.7326/0003-4819-140-9-200405040-00028. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol. 2012;12:203–213. doi: 10.1016/j.meegid.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Mattia D, Del Vecchio GC, Russo G, De Santis A, Ramenghi U, Notarangelo L, Jankovic M, Molinari AC, Zecca M, Nobili B, et al. Management of chronic childhood immune thrombocytopenic purpura: AIEOP consensus guidelines. Acta Haematol. 2010;123:96–109. doi: 10.1159/000268855. [DOI] [PubMed] [Google Scholar]
- 29.Rajantie J, Klemola T. Helicobacter pylori and idiopathic thrombocytopenic purpura in children. Blood. 2003;101:1660. doi: 10.1182/blood-2002-10-3201. [DOI] [PubMed] [Google Scholar]
- 30.Jaing TH, Yang CP, Hung IJ, Chiu CH, Chang KW. Efficacy of Helicobacter pylori eradication on platelet recovery in children with chronic idiopathic thrombocytopenic purpura. Acta Paediatr. 2003;92:1153–1157. doi: 10.1080/08035250310005648. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi H, Okuda M, Aoyagi N, Yoshiyama M, Miyashiro E, Kounami S, Yoshikawa N. Helicobacter pylori infection in children with chronic idiopathic thrombocytopenic purpura. Pediatr Int. 2005;47:292–295. doi: 10.1111/j.1442-200x.2005.02058.x. [DOI] [PubMed] [Google Scholar]
- 32.Yetgin S, Demir H, Arslan D, Unal S, Koçak N. Autoimmune thrombocytopenic purpura and Helicobacter pylori infection effectivity during childhood. Am J Hematol. 2005;78:318. doi: 10.1002/ajh.20302. [DOI] [PubMed] [Google Scholar]
- 33.Jaing TH, Tsay PK, Hung IJ, Chiu CH, Yang CP, Huang IA. The role of Helicobacter pylori infection in children with acute immune thrombocytopenic purpura. Pediatr Blood Cancer. 2006;47:215–217. doi: 10.1002/pbc.20577. [DOI] [PubMed] [Google Scholar]
- 34.Loffredo G, Marzano MG, Migliorati R, Miele E, Menna F, Poggi V, Staiano A. The relationship between immune thrombocytopenic purpura and Helicobacter pylori infection in children: where is the truth? Eur J Pediatr. 2007;166:1067–1068. doi: 10.1007/s00431-006-0344-4. [DOI] [PubMed] [Google Scholar]
- 35.Neefjes VM, Heijboer H, Tamminga RY. H. pylori infection in childhood chronic immune thrombocytopenic purpura. Haematologica. 2007;92:576. doi: 10.3324/haematol.10940. [DOI] [PubMed] [Google Scholar]
- 36.Wu KS, Hsiao CC, Yu HR, Huang EY, Mai WL, Sheen JM. Helicobacter pylori infection and childhood idiopathic thrombocytopenic purpura. Acta Paediatr Taiwan. 2007;48:263–266. [PubMed] [Google Scholar]
- 37.Bisogno G, Errigo G, Rossetti F, Sainati L, Pusiol A, Da Dalt L, Colleselli P, Grotto P, Carli M. The role of Helicobacter pylori in children with chronic idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2008;30:53–57. doi: 10.1097/MPH.0b013e3181615613. [DOI] [PubMed] [Google Scholar]
- 38.Hamidieh AA, Arzanian MT, Gachkar L, Pasha F. Helicobacter pylori infection in children with chronic idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2008;30:96–97. doi: 10.1097/MPH.0b013e3181615600. [DOI] [PubMed] [Google Scholar]
- 39.Treepongkaruna S, Sirachainan N, Kanjanapongkul S, Winaichatsak A, Sirithorn S, Sumritsopak R, Chuansumrit A. Absence of platelet recovery following Helicobacter pylori eradication in childhood chronic idiopathic thrombocytopenic purpura: a multi-center randomized controlled trial. Pediatr Blood Cancer. 2009;53:72–77. doi: 10.1002/pbc.21991. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara M, Capozzi L, Russo R. Effect of Helicobacter pylori eradication on platelet count in children with chronic idiopathic thrombocytopenic purpura. Hematology. 2009;14:282–285. doi: 10.1179/102453309X12473408860181. [DOI] [PubMed] [Google Scholar]
- 41.Maghbool M, Maghbool M, Shahriari M, Karimi M. Does Helicobacter pylori play a role in the pathogenesis of childhood chronic idiopathic thrombocytopenic purpura? Pediatr Rep. 2009;1:e2. doi: 10.4081/pr.2009.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo G, Miraglia V, Branciforte F, Matarese SM, Zecca M, Bisogno G, Parodi E, Amendola G, Giordano P, Jankovic M, et al. Effect of eradication of Helicobacter pylori in children with chronic immune thrombocytopenia: a prospective, controlled, multicenter study. Pediatr Blood Cancer. 2011;56:273–278. doi: 10.1002/pbc.22770. [DOI] [PubMed] [Google Scholar]
- 43.Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2–S14. doi: 10.1053/j.seminhematol.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 45.Veneri D, De Matteis G, Solero P, Federici F, Zanuso C, Guizzardi E, Arena S, Gaio M, Pontiero P, Ricetti MM, et al. Analysis of B- and T-cell clonality and HLA class II alleles in patients with idiopathic thrombocytopenic purpura: correlation with Helicobacter pylori infection and response to eradication treatment. Platelets. 2005;16:307–311. doi: 10.1080/09537100400028685. [DOI] [PubMed] [Google Scholar]
- 46.Satoh T, Pandey JP, Okazaki Y, Asahi A, Kawakami Y, Ikeda Y, Kuwana M. Single nucleotide polymorphism of interleukin-1beta associated with Helicobacter pylori infection in immune thrombocytopenic purpura. Tissue Antigens. 2009;73:353–357. doi: 10.1111/j.1399-0039.2009.01214.x. [DOI] [PubMed] [Google Scholar]
- 47.Hashino S, Mori A, Suzuki S, Izumiyama K, Kahata K, Yonezumi M, Chiba K, Kondo T, Ota S, Toyashima N, et al. Platelet recovery in patients with idiopathic thrombocytopenic purpura after eradication of Helicobacter pylori. Int J Hematol. 2003;77:188–191. doi: 10.1007/BF02983220. [DOI] [PubMed] [Google Scholar]
- 48.Nomura S, Inami N, Kanazawa S. The effects of Helicobacter pylori eradication on chemokine production in patients with immune thrombocytopenic purpura. Eur J Haematol. 2004;72:304–305. doi: 10.1111/j.1600-0609.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- 49.Stasi R, Rossi Z, Stipa E, Amadori S, Newland AC, Provan D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. Am J Med. 2005;118:414–419. doi: 10.1016/j.amjmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Kodama M, Kitadai Y, Ito M, Kai H, Masuda H, Tanaka S, Yoshihara M, Fujimura K, Chayama K. Immune response to CagA protein is associated with improved platelet count after Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter. 2007;12:36–42. doi: 10.1111/j.1523-5378.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 51.Ando K, Shimamoto T, Tauchi T, Ito Y, Kuriyama Y, Gotoh A, Miyazawa K, Kimura Y, Kawai T, Ohyashiki K. Can eradication therapy for Helicobacter pylori really improve the thrombocytopenia in idiopathic thrombocytopenic purpura? Our experience and a literature review. Int J Hematol. 2003;77:239–244. doi: 10.1007/BF02983780. [DOI] [PubMed] [Google Scholar]
- 52.Inaba T, Mizuno M, Take S, Suwaki K, Honda T, Kawai K, Fujita M, Tamura T, Yokota K, Oguma K, et al. Eradication of Helicobacter pylori increases platelet count in patients with idiopathic thrombocytopenic purpura in Japan. Eur J Clin Invest. 2005;35:214–219. doi: 10.1111/j.1365-2362.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 53.Emilia G, Luppi M, Zucchini P, Morselli M, Potenza L, Forghieri F, Volzone F, Jovic G, Leonardi G, Donelli A, et al. Helicobacter pylori infection and chronic immune thrombocytopenic purpura: long-term results of bacterium eradication and association with bacterium virulence profiles. Blood. 2007;110:3833–3841. doi: 10.1182/blood-2006-12-063222. [DOI] [PubMed] [Google Scholar]
- 54.Sato R, Murakami K, Watanabe K, Okimoto T, Miyajima H, Ogata M, Ohtsuka E, Kodama M, Saburi Y, Fujioka T, et al. Effect of Helicobacter pylori eradication on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura. Arch Intern Med. 2004;164:1904–1907. doi: 10.1001/archinte.164.17.1904. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki T, Matsushima M, Shirakura K, Koike J, Masui A, Takagi A, Shirasugi Y, Ogawa Y, Shirai T, Mine T. Association of inflammatory cytokine gene polymorphisms with platelet recovery in idiopathic thrombocytopenic purpura patients after the eradication of Helicobacter pylori. Digestion. 2008;77:73–78. doi: 10.1159/000121392. [DOI] [PubMed] [Google Scholar]
- 56.Satoh T, Miyazaki K, Shimohira A, Amano N, Okazaki Y, Nishimoto T, Akahoshi T, Munekata S, Kanoh Y, Ikeda Y, et al. Fcγ receptor IIB gene polymorphism in adult Japanese patients with primary immune thrombocytopenia. Blood. 2013;122:1991–1992. doi: 10.1182/blood-2013-05-501858. [DOI] [PubMed] [Google Scholar]
- 57.Scandellari R, Allemand E, Vettore S, Plebani M, Randi ML, Fabris F. Platelet response to Helicobacter pylori eradication therapy in adult chronic idiopathic thrombocytopenic purpura seems to be related to the presence of anticytotoxin-associated gene A antibodies. Blood Coagul Fibrinolysis. 2009;20:108–113. doi: 10.1097/MBC.0b013e32832315d8. [DOI] [PubMed] [Google Scholar]
- 58.Sato R, Murakami K, Okimoto T, Watanabe K, Kodama M, Fujioka T. Development of corpus atrophic gastritis may be associated with Helicobacter pylori-related idiopathic thrombocytopenic purpura. J Gastroenterol. 2011;46:991–997. doi: 10.1007/s00535-011-0416-8. [DOI] [PubMed] [Google Scholar]
- 59.Matsukawa Y, Iwamoto M, Kato K, Mizuno S, Gon Y, Hemmi A, Shirinskaya N, Takeuchi J, Sawada S. Long term changes in platelet counts after H. pylori eradication in non-ITP patients. Platelets. 2010;21:628–631. doi: 10.3109/09537104.2010.510894. [DOI] [PubMed] [Google Scholar]
- 60.Bai Y, Wang Z, Bai X, Yu Z, Cao L, Zhang W, Ruan C. Cross-reaction of antibody against Helicobacter pylori urease B with platelet glycoprotein IIIa and its significance in the pathogenesis of immune thrombocytopenic purpura. Int J Hematol. 2009;89:142–149. doi: 10.1007/s12185-008-0247-4. [DOI] [PubMed] [Google Scholar]
- 61.Yamanishi S, Iizumi T, Watanabe E, Shimizu M, Kamiya S, Nagata K, Kumagai Y, Fukunaga Y, Takahashi H. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect Immun. 2006;74:248–256. doi: 10.1128/IAI.74.1.248-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellicano R, Touscoz GA, Smedile A, Berrutti M, Saracco G, Repici A, Ponzetto A, Rizzetto M. Prevalence of non-organ-specific autoantibodies in patients suffering from duodenal ulcer with and without Helicobacter pylori infection. Dig Dis Sci. 2004;49:395–398. doi: 10.1023/b:ddas.0000020491.78450.82. [DOI] [PubMed] [Google Scholar]
- 63.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–1402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuwana M, Ikeda Y. The role of autoreactive T-cells in the pathogenesis of idiopathic thrombocytopenic purpura. Int J Hematol. 2005;81:106–112. doi: 10.1532/ijh97.04176. [DOI] [PubMed] [Google Scholar]
- 65.Nishimoto T, Kuwana M. CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol. 2013;50 Suppl 1:S43–S49. doi: 10.1053/j.seminhematol.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost. 2009;7:322–329. doi: 10.1111/j.1538-7836.2008.03161.x. [DOI] [PubMed] [Google Scholar]
- 67.Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, Ikeda Y, Kuwana M. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118:2939–2949. doi: 10.1172/JCI34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Z, Zhou J, Prsoon P, Wei X, Liu X, Peng B. Low expression of FCGRIIB in macrophages of immune thrombocytopenia-affected individuals. Int J Hematol. 2012;96:588–593. doi: 10.1007/s12185-012-1187-6. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki H, Mori M, Seto K, Kai A, Kawaguchi C, Suzuki M, Suematsu M, Yoneta T, Miura S, Ishii H. Helicobacter pylori-associated gastric pro- and antioxidant formation in Mongolian gerbils. Free Radic Biol Med. 1999;26:679–684. doi: 10.1016/s0891-5849(98)00248-2. [DOI] [PubMed] [Google Scholar]
- 70.Ferrero RL. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. Mol Immunol. 2005;42:879–885. doi: 10.1016/j.molimm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 72.Liu XG, Ma SH, Sun JZ, Ren J, Shi Y, Sun L, Dong XY, Qin P, Guo CS, Hou M, et al. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood. 2011;117:2061–2069. doi: 10.1182/blood-2010-07-295477. [DOI] [PubMed] [Google Scholar]
- 73.Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854. doi: 10.1016/s0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]


