Abstract
AIM: To evaluate the expression status of S-phase kinase-associated protein 2 (Skp2)/cyclin-dependent kinases regulatory subunit 1 (Cks1) and p27kip1, and assess the prognostic significance of Skp2/Cks1 expression with p27kip1 in patients with extrahepatic cholangiocarcinoma.
METHODS: Seventy-six patients who underwent curative resection for histologically confirmed extrahepatic cholangiocarcinoma at our institution from December 1994 to March 2008 were enrolled. Immunohistochemical staining for Skp2, Cks1, p27kip1, and Ki67, along with other relevant molecular biologic experiments, were performed.
RESULTS: By Cox regression analyses, advanced age (> 65 years), advanced AJCC tumor stage, poorly differentiated histology, and higher immunostaining intensity of Skp2 were identified as independent prognostic factors in patients with extrahepatic cholangiocarcinoma. Exogenous epidermal growth factor (EGF, especially 0.1-10 ng/mL) significantly increased the proliferation indices by MTT assay and the mRNA levels of Skp2/Cks1 and p27kip1 in SNU-1196, SNU-1079, and SNU-245 cells. The protein levels of Skp2/Cks1 (from nuclear lysates) and p27kip1 (from cytosolic lysate) were also significantly increased in these cells. There were significant reductions in the protein levels of Skp2/Cks1 and p27kip1 (from nuclear lysate) after the treatment of LY294002. By chromatin immunoprecipitation assay, we found that E2F1 transcription factor directly binds to the promoter site of Skp2.
CONCLUSION: Higher immunostaining intensity of Skp2/Cks1 was an independent prognostic factor for patients with extrahepatic cholangiocarcinoma. EGF upregulates the mRNA and protein levels of Skp2/Cks1 and p27kip1 via the PI3K/Akt pathway and direct binding of E2F1 transcription factor with the Skp2 promoter.
Keywords: S-phase kinase-associated protein 2, Cyclin-dependent kinases regulatory subunit 1, P27kip1, Cholangiocarcinoma, E2F1, PI3K/Akt
Core tip: Based on the idea that S-phase kinase-associated protein 2 (Skp2) and p27kip1 might play a role in the pathogenesis and disease progression of patients with extrahepatic cholangiocarcinoma, immunohistochemical staining for Skp2, cyclin-dependent kinases regulatory subunit 1 (Cks1), p27kip1, and Ki67, along with other relevant molecular biologic experiments, were performed in tissue samples and human cholangiocarcinoma cell lines. Higher immunostaining intensity of Skp2 was an independent prognostic factor in patients with extrahepatic cholangiocarcinoma, and exogenous epidermal growth factor upregulates mRNA and protein levels of Skp2/Cks1 and p27kip1 in SNU-1196, SNU-1079, and SNU-245 cells. By chromatin immunoprecipitation assay, we found that E2F1 transcription factor directly binds to the promoter site of Skp2.
INTRODUCTION
Human extrahepatic cholangiocarcinoma (CC) is a highly malignant epithelial cancer of the biliary tract with high morbidity and mortality. Although certain conditions are associated with greater risk (i.e., sclerosing cholangitis[1] and hepatolithiasis[2,3]), most cases are sporadic. Despite the current knowledge of etiology and pathology of CC, its cellular and molecular pathogenesis is still poorly understood.
Ubiquitin-directed protein degradation plays an important role in regulating a broad spectrum of biologic processes, including transcription, signal transduction, cell cycle progression, apoptosis, cell growth, differentiation, and development. Proteins that are targeted for degradation by this mechanism undergo a series of ubiquitin-modification steps carries out by the successive activity of E1 (activating), E2 (conjugating), and E3 (ligating) ubiquitin enzymes[4,5]. SCF (Skp, Cullin, F-box containing complex) complexes belong to a large family of multi-subunit E3 ubiquitin ligases that select and ubiquitinate specific proteins for targeted destruction by the 26S proteasome.
S-phase kinase associated protein 2 (Skp2) is the substrate-recognition subunit of the SCFskp2 E3 ligase complex. Several lines of evidence from both biochemical and genetic studies have shown that Skp2 is required for cell cycle progression at multiple stages, including G1/S transition, S phase progression, and S/G2 transition[6-8]. A principal substrate responsible for these activities of Skp2 in the cell cycle phases is p27kip1, an inhibitor of cdk2 and cdk1 activities that promote entry into DNA synthesis and mitosis, respectively[8-10]. The important role of Skp2 in promoting entry into the S phase, mainly by promoting p27kip1 destruction, was observed in cell-free systems, cell cultures, and in animal models[9-12]. Those findings raised the question of whether the low levels of p27kip1 in human cancers are caused by increased expression of Skp2.
Cyclin kinase subunit 1 (Cks1) is an additional protein that has a role in efficient interaction between the Skp2 ubiquitin ligase complex and its substrate p27kip1. The critical role of Cks1 in targeting p27kip1 was indicated in the studies which demonstrated the lack of p27kip1 ubiquitylation and breakdown in the absence of Cks1 in vitro in cell-free systems[13] and the slow cell proliferation and accumulation of p27kip1 in Cks1 nullizygous mice in vivo[14]. The expression of Cks1 mRNA and protein levels is strongly correlated with the expression of Skp2 protein levels and inversely with those of p27kip1 levels in colorectal, breast, gastric, prostate, oral epithelial, and non-small cell lung cancers[15-21]. In some of these cancers, including breast, colorectal, and gastric cancers, Skp2 and Cks1 expression were independent prognostic markers and provided additional prognostic information in these cancer patients[22-25].
To our knowledge, there have been no reports concerning the expression status and the prognostic implication of Skp2 and Cks1 in extrahepatic cholangiocarcinoma. The overall objective of our study is to evaluate the expression status of Skp2/Cks1 and p27kip1, and assess the significance of Skp2/Cks1 expression with p27kip1 as a predictive prognostic marker in patients with extrahepatic cholangiocarcinoma. Additionally, we explored the molecular biologic mechanisms for the overexpression of Skp2/Cks1 and down-regulation of p27kip1 using the intrahepatic and extrahepatic cholangiocarcinoma cell lines.
MATERIALS AND METHODS
Patients
Archived paraffin embedded tissue blocks of 76 cases of extrahepatic cholangiocarcinoma who underwent curative surgery at Kangbuk Samsung Hospital from January 1994 to March 2008 were included in our study. Medical records and laboratory data of each patient were retrospectively reviewed. Survival data (including overall and recurrence free survival) were collected by review of medical records and contact with patients or their relatives by telephone interview. This study was conducted in accordance with the principles of the Declaration of Helsinki. Our study protocol was approved by the Ethics Committee of Kangbuk Samsung Hospital (C0653, approved at 2009-4-23).
Materials
The following primary or secondary antibodies were used: anti-Skp2 (Invitrogen, 32-3300), anti-p27kip1 (BD Biosciences, 610241), anti-Cks1 (Invitrogen, 36-6800), anti-Ki-67 (Dako), monoclonal mouse anti-E2F1 (Abcam, ab483), and monoclonal mouse anti-β-actin (Abcam, ab8226). PI3K inhibitor LY294002 and mitogen activated protein kinase (MAPK) inhibitor PD169316 were purchased from Calbiochem.
Immunohistochemistry
Immunohistochemical staining for Skp2, Cks1, p27kip1, and Ki67 were performed using the relevant microarray tissue blocks of cholangiocarcinoma. The tissue sections (4 μm) were cut, placed on silane-pretreated slides, deparaffinized, and rehydrated through graded alcohols. Antigen retrieval was performed by microwave heating at high power (750 W) in 10 mmol/L sodium citrate buffer (pH = 6) for 4 cycles of 5 min each. Slides were then allowed to cool for 30 min prior to incubation with either the primary antibodies targeting Skp2 (2C8D9, 1:100, Zymed), Cks1 (4G12G7, 1:250, Zymed), p27kip1 (1B4, 1:20, Zymed), or Ki-67 (MIB-1, 1:100, DAKO) for 1 h at room temperature. Staining was performed with the Envision Monoclonal System (Dako, Carpinteria, CA, United Sates). The reaction was developed with DAB. Slides were counterstained with Mayer’s hematoxylin and mounted. Incubation without the primary antibody was used as a negative control.
Two pathologists (Sohn JH and Chae SW) blinded to the follow-up data independently evaluated the immunohistochemically stained slides. If there were discrepancies between them, the results were read again by both pathologists together. The staining intensities of Skp2, Cks1, and p27kip1 were semi-quantitatively measured at × 400 magnification, and staining intensity of the nucleus and cytoplasm was independently categorized as no staining (intensity = 0), weak (intensity = 1), moderate (intensity = 2), or strong (intensity = 3, Figure 1). The number of staining positive cells was expressed as a percentage of the total number of epithelial cells and assigned to the one of five categories: score 0, < 5%; score 1, 5%-25%; score 2, 26%-50%; score 3, 51%-75%; score 4, > 75%. The percentage score of staining positive tumor cells and the staining intensity were multiplied to produce an immunoreactive score (IS) for each tumor specimen. Ki-67 immunostaining results (Ki-67 LI) were recorded as the percentage of immunoreactive cells over at least 2000 tumor cells randomly selected from the periphery of each of the invasive carcinoma in the surgical specimens. The slides were examined under a Leica DMR microscope and images were captured using a Leica digital camera (Leica Microsystems Inc., 1700 Leider Lane, Buffalo Grove, IL, United States). Immunoreactive cells were counted randomly with a minimum of 20 high-power fields (× 400), with the help of an image computer analyzer (ImageJ, Image Processing and Analysis in Java).
Figure 1.
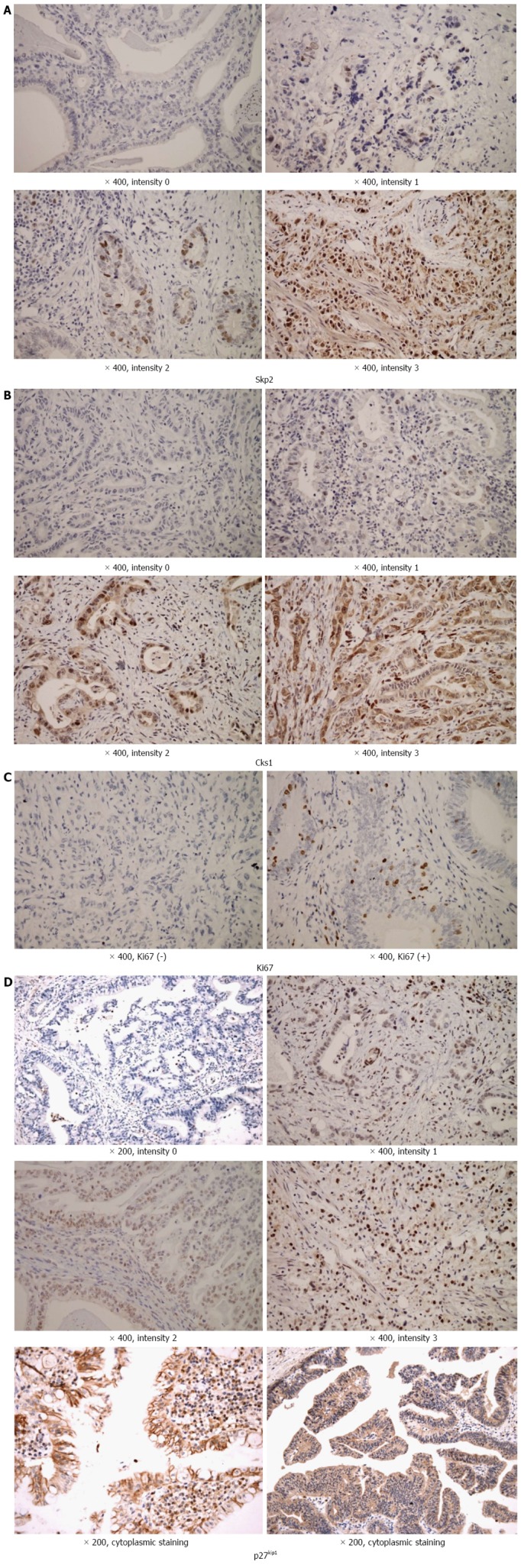
Results of immunohistochemical staining. A-C: Immunohistochemistry for (A) Skp2, (B) Cks1, and (C) Ki67 proteins showed that stains were mainly expressed in the nuclei of cancer cells; D: The staining pattern of p27kip1 was somewhat heterogeneous. The pattern of nuclear staining was mainly noticed, and some of them were expressed as diffuse cytoplasmic staining. The staining intensity of Skp2, Cks1, and p27kip1 was classified as follows: 0, no staining; 1, weak; 2, moderate; 3, strong. The staining pattern of Ki-67 was nuclear and classified as positive or negative. At least 20 high-power fields will be randomly chosen, and 2000 cells will be always counted. Skp2: S-phase kinase-associated protein 2; Cks1: Cyclin-dependent kinases regulatory subunit 1.
Cell lines and culture
Human intrahepatic duct cancer cells SNU-1079 (KCLB No. 01079), human hepatic duct bifurcation site cancer cells SNU-1196 (KCLB No. 01196), and human common bile duct cancer cells SNU-245 (KCLB No. 00245) were maintained in RPMI1640 medium supplemented with L-glutamine (300 mg/L), 25 mmol/L HEPES, 25 mmol/L NaHCO3 (90%), and 10 % heat inactivated fetal bovine serum[26].
Cell proliferation assay
A total of 3 × 103 cells were seeded into 96-well plates and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, M5655, Sigma, St. Louis, MO, United States] was added to each well every 24 h. The plates were incubated at 37 °C for 4 h followed by addition of 100 μL lysate (10% SDS in 0.01 mol/L HCl). The absorbance was measured at 490 nm using an ELISA microplate reader.
Quantitative polymerase chain reaction
Total RNA was extracted from the cells using the TRIzol Reagent (Invitrogen), and was reverse-transcribed into complementary DNA with the Takara RNA PCR Kit (TAKARA Bio Inc., Otsu, Shiga, Japan). Quantification of the complementary DNA template was performed with a quantitative polymerase chain reaction (qPCR, T3000 thermocycler, Biometra GmbH, Goettingen, Germany) using Thermocycler Manager Software (V4.11, Biometra GmbH, Goettingen, Germany). PCR primers are as follows: Skp2: forward, 5’-CCAGCAAGACTTCTGAAC-3’, reverse, 5’-GGAGGCACAGACAGGAAA-3’; p27kip1: forward, 5’-GCAACCGACGATTCTTCTAC-3’, reverse, 5’-GTCCATTCCATGAAGTCAGC-3’; Cks1: forward, 5’-CCCACTACCCAAGAAACCAA-3’, reverse, 5’-CCGCAAGTCACCACACATAC-3’; E2F1 forward: 5’-CGTGAGCGTCATGGCCTTGG-3’, reverse: 5’-GGCGTCCCTGGGGTCCGTAC-3’; β-actin forward: 5’-CAAGAGATGGCCACGGCTGCT-3’, reverse: 5’-TCCTTCTGCATCCTGTCGGCA-3’.
Immunoblot analysis
Various concentration of exogenous EGF (0.01-100 ng/mL) were added onto cultured tumor cell lines. Cells were directly lysed for 30 min on ice with lysis buffer [50 mmol/L Tris-HCl (pH = 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 mmol/L Na3VO4, and 1 mmol/L NaF). After centrifugation at 13000 g for 15 min, protein concentrations were measured using Bradford’s reagent (Bio-Rad, Hercules, CA, United States), and protein was denatured by boiling for 10 min. Protein (25 μg) was loaded onto 10% SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking with 5% milk in TBST (137 mmol/L NaCl, 25 mmol/L Tris and 1 mmol/L disodium ethylenediaminetetraacetate containing 0.1% Tween-20), the membranes were incubated with appropriate primary antibodies at a dilution of 1:1000 at 4 °C overnight. After washing with TBST three times (each for 10 min), the membranes were incubated with their corresponding horseradish peroxidase-conjugated secondary antibodies at a dilution of 1:1000 at room temperature for 1 h. After washing with TBST three times (each for 10 min), bound antibodies were visualized using enhanced chemiluminescent substrates (Amersham, Arlington Heights, IL, United States). For nuclear and cytoplasmic extracts, cells were processed using the Thermo Scientific NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific Inc. Rockford, IL, United States), according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was performed using a commercially available assay kit (#17-229, Upstate Biotechnology, Lake Placid, NY, United States) according to the manufacturer’s instructions. Cells were briefly incubated with 1% formaldehyde in media at 37 °C for 15 mitosis cross-link DNA and DNA-binding proteins. After washing in PBS, cells were collected and lysed within the 200 μL sodium dodecyl sulfate lysis buffer supplied by the kit. The cell extracts were sonicated with four sets of 10-s pulses using a sonicator (Sonifier cell disruptor; Heat Systems Ultrasonics, Plainview, NY, United States) equipped with a 2-mm tip and set to 30% of maximum power to shear the DNA into 200- to 1000-bp fragments, and then centrifuged at 13000 g for 10 min. The DNA concentration in the supernatant was quantitated by measuring absorbance at 260 nm. Samples containing 200 μg DNA was diluted with the chromatin immunoprecipitation dilution buffer to a final volume of 2050 μL. Fifty microliters from each sample was removed as a non-immunoprecipitation input control. After preclearing the sample with 75 μL salmon sperm DNA/protein A agarose slurry at 4 °C for 30 min, the target protein E2F1 was immunoprecipitated with 2 μg anti-E2F1 monoclonal antibody at 4 °C overnight with rotation using a multipurpose rotator (Model 151; Scientific Industries, Bohemia, NY, United States). A mock immunoprecipitation without antibody was also performed. Next, 60 μL of the salmon sperm DNA/protein A agarose slurry was added and incubated at 4 °C for 60 min. After washing the agarose beads, the protein A-agarose immune complexes were eluted, in two separate 250-μL aliquots, in elution buffer (1% SDS, 0.1 mol/L NaHCO3) at room temperature for 30 min. Twenty-five microliters of each eluent was used for immunoblot analysis to determine the immunoprecipitation efficiency. Following the addition of 5 mol/L NaCl (20 μL), protein-DNA cross-linking was reversed by heating at 65 °C for 5 h. After digestion with 2 μL of 10 mg/mL proteinase K at 45 °C for 1 h, DNA fragments were purified via phenol/chloroform extraction and ethanol precipitation, and the DNA pellet was dissolved in 20 μL water. The following PCR primers were employed to amplify the 238-bp product spanning the E2F1-specific binding site identified in the 5’-flanking region of the human Skp2 gene: forward, 5’-GAGAGAGACAGGGCAATCATACAC-3’; reverse, 5’-ATCACCAGAAGGCCTGCGGGCT-3’. The copy number for amplified PCR products was quantitated using qPCR as described in the qPCR section.
Statistical analysis
Continuous variables were compared by Student’s t test and one-way ANOVA. Categorical variables were evaluated using the χ2 or two-tailed Fisher exact test. For comparison of overall survival between the patient group with each immunohistochemical staining intensity and IS, Kaplan-Meier analyses with Log-rank comparisons were performed. Multivariate logistic regression analysis was performed to identify independent prognostic factors. All tests were 2-sided, and a P value of less than 0.05 was considered statistically significant. Statistical analysis was conducted using the PASW statistics package 18 (IBM, Armonk, New York, NY, United States).
RESULTS
Clinicopathologic characteristics and immunohistochemical expressions of Skp2, Cks1, Ki-67, and p27kip1
The baseline clinicopathologic characteristics of the 76 patients with extrahepatic cholangiocarcinoma enrolled in the current study are listed in Table 1. Immunohistochemical staining showed that Skp2, Cks1, and Ki67 proteins were expressed in the nuclei of cancer cells (Figure 1A-C). The staining pattern of p27kip1 was somewhat heterogeneous. The pattern of nuclear staining was most noticeable, some of which were expressed as diffuse cytoplasmic staining (Figure 1D). The results of the immunohistochemical staining for Skp2, Cks1, Ki67, and p27kip1 were as follows. Skp2 intensity: 0, 32 (42.1%); 1, 21 (27.6%); 2, 16 (21.1%); 3, 7 (9.2%); Cks1 intensity: 0, 9 (11.8%); 1, 24 (31.6%); 2, 37 (48.7%); 3, 6 (7.9%); p27kip1 intensity: 0, 23 (30.3%); 1, 31 (40.8%); 2, 15 (19.7%); 3, 7 (9.2%); Ki67: negative, 33 (43.4%); positive, 43 (56.6%).
Table 1.
Clinicopathologic characteristics and immunohistochemical expressions in 76 patients with extrahepatic cholangiocarcinoma
| Parameters | n (%) | |
| Sex | Male | 47 (61.8) |
| Female | 29 (38.2) | |
| Age | mean ± SD | 63.1 ± 9.3 |
| Surgical resection | CBD segmental Resection | 32 (42.1) |
| Whipple or PPPD | 44 (57.9) | |
| Tumor size | ≥ 2 cm | 48 (63.2) |
| < 2 cm | 28 (36.8) | |
| American Joint Commission on Cancer stage | IA | 10 (13.2) |
| IB | 18 (23.7) | |
| IIA | 20 (26.3) | |
| IIB | 26 (34.2) | |
| III | 1 (1.3) | |
| IV | 1 (1.3) | |
| Histologic type | Well-differentiated | 19 (25.0) |
| Moderately-differentiated | 46 (60.5) | |
| Poorly-differentiated | 11 (14.5) | |
| Resection margin | R0 | 57 (75.0) |
| R1 | 19 (25.0) | |
| Angiolymphatic invasion | Absent | 31 (40.8) |
| Present | 45 (59.2) | |
| Perineural invasion | Absent | 36 (47.4) |
| Present | 40 (52.6) | |
| Regional lymph nodes involvement | Absent | 49 (64.5) |
| Present | 27 (35.5) | |
| Skp2 immunostaining | Intensity | |
| 0 | 32 (42.1) | |
| 1 | 21 (27.6) | |
| 2 | 16 (21.1) | |
| 3 | 7 (9.2) | |
| Immunoreactive score | ||
| 0 | 43 (56.6) | |
| 1 | 10 (13.2) | |
| 2 | 15 (19.7) | |
| 3 | 6 (7.9) | |
| 6 | 2 (2.6) | |
| Cyclin-dependent kinases regulatory subunit 1 immunostaining | Intensity | |
| 0 | 9 (11.8) | |
| 1 | 24 (31.6) | |
| 2 | 37 (48.7) | |
| 3 | 6 (7.9) | |
| Immunoreactive score | ||
| 0 | 24 (31.6) | |
| 1 | 12 (15.8) | |
| 2 | 26 (34.2) | |
| 3 | 1 (1.3) | |
| 4 | 8 (10.5) | |
| 6 | 4 (5.3) | |
| 9 | 1 (1.3) | |
| p27kip1 immunostaining | Intensity | |
| 0 | 23 (30.3) | |
| 1 | 31 (40.8) | |
| 2 | 15 (19.7) | |
| 3 | 7 (9.2) | |
| Immunoreactive score | ||
| 0 | 29 (38.2) | |
| 1 | 22 (28.9) | |
| 2 | 6 (7.9) | |
| 3 | 2 (2.6) | |
| 4 | 8 (10.5) | |
| 6 | 7 (9.2) | |
| 9 | 2 (2.6) | |
| Ki-67 LI | mean ± SD | 4.0 ± 6.7 |
CBD: Common bile duct; LI: Labeling index; PPPD: Pylorus preserving pancreatoduodenectomy; Skp2: S-phase kinase-associated protein 2.
The staining intensity of Skp2 and Cks1 showed significant linear correlation (Pearson’s r = 0.427, P < 0.01). The staining intensity of Skp2 and Cks1 also showed significant linear correlations with the staining intensity of p27kip1 (Pearson’s r = 0.314, P < 0.01 for Skp2 and p27kip1; Pearson’s r = 0.403, P < 0.01 for Cks1 and p27kip1). The Ki-67 labeling index (LI) significantly increased as the staining intensity of Skp2 and Cks1 increased (P < 0.01). However, the staining intensity of p27kip1 did not correlate with the Ki-67 LI (Figure 2).
Figure 2.
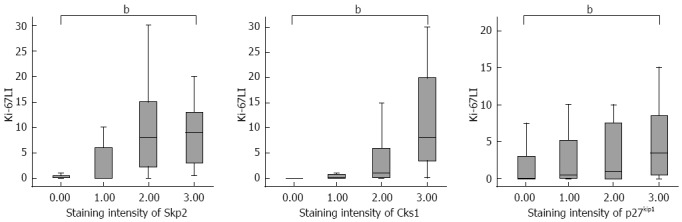
Relationship among the immunostaining intensity for S-phase kinase-associated protein 2, Cyclin-dependent kinases regulatory subunit 1, p27kip1, and Ki-67 LI. Ki-67 LI significantly increased as the staining intensity of Skp2 and Cks1 increased. Ki-67 LI showed no significant correlation with the staining intensity of p27kip1. Statistical analyses were performed by one-way ANOVA. bP < 0.01 between groups. Skp2: S-phase kinase-associated protein 2; Cks1: Cyclin-dependent kinases regulatory subunit 1.
By Kaplan-Meier analyses, a tumor size larger than 2 cm (P < 0.01, Figure 3A), advanced AJCC stage (P < 0.01, Figure 3B), moderately and poorly differentiated histology (P < 0.01, Figure 3C), R1 resection (P < 0.01, Figure 3D), presence of angiolymphatic tumor invasion (P = 0.036, Figure 3E), and stronger immunostaining intensity of Skp2 (P < 0.01, Figure 3F) were significantly associated with shorter survival for patients with extrahepatic cholangiocarcinoma.
Figure 3.
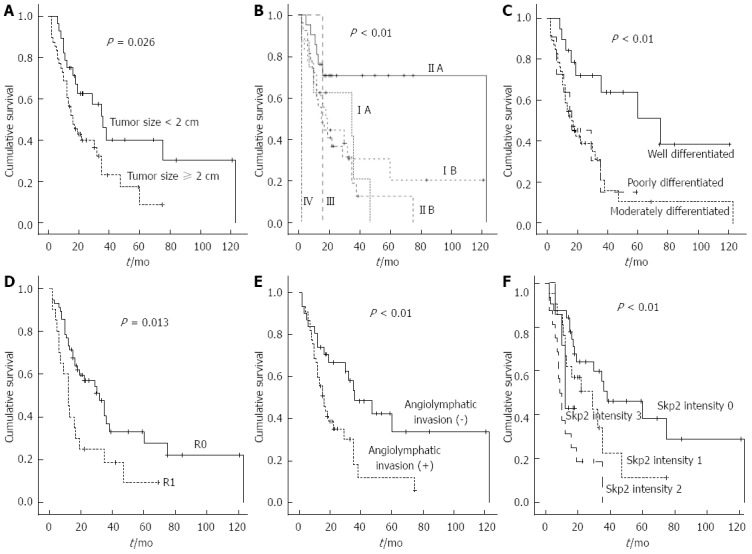
Results of Kaplan-Meier survival analysis. By Kaplan-Meier survival analysis, tumor size larger than 2 cm (A), advanced American Joint Commission on Cancer stage (B), tumor differentiation (C), R1 resection (D), angiolymphatic tumor invasion (E), and the intensity of Skp2 immunostaining (F) were significantly associated with overall survival in patients with extrahepatic cholangiocarcinoma. Skp2: S-phase kinase-associated protein 2.
To explore the independent and significant prognostic factors for patients with extrahepatic cholangiocarcinoma, we performed Cox regression analyses with the forward stepwise conditional method. As a result, advanced age (> 65 years), advanced AJCC tumor stage, histologic type of tumor, and immunostaining intensity of Skp2 were identified as independent and significant prognostic factors for patients with extrahepatic cholangiocarcinoma (Table 2).
Table 2.
Independent and significant prognostic factors for patients with extrahepatic cholangiocarcinoma
| Parameters | Category | OR | 95%CI | P value |
| Age | > 65 yr | 2.707 | 1.341-5.464 | < 0.01 |
| AJCC stage | IA | |||
| IB | 2.543 | 0.695-9.306 | 0.159 | |
| IIA | 0.801 | 0.185-3.477 | 0.767 | |
| IIB | 3.534 | 1.041-11.997 | 0.043 | |
| III | 16.446 | 1.370-197.470 | 0.027 | |
| IV | 92.965 | 5.585-1547.531 | < 0.010 | |
| Histologic type | Well-differentiated | |||
| Moderately-differentiated | 6.041 | 2.146-17.006 | < 0.010 | |
| Poorly-differentiated | 5.620 | 1.586-19.917 | < 0.010 | |
| Immunostaining intensity of Skp2 | 0 | |||
| 1 | 1.308 | 0.571-2.994 | 0.526 | |
| 2 | 3.782 | 1.581-9.051 | < 0.010 | |
| 3 | 2.312 | 1.127-6.941 | 0.048 |
AJCC: American Joint Commission on Cancer; Skp2: S-phase kinase-associated protein 2.
Exogenous EGF increased the proliferation indices, mRNA, and protein levels of SKP2/Cks1 and p27kip1 in SNU-1196, SNU-1079, and SNU-245 cells
qPCR and western blotting for Skp2/Cks1 and p27kip1 after the exogenous treatment of variable concentrations of EGF into the cell culture mediums were subsequently performed. Exogenous EGF (especially 0.1-10 ng/mL) significantly increased the proliferation indices of SNU-1196, SNU-1079, and SNU-245 cells by MTT assay (Figure 4).
Figure 4.
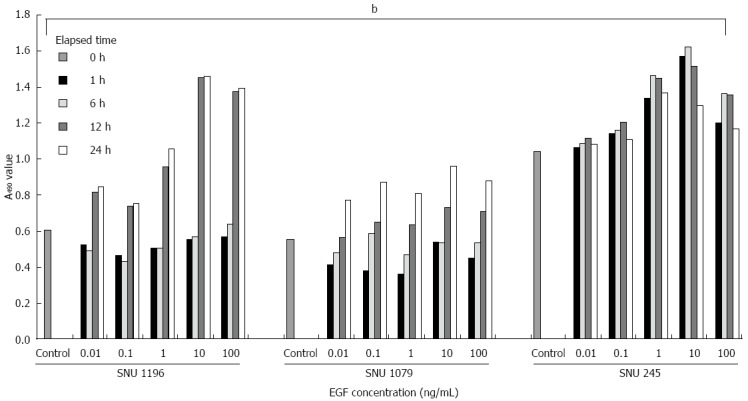
MTT assay after the addition of exogenous epidermal growth factor. Exogenous epidermal growth factor (EGF) (especially 0.1-10 ng/mL) significantly increased the proliferation indices of SNU-1196, SNU-1079, and SNU-245 cells. Statistical analyses were performed by one-way ANOVA. bP < 0.01 vs control. Data are representative of triplicate experiments.
Exogenous EGF (especially 0.1-10 ng/mL) significantly upregulated the mRNA levels of SKP2/Cks1 and p27kip1 in SNU-1196 (Figure 5A), SNU-1079 (Figure 5B), and SNU-245 cells (Figure 5C).
Figure 5.
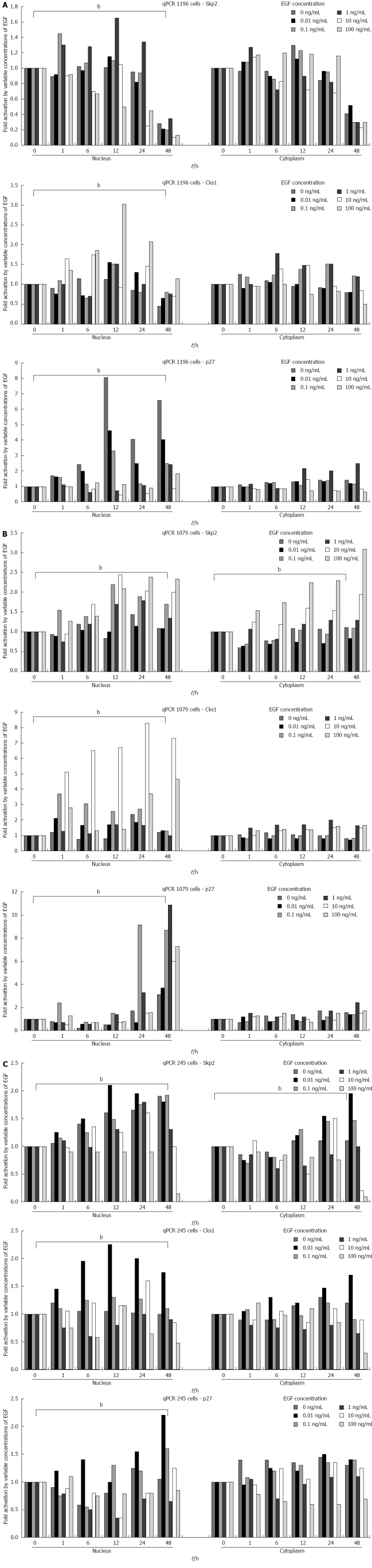
Results of quantitative polymerase chain reaction for S-phase kinase-associated protein 2, Cyclin-dependent kinases regulatory subunit 1, and p27kip1 after the addition of exogenous epidermal growth factor in (A) SNU-1196, (B) SNU-1079, and (C) SNU-245 cells. Data are representative of triplicate experiments. Statistical analyses were performed by one-way ANOVA. bP < 0.01 vs control. EGF: Exogenous epidermal growth factor; Skp2: S-phase kinase-associated protein 2; Cks1: Cyclin-dependent kinases regulatory subunit 1.
The protein levels of SKP2/Cks1 (from nuclear lysates) and p27kip1 (from cytosolic lysate) were significantly increased in SNU-1196 (Figure 6A), SNU-1079 (Figure 6B), and SNU-245 cells (Figure 6C) after exogenous treatment of variable concentrations of EGF into the cell culture mediums.
Figure 6.
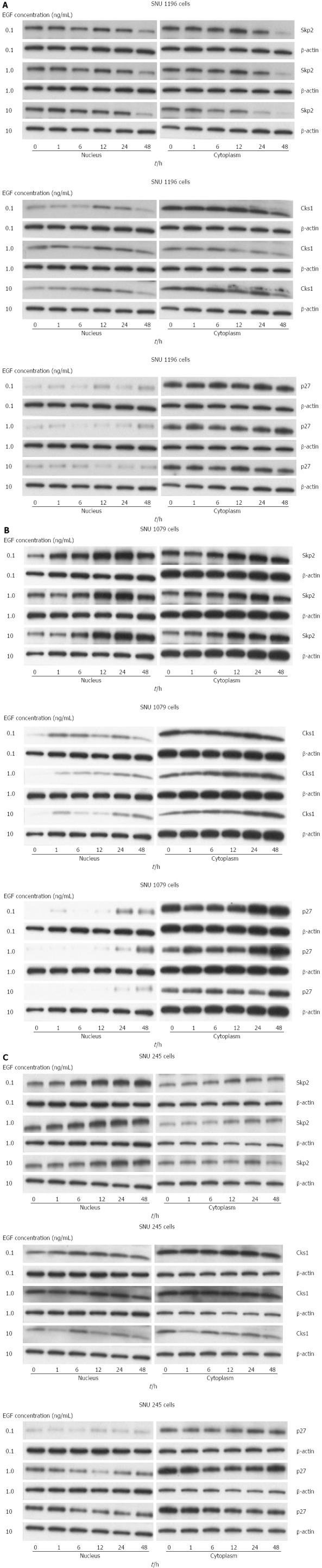
Results of western blotting for S-phase kinase-associated protein 2, Cyclin-dependent kinases regulatory subunit 1, and p27kip1 after the addition of exogenous epidermal growth factor in (A) SNU-1196, (B) SNU-1079, and (C) SNU-245 cells. Data are representative of triplicate experiments. Skp2: S-phase kinase-associated protein 2; Cks1: Cyclin-dependent kinases regulatory subunit 1.
Protein levels of SKP2/Cks1 were dependent upon PI3K/Akt pathway
To explore the intracellular signal transduction pathways responsible for the upregulation of the mRNA and protein levels of Skp2/Cks1, we treated the 3 cholangiocarcinoma cell lines with LY294002 (PI3K inhibitor, 5 μmol/L) and PD0325901 (MEK1/2 inhibitor, 100 nmol/L). There were significant reductions in the protein levels of SKP2/Cks1 and p27kip1 (from nuclear lysate) after the treatment of LY294002. However, there were no significant changes in the protein levels of SKP2/Cks1 and p27kip1 (from nuclear lysate) after the treatment of PD0325901 (Figure 7).
Figure 7.
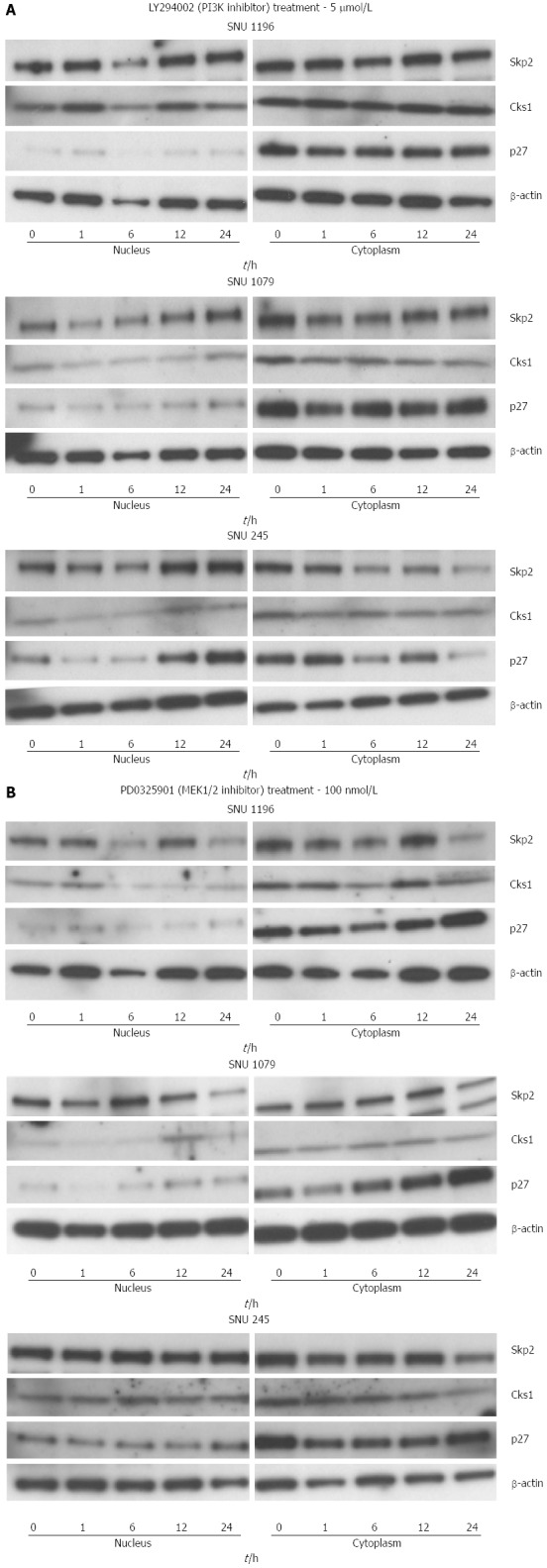
Effect of LY294002 (PI3K inhibitor, 5 μmol/L), and PD0325901 (MEK1/2 inhibitor, 100 nmol/L) on the protein levels of S-phase kinase-associated protein 2/Cyclin-dependent kinases regulatory subunit 1 and p27kip1 in SNU-1196, SNU-1079, and SNU-245 cells. Data are representative of triplicate experiments. Skp2: S-phase kinase-associated protein 2; Cks1: Cyclin-dependent kinases regulatory subunit 1.
E2F1 transcription factor binds to the promoter region of Skp2 gene
Because the PI3K signaling pathway regulates Skp2 transcription and the molecular mechanism linking PI3K signaling to the Skp2 gene is unclear, we investigated Skp2 regulation more precisely. To show transcriptional control of Skp2 after the addition of 10 ng/mL of EGF, we did chromatin immunoprecipitation (ChIP) assays. As shown in Figure 8, E2F1 transcription factor directly binds to the promoter site of Skp2.
Figure 8.
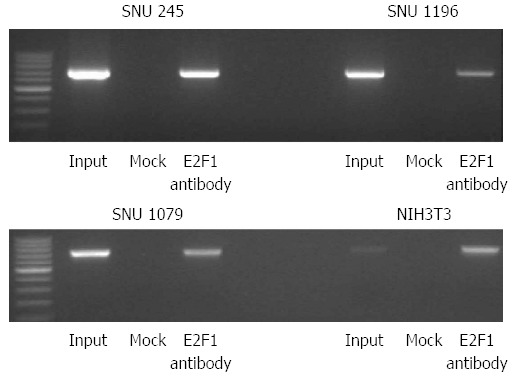
Binding of E2F1 transcription factor to the promoter region of Skp2 gene. Chromatin immunoprecipitation assay shows the direct binding of E2F1 transcription factor to the promoter region of the Skp2 gene after the addition of 10 ng/mL of EGF in SNU-245, SUN-1196, SUN-1079, and NIH3T3 cells.
DISCUSSION
Skp2 and Cks1, the ubiquitin ligase subunits that target p27kip1 for degradation, are commonly overexpressed in human cancers[21-25]. p27kip1 is a negative regulator of the cell cycle that plays an important role in tumor suppression. Loss of p27kip1 secondary to enhanced ubiquitin-mediated degradation results in uncontrolled proliferation and promotes tumor progression. In the present study, the positive immunoreactivity of Skp2 and Cks1 were noted in a significant proportion of patients with extrahepatic cholangiocarcinoma, and the positive immunoreactivity of Skp2 was a significant and independent prognostic factor in these patients. The expression of Skp2 was examined in a previous study by Sanada et al[27], in which they found a significant association between increased levels of Skp2 protein and poor prognosis in patients with biliary tract carcinoma. However, in this study, the enrolled number of patients was relatively small (n = 33) and enrolled heterogeneous groups of patients, including extrahepatic bile duct and gallbladder cancers. In the current study, the staining intensity of Skp2 had significant linear correlations with those of Cks1 and nuclear p27kip1. Notably, Ki-67 LI significantly increased as the staining intensity of Skp2 and Cks1 increased (P < 0.01, Figure 2). However, Ki-67 LI showed no significant correlations with the staining intensity of p27kip1.
The lack of an inverse correlation between Skp2 and p27kip1 has been reported in several other types of solid tumors[28-30], as well as in biliary tract cancer[27]. These observations might be explained, at least partly, by additional oncogenic properties of Skp2 when it acts on target molecules other than p27kip1 and/or by additional molecular events involving p27kip1 proteolysis. Interestingly, the staining intensity of Ki67, a marker of cell proliferation, was significantly associated with that of nuclear p27kip1. Our results indicate that the observations in which low levels of p27kip1 in aggressive human cancers may be caused by increased expression of Skp2 that targets p27kip1 for ubiquitin-mediated degradation did not apply to extrahepatic cholangiocarcinoma. The specific findings in need of alternative explanatory hypotheses in extrahepatic cholangiocarcinoma are the lack of an inverse correlation between p27kip1 and Skp2 levels and the apparent paradox of both increased expression of Skp2 and p27kip1. Plausible explanations for these observations need further experiments. However, an independent mechanism of action for oncogenic Skp2 protein and tumor-suppressive p27kip1 in extrahepatic cholangiocarcinoma may be involved.
Conversely, it seems evident that oncoprotein Skp2 is an independent prognostic marker in patients with extrahepatic cholangiocarcinoma, like many other solid tumors[16-24]. It was repeatedly shown that Skp2 is an accurate and independent prognostic marker for disease-free and overall survival. Nevertheless, despite the increasing evidence showing that Skp2 may more accurately stratify and subgroup patients at risk, there are no studies at present that have clinically implicated its application in the clinical decision process, such as adding adjuvant chemotherapy in patients with extrahepatic cholangiocarcinoma who underwent cure-intent surgical resection.
The precise mechanisms that regulate Skp2 expression in cancer are not fully understood at present. Identification of the regulatory mechanism(s) leading to increased Skp2 expression may offer new insight into the control of tumor cell proliferation. A previous report[29] showed that Skp2 gene amplification is likely to be associated with an advanced stage of tumor progression, while other control mechanisms not involving a gross genomic alteration are involved in the initial accumulation of Skp2 mRNA during oncogenesis. This implies a change in the rate of either transcription or mRNA degradation. Their analyses revealed no significant differences in the half-life of Skp2 mRNA between non-transformed and transformed human cell lines. Therefore, one plausible mechanism is a dysregulated transcription of Skp2 mRNA and a resultant increased level of Skp2 protein. The roles of EGF in human cholangiocarcinoma cells were reported to be sustained EGF receptor (EGFR) activation, extended -42/44 MAPK activation, and increased cell proliferation[31]. EGFR kinase inhibitors also effectively attenuated cellular growth of human cholangiocarcinoma cells. In this study, we show for the first time that transcription of Skp2 mRNA and translation of Skp2 protein were significantly increased after the exogenous addition of variable concentrations of EGF in human cholangiocarcinoma cell lines. A previous report[32] showed that Skp2 is a novel target of E2F regulation and that the SKP2 gene contains a functional E2F response element. Moreover, an increase in DP-1, a coactivator of the E2F family and E2F3, was reported to be involved in the proliferation-enhancing effect of EGF[33]. In order to elucidate the transcriptional control of Skp2 in human cholangiocarcinoma cells, we performed ChIP assays after the addition of 10 ng/mL EGF, with the results showing that the E2F1 transcription factor directly binds to the promoter site of Skp2 (Figure 5). E2F1 transcription factor is known to be a downstream target of variable growth factors, such as EGF and TGF-α. Disruption of the E2F/RB regulatory pathway is known to be a major contributor to increased E2F expression in many human tumors. Our finding that E2F1 that directly binds to the SKP2 promoter offers a relevant mechanism for the upregulated Skp2 expression in human cholangiocarcinoma cells and may be served as possible therapeutic targets in this devastating malignancy.
The PI3K/AKT signaling pathway controls fundamental processes of cancer cell biology like proliferation and cell survival[34]. The PI3K/AKT pathway is activated in cholangiocarcinoma cells[35]. So far, the molecular mechanisms linking PI3K/AKT signaling to the cell cycle machinery in cholangiocarcinoma cells have not been investigated in detail. Using the PI3K inhibitor LY294002, we show that Skp2 is regulated by the PI3K/AKT pathway in cholangiocarcinoma cells. At the molecular level, the control of Skp2 protein expression is thought to be due to the regulation of E2F1 binding to the Skp2 gene promoter.
In summary, higher immunostaining intensity of Skp2 was an independent prognostic factor for patients with extrahepatic cholangiocarcinoma. EGF upregulates the mRNA and protein levels of Skp2/Cks1 and p27kip1 via the PI3K/Akt pathway and direct binding of E2F1 transcription factor with the Skp2 promoter.
COMMENTS
Background
Human extrahepatic cholangiocarcinoma (CC) is a highly malignant epithelial cancer of the biliary tract with high morbidity and mortality. Despite the current knowledge of etiology and pathology of CC, its cellular and molecular pathogenesis is still poorly understood. S-phase kinase associated protein 2 (Skp2) is the substrate-recognition subunit of the SCFskp2 E3 ligase complex. The important role of Skp2 in promoting entry into the S-phase, mainly by promoting p27kip1 destruction, was observed in cell-free systems, cell cultures, and animal models. In some cancers, including breast, colorectal, and gastric cancers, Skp2 and cyclin-dependent kinases regulatory subunit 1 (Cks1) expression were independent prognostic markers and provided additional prognostic information in these cancer patients. To our knowledge, there have been no reports concerning the expression status and the prognostic implication of Skp2 and Cks1 in extrahepatic cholangiocarcinoma.
Research frontiers
The research hotspot is exploring the cellular and molecular pathogenetic mechanisms of extrahepatic cholangiocarcinoma, which is highly malignant tumor with few or no palliative medical treatments available for unresectable patients.
Innovations and breakthroughs
The precise mechanisms that regulate Skp2 expression in cancer are not fully understood at present. Identification of the regulatory mechanism(s) leading to increased Skp2 expression may offer new insight into the control of tumor cell proliferation. The roles of exogenous epidermal growth factor (EGF) in human cholangiocarcinoma cells were reported to be sustained EGF receptor (EGFR) activation, extended -42/44 MAPK activation, and increased cell proliferation. EGFR kinase inhibitors also effectively attenuated cellular growth of human cholangiocarcinoma cells. In this study, the authors show for the first time that transcription of Skp2 mRNA and translation of Skp2 protein were significantly increased after the exogenous addition of variable concentrations of EGF in human cholangiocarcinoma cell lines. In order to elucidate the transcriptional control of Skp2 in human cholangiocarcinoma cells, the authors performed chromatin immunoprecipitation assays after the addition of 10 ng/mL EGF, and the results showed that E2F1 transcription factor directly binds to the promoter site of Skp2. The finding that E2F1 directly binds to the Skp2 promoter offers a relevant mechanism for the upregulated Skp2 expression in human cholangiocarcinoma cells, and may be served as a possible therapeutic target in this devastating malignancy.
Applications
The study results suggest that EGFR, the PI3K-Akt pathway, SKP2, and E2F1 can be candidate therapeutic targets in patients with extrahepatic cholangiocarcinoma
Terminology
Skp2: S-phase kinase associated protein 2, the substrate-recognition subunit of the SCFskp2 E3 ligase complex; Cks1: Cyclin kinase subunit 1, an additional protein that has a role in efficient interaction between the Skp2 ubiquitin ligase complex and its substrate p27kip1; p27kip1: Cyclin-dependent kinase inhibitor 1B, a protein which belongs to the Cip/Kip family of cyclin dependent kinase inhibitor proteins; PI3K: Phosphoinositide 3-kinases, a family of related intracellular signal transducer enzymes capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol.
Peer review
This study investigated the role of the Skp2 signaling pathway in the pathogenesis of human extrahepatic cholangiocarcinoma. The paper is well written and results are interesting.
Footnotes
Supported by A grant from Samsung Biomedical Research Institute, No. C-A9-210-1
P- Reviewer: Wu WD S- Editor: Zhai HH L- Editor: Rutherford A E- Editor: Ma S
References
- 1.MacFaul GR, Chapman RW. Sclerosing cholangitis. Curr Opin Gastroenterol. 2005;21:348–353. doi: 10.1097/01.mog.0000155359.43763.cc. [DOI] [PubMed] [Google Scholar]
- 2.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 3.Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386–393. doi: 10.1016/j.surg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 5.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 7.Koepp DM, Harper JW, Elledge SJ. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell. 1999;97:431–434. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutterlüty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 11.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 12.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 13.Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 14.Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Slotky M, Shapira M, Ben-Izhak O, Linn S, Futerman B, Tsalic M, Hershko DD. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res. 2005;7:R737–R744. doi: 10.1186/bcr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapira M, Ben-Izhak O, Bishara B, Futerman B, Minkov I, Krausz MM, Pagano M, Hershko DD. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer. 2004;100:1615–1621. doi: 10.1002/cncr.20172. [DOI] [PubMed] [Google Scholar]
- 18.Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, Utsunomiya T, Mori M. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res. 2003;9:5693–5698. [PubMed] [Google Scholar]
- 19.Kitajima S, Kudo Y, Ogawa I, Bashir T, Kitagawa M, Miyauchi M, Pagano M, Takata T. Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am J Pathol. 2004;165:2147–2155. doi: 10.1016/S0002-9440(10)63264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inui N, Kitagawa K, Miwa S, Hattori T, Chida K, Nakamura H, Kitagawa M. High expression of Cks1 in human non-small cell lung carcinomas. Biochem Biophys Res Commun. 2003;303:978–984. doi: 10.1016/s0006-291x(03)00469-8. [DOI] [PubMed] [Google Scholar]
- 21.Shapira M, Ben-Izhak O, Slotky M, Goldin O, Lahav-Baratz S, Hershko DD. Expression of the ubiquitin ligase subunit cyclin kinase subunit 1 and its relationship to S-phase kinase protein 2 and p27Kip1 in prostate cancer. J Urol. 2006;176:2285–2289. doi: 10.1016/j.juro.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Traub F, Mengel M, Lück HJ, Kreipe HH, von Wasielewski R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res Treat. 2006;99:185–191. doi: 10.1007/s10549-006-9202-3. [DOI] [PubMed] [Google Scholar]
- 23.Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103:1336–1346. doi: 10.1002/cncr.20917. [DOI] [PubMed] [Google Scholar]
- 24.Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819–3825. [PubMed] [Google Scholar]
- 25.Reichert M, Saur D, Hamacher R, Schmid RM, Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007;67:4149–4156. doi: 10.1158/0008-5472.CAN-06-4484. [DOI] [PubMed] [Google Scholar]
- 26.Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, Kim SW, Park YH, Hwang JH, Yoon YB, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87:187–193. doi: 10.1038/sj.bjc.6600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanada T, Yokoi S, Arii S, Yasui K, Imoto I, Inazawa J. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 2004;95:969–976. doi: 10.1111/j.1349-7006.2004.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penin RM, Fernandez-Figueras MT, Puig L, Rex J, Ferrandiz C, Ariza A. Over-expression of p45(SKP2) in Kaposi’s sarcoma correlates with higher tumor stage and extracutaneous involvement but is not directly related to p27(KIP1) down-regulation. Mod Pathol. 2002;15:1227–1235. doi: 10.1097/01.MP.0000036589.99516.D6. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira AM, Okuno SH, Nascimento AG, Lloyd RV. Skp2 protein expression in soft tissue sarcomas. J Clin Oncol. 2003;21:722–727. doi: 10.1200/JCO.2003.05.112. [DOI] [PubMed] [Google Scholar]
- 30.Dowen SE, Scott A, Mukherjee G, Stanley MA. Overexpression of Skp2 in carcinoma of the cervix does not correlate inversely with p27 expression. Int J Cancer. 2003;105:326–330. doi: 10.1002/ijc.11066. [DOI] [PubMed] [Google Scholar]
- 31.Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41:808–814. doi: 10.1016/j.jhep.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Wang C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene. 2006;25:2615–2627. doi: 10.1038/sj.onc.1209286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimer D, Sadr S, Wiedemair A, Concin N, Hofstetter G, Marth C, Zeimet AG. Heterogeneous cross-talk of E2F family members is crucially involved in growth modulatory effects of interferon-gamma and EGF. Cancer Biol Ther. 2006;5:771–776. doi: 10.4161/cbt.5.7.2750. [DOI] [PubMed] [Google Scholar]
- 34.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 35.Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–722. doi: 10.3748/wjg.v16.i6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]


