Abstract
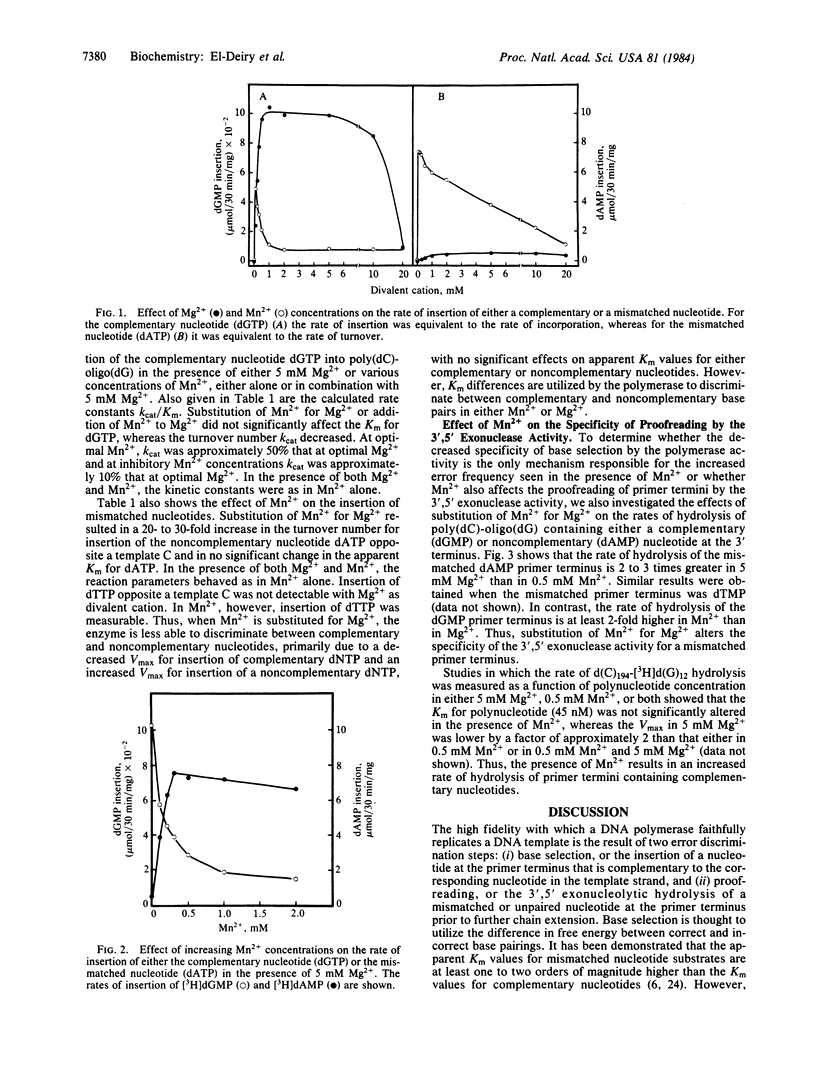
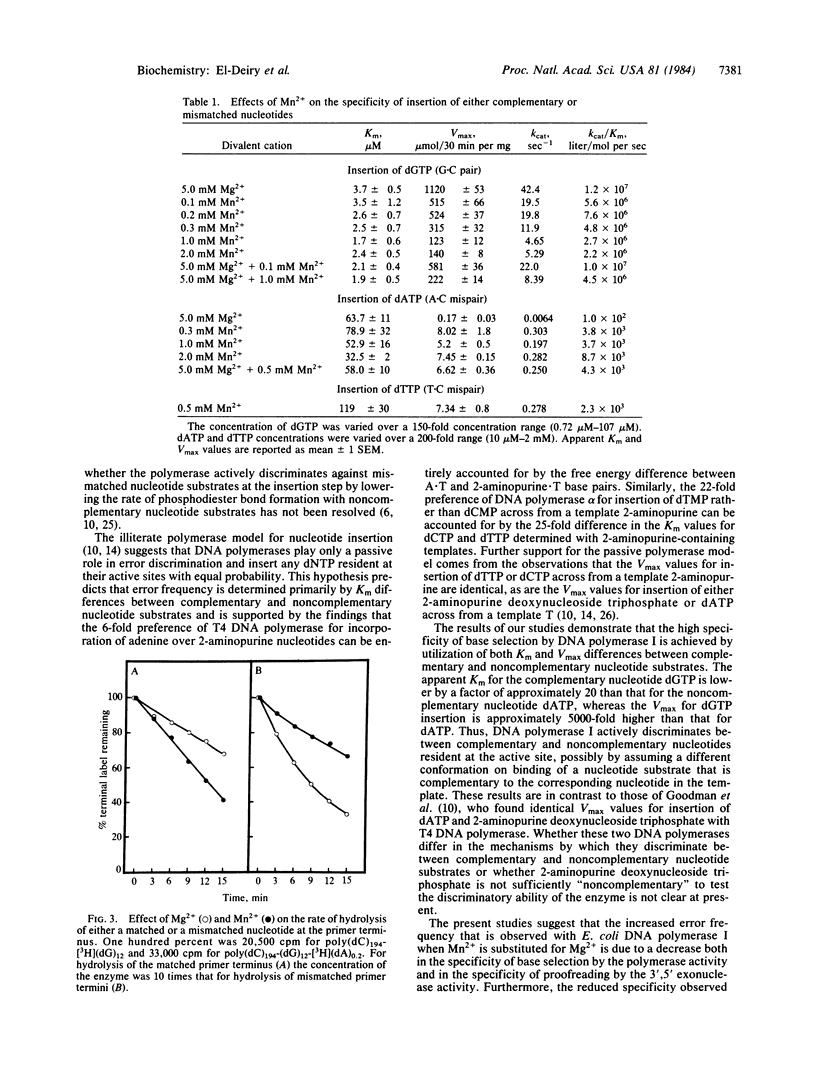
The mechanism by which DNA polymerase discriminates between complementary and noncomplementary nucleotides for insertion into a primer terminus has been investigated. Apparent kinetic constants for the insertion of dGTP and dATP into the hook polymer d(C)194-d(G)12 with Escherichia coli DNA polymerase I (large fragment) were determined. The results suggest that the high specificity of base selection by DNA polymerase I is achieved by utilization of both Km and Vmax differences between complementary and noncomplementary nucleotides. The molecular basis for the increased error frequency observed with DNA polymerase I in the presence of Mn2+ has also been investigated. Our studies demonstrate that when Mn2+ is substituted for Mg2+, there is a higher ratio of insertion of incorrect to correct dNTP by the polymerase activity, accompanied by a decreased hydrolysis of a mismatched dNMP relative to a matched dNMP at the primer terminus by the 3',5' exonuclease activity. Kinetic analysis revealed that in the presence of Mn2+, the kcat for insertion of a complementary dNTP is reduced, whereas the catalytic rate for the insertion of a mismatched nucleotide is increased. The apparent Km values for either complementary or noncomplementary nucleotide substrates are not significantly altered when Mg2+ is replaced by Mn2+. The rate of hydrolysis of a mismatched dNMP at the primer terminus is greater in the presence of Mg2+ vs. Mn2+, whereas the rate of hydrolysis of a properly base-paired terminal nucleotide is greater in Mn2+ vs. Mg2+. These studies demonstrate that both the accuracy of base selection by the polymerase activity and the specificity of hydrolysis by the 3',5' exonuclease activity are altered by the substitution of Mn2+ for Mg2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNARDI G. CHROMATOGRAPHY OF NUCLEOSIDE MONO- AND POLYPHOSPHATES ON HYDROXYAPATITE. Biochim Biophys Acta. 1964 Dec 16;91:686–688. doi: 10.1016/0926-6550(64)90030-1. [DOI] [PubMed] [Google Scholar]
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Tsui W. C. Kinetics of base misinsertion by DNA polymerase I of Escherichia coli. J Mol Biol. 1983 Apr 25;165(4):655–667. doi: 10.1016/s0022-2836(83)80272-1. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. Enzymological characterization of KB cell DNA polymerase-alpha. III. The polymerization reaction with single-stranded DNA. J Biol Chem. 1979 Nov 10;254(21):11040–11046. [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. II. Accuracy of nucleotide selection by the L88 mutator, CB120 antimutator, and wild type phage T4 DNA polymerases. J Biol Chem. 1976 Sep 10;251(17):5225–5232. [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. T4 DNA polymerase has a lower apparent Km for deoxynucleoside triphosphates complementary rather than noncomplementary to the template. Biochem Biophys Res Commun. 1975 May 19;64(2):457–464. doi: 10.1016/0006-291x(75)90343-5. [DOI] [PubMed] [Google Scholar]
- Goodman M. F., Keener S., Guidotti S., Branscomb E. W. On the enzymatic basis for mutagenesis by manganese. J Biol Chem. 1983 Mar 25;258(6):3469–3475. [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. An in vitro transversion by a mutationally altered T4-induced DNA polymerase. J Mol Biol. 1968 Sep 28;36(3):321–333. doi: 10.1016/0022-2836(68)90158-7. [DOI] [PubMed] [Google Scholar]
- Hanson K. R., Ling R., Havir E. A computer program for fitting data to the Michaelis-Menten equation. Biochem Biophys Res Commun. 1967 Oct 26;29(2):194–197. doi: 10.1016/0006-291x(67)90586-4. [DOI] [PubMed] [Google Scholar]
- Hibner U., Alberts B. M. Fidelity of DNA replication catalysed in vitro on a natural DNA template by the T4 bacteriophage multi-enzyme complex. Nature. 1980 May 29;285(5763):300–305. doi: 10.1038/285300a0. [DOI] [PubMed] [Google Scholar]
- Kwiram A. L., McConnell H. M. RADIATION DAMAGE IN ORGANIC CRYSTALS: AUFBAU PROCESSES. Proc Natl Acad Sci U S A. 1962 Apr;48(4):499–500. doi: 10.1073/pnas.48.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Miyaki M., Murata I., Osabe M., Ono T. Effect of metal cations on misincorporation by E. coli DNA polymerases. Biochem Biophys Res Commun. 1977 Aug 8;77(3):854–860. doi: 10.1016/s0006-291x(77)80056-9. [DOI] [PubMed] [Google Scholar]
- Orgel A., Orgel L. E. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol Biol. 1965 Dec;14(2):453–457. doi: 10.1016/s0022-2836(65)80195-4. [DOI] [PubMed] [Google Scholar]
- Patten J. E., So A. G., Downey K. M. Effect of base-pair stability of nearest-neighbor nucleotides on the fidelity of deoxyribonucleic acid synthesis. Biochemistry. 1984 Apr 10;23(8):1613–1618. doi: 10.1021/bi00303a005. [DOI] [PubMed] [Google Scholar]
- Que B. G., Downey K. M., So A. G. Mechanisms of selective inhibition of 3' to 5' exonuclease activity of Escherichia coli DNA polymerase I by nucleoside 5'-monophosphates. Biochemistry. 1978 May 2;17(9):1603–1606. doi: 10.1021/bi00602a004. [DOI] [PubMed] [Google Scholar]
- Que B. G., Downey K. M., So A. G. Selective inhibition of the polymerase activity of DNA polymerase. I. Further evidence for separate active sites for polymerase and 3' to 5' exonuclease activities. Biochemistry. 1979 May 15;18(10):2064–2068. doi: 10.1021/bi00577a034. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Metal activation of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):107–111. [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. On the fidelity of DNA replication. Effect of metal activators during synthesis with avian myeloblastosis virus DNA polymerase. J Biol Chem. 1977 Jun 10;252(11):3605–3610. [PubMed] [Google Scholar]
- Stoner G. D., Shimkin M. B., Troxell M. C., Thompson T. L., Terry L. S. Test for carcinogenicity of metallic compounds by the pulmonary tumor response in strain A mice. Cancer Res. 1976 May;36(5):1744–1747. [PubMed] [Google Scholar]
- Wang T. S., Eichler D. C., Korn D. Effect of Mn2+ on the in vitro activity of human deoxyribonucleic acid polymerase beta. Biochemistry. 1977 Nov 1;16(22):4927–4934. doi: 10.1021/bi00641a029. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Goodman M. F. Kinetic measurement of 2-aminopurine X cytosine and 2-aminopurine X thymine base pairs as a test of DNA polymerase fidelity mechanisms. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6429–6433. doi: 10.1073/pnas.79.21.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]



