Abstract
AIM: To study the natural history, patterns and clinical characteristics of inflammatory bowel diseases (IBD) in Egypt.
METHODS: We designed a case-series study in the gastroenterology centre of the Internal Medicine department of Cairo University, which is a tertiary care referral centre in Egypt. We included all patients in whom the diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) was confirmed by clinical, laboratory, endoscopic, histological and/or radiological criteria over the 15 year period from 1995 to 2009, and we studied their sociodemographic and clinical characteristics. Endoscopic examinations were performed by 2 senior experts. This hospital centre serves patients from Cairo, as well as patients referred from all other parts of Egypt. Our centre received 24156 patients over the described time period for gastro-intestinal consultations and/or interventions.
RESULTS: A total of 157 patients with established IBD were included in this study. Of these, 135 patients were diagnosed with UC (86% of the total), and 22 patients, with CD (14% of the total). The mean ages at diagnosis were 27.3 and 29.7, respectively. Strikingly, we noticed a marked increase in the frequency of both UC and CD diagnoses during the most recent 10 years of the 15 year period studied. Regarding the gender distribution, the male:female ratio was 1:1.15 for UC and 2.6:1 for CD. The mean duration of follow up for patients with UC was 6.2 ± 5.18 years, while the mean duration of follow up for patients with CD was 5.52 ± 2.83 years. For patients with UC we found no correlation between the severity of the disease and the presence of extraintestinal manifestations. Eleven patients had surgical interventions during the studied years: 4 cases of total colectomy and 7 cases of anal surgery.
CONCLUSION: We observed a ratio of 6:1 for UC to CD in our series. The incidence of IBD seems to be rising in Egypt.
Keywords: Natural history of Inflammatory bowel diseases, Epidemiology of Ulcerative colitis, Epidemiology of Crohn’s disease, Epidemiology of inflammatory bowel diseases in Egypt, inflammatory bowel diseases Prevalence, Incidence of ulcerative colitis, Incidence of Crohn’s disease
Core tip: The precise aetiology of inflammatory bowel disease (IBD) remains obscure. In our study, the ratio of patients diagnosed with ulcerative colitis (UC) to those diagnosed with Crohn’s disease (CD) was approximately 6:1. The total colectomy rate in our study was 2.9%, after a follow up period of 5-15 years, which is far lower than the rates of Western countries. We found that the characteristics of IBD in the Egyptian population were more similar to Asian and African IBD patterns. We noticed a marked increase in the frequency of UC and CD diagnoses over the past 10 years, which indicates an increasing incidence of IBD in Egypt.
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are collectively referred to as inflammatory bowel diseases (IBD)[1]. They mainly affect young populations, majorly altering their quality of life and increasing morbidity, compared to the general population. Although the exact aetiology of IBD has still not been exactly identified[2-5], it is believed that the pathogenesis of IBD includes immune deregulation secondary to environmental factors in genetically susceptible individuals. This results in a mounted immune response to the normally existing intestinal flora or epithelial antigens[2,6-8].
IBD occur with different frequencies around the world. The countries reporting for the highest incidence of UC are the United States, the United Kingdom and Sweden[9-11].
IBD have always seemed to be rare in the Middle East and Northern Africa. No accurate registry or cohort of patients had ever studied the exact prevalence of CD and UC in these populations. In Mediterranean countries, the prevalence of UC was estimated at 5/100000 in urban areas[12].
Recent data from a few single-centre studies have pointed to a change in the disease incidence that is usually explained by lifestyle changes, such as urbanisation, and changes in alimentary habits, such as greater consumption of fast food, greater consumption of carbohydrates, and a lower daily intake of alimentary fibres.
In a recent review of the natural history of IBD, it was noted that as countries become Westernised, the incidence of UC increases first and is later followed by CD[13-15]. Asia had a high ratio of UC/CD incidence in the 1980s and 1990s, but in 2000, the incidence of CD increased. Both diseases have emerged in countries in which they had rarely been previously reported, including Japan, South Korea, India, Iran, Lebanon, Thailand, the French West Indies, and North Africa[16-18]. In these countries, the occurrence of UC preceded that of CD by approximately 10 years. The overall incidence of IBD can be broken down into several geographic zones: those with a high incidence, those with a moderate incidence, those with low incidence 15 years ago but with a consistently increasing incidence, and those with an unknown incidence[13].
Overall, a pattern can be drawn for IBD frequency in the developing world: first, a low UC incidence; then, an increase in UC, while the CD incidence remains low; and finally, a CD incidence that approaches UC levels.
In this study, we studied the sociodemographic and clinical characteristics of patients diagnosed with CD and UC in the gastroenterology centre of the Internal Medicine department of Cairo University, which is a tertiary care referral centre in Cairo. To the best of our knowledge, this is the first trial establishing a cohort of IBD patients and starting a registry for data collection and analysis in Egypt.
MATERIALS AND METHODS
The study aimed to identify the socio-demographic and clinical characteristics of IBD patients in a very diverse population (Cairo agglomeration).
In the Middle East, as well as in most of the African countries, data on IBD patients are lacking, and there are no solid databases or registries to follow up the pattern of the disease.
We included all patients in whom the diagnosis of UC or CD was confirmed by clinical, laboratory, endoscopic and histological examination over the 15 year period from 1995 to 2009.
Our hospital gastroenterology centre serves patients from Cairo and also patients referred from all other parts of Egypt. Our centre received 24156 patients over the same duration of time who were referred for gastro-intestinal consultations and/or interventions.
First, we identified the presenting complaint of the patients who consulted our centre and the reason for which the endoscopic exploration was ordered. We considered patients presenting with chronic diarrhoea, rectal bleeding, recurrent abdominal pains or discomfort, melena, weight loss, and/or perianal fistula or abscess. The diagnosis of IBD was established by clinical, endoscopic, histological, and/or radiological criteria.
CD was diagnosed if skip lesions were found at endoscopy; a cobblestone appearance was evident; mucosal ulceration was found upon colonoscopy; or aphthous lesions were found at endoscopy. Deep inflammation or chronic terminal ileal inflammation, with or without radiologic evidence of skip lesions, stricturing disease, fistulising disease, existence of perianal disease (skin tags, abscess, fistula), small intestinal involvement or non-caseating granulomas, was also included in the diagnosis. Endoscopic examinations were performed by 2 senior experts.
Extraintestinal manifestations included musculoskeletal, mucocutaneous, hepatic, ophthalmic, and urinary tract involvements.
UC was diagnosed when there was evidence of a diffuse mucosal disease of colon with different proximal extensions from the rectum, superficial inflammation, crypt abscess, cryptitis, and rectal involvement without any evidence of small bowel involvement other than backwash ileitis.
We included patients who had an established diagnosis of IBD over the 15 years from 1995 to 2009 and who were referred to our centre. The following data were gathered for assessment: demographics, clinical features, area of residency, living conditions (city or countryside), smoking, family history of IBD, disease characteristics, extraintestinal manifestations, medical treatment used, and surgical interventions. Clinical information was obtained from medical records and patient interviews.
A diagnosis of IBD was established according to the corresponding criteria. For cases of UC, the true love classification was used to assess severity, and the Montreal classification was used to assess the extent of the disease[19]. Endoscopic grades were assigned in the form of mild (erythematous oedematous rectal mucosa, absent or distorted vascular pattern), moderate (marked oedema, spontaneously bleeding mucosa, purulent exudates) and severe (frank ulcerations) degrees. The histopathological findings included the following: vascular congestion, crypt abscesses, mucin depletion, cellular infiltrate, cryptitis, and crypt branching.
For the CD cases, the Crohn’s disease activity index was used to assess the disease activity. The Montreal classifications and endoscopic grades assessed the activity as follows: (1) inactive (the vascular pattern is only slightly distorted and there is, fine granularity without friability or epithelial defects); (2) mildly active (there is unequivocal erythema, either focal or confluent, and some friability without epithelial necrosis); (3) moderately active (a few aphthoid erosions or small ulcers are noted); or (4) severe (ulcers are larger and more numerous). The histopathological findings included the following: cellular infiltrate, focal inflammation, microfistulisation, non-caseating granulomas, cobblestoning, and lymphoid hyperplasia.
Finally, as Egypt is currently in an endemic for parasitic infestations, a stool analysis was performed for all patients; we only included patients with non-complicated or evolving parasitic infections.
Statistical analysis
Data analysis was performed by the χ2 test, and statistical significance was set at a P value of 0.05. The protocol of this study was approved by the review board of the department of Internal Medicine, according to the Declaration of Helsinki.
RESULTS
A total of 157 patients with established IBD were included in this study. From those, 135 patients were diagnosed with UC (86% of the total), and 22 patients, with CD (14% of the total). The mean ages at diagnosis were, respectively, 27.3 and 29.7. The mean age of diagnosis for UC patients residing in Cairo was 27.9, while it was 25.9 years for those living outside of Cairo, with no statistically significant difference (Table 1).
Table 1.
Demographic characteristics of ulcerative colitis patients in Cairo
| Ulcerative colitis patients | |
| Mean age at presentation (yr) | 27.3 (± 12) |
| Mean age at presentation (yr) (male:female) | 29.2 (± 13.4):25.7 (± 10.6) P = 0.093 |
| Mean age at presentation (yr) (urban:rural) | 25.9 (± 9.7):27.9 (± 12.7) P = 0.394 |
As for gender distribution, the male: female ratio was 1:1.15 for UC and 2.6:1 for CD. Among UC patients, only 38 patients (27.9% of the total) were not residents of Cairo (rural area of residency). As for CD, they were almost all residents of Cairo (except for one patient). Being a resident of Cairo or not was not correlated with the type of disease (UC or CD).
Strikingly, we noticed a marked increase in the frequency of both CD and UC diagnoses in the last 5 years, stronger than had occurred in the middle five years and much stronger than in the first 5 years, as shown in Figure 1. We analysed the association of cigarette smoking with both diseases; for UC patients, there were 121 patients with no active smoking habits at the date of diagnosis (88.9% of the total). For CD, only 2 patients were active smokers at the date of diagnosis.
Figure 1.
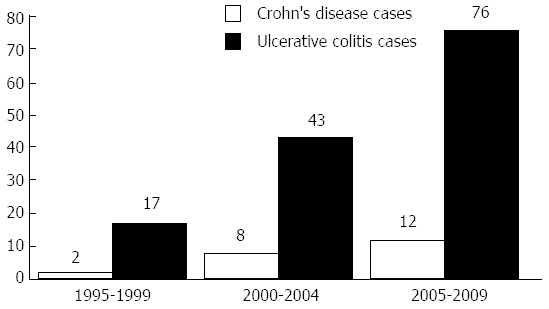
Number of ulcerative colitis and Crohn’s disease cases over the first, second and last five years of the 15 years duration.
For patients with UC, we found no correlation between the severity of the disease, the presence of extraintestinal manifestations (especially hepatobiliary associations) in either the area of residency or the smoking habits.
The mean duration of follow up for patients with UC was 6.2 ± 5.18 years, while for CD patients, the mean duration of follow up was 5.52 ± 2.83 years. There was no statistically significant difference between either the genders or between those patients residing in or outside of Cairo. For UC patients residing in Cairo, the mean duration of follow up was 6.2 years, while it was only 6 years for those living outside of Cairo, with no statistically significant difference (Tables 2 and 3).
Table 2.
Sociodemographic and clinical data of ulcerative colitis patients
| Total number | 135 |
| Gender (M/F) | 63/72 |
| Age at first presentation (mean ± SD) | 27.3 ± 11.7 |
| Resident of Cairo ( yes/no) | 97/38 |
| Smoking (yes/no) | 14/121 |
| Main presenting symptoms included: rectal bleeding, diarrhea, mucous, abdominal pain, loss of weight, fever | 128/113/72/76/8/8 |
| Follow up duration (mean ± SD) | 6.2 ± 5.18 |
| Severity of symptoms (true love classification) mild/moderate/severe | 66/52/17 |
| Anoperineal lesions (yes/no) | 6/129 |
| Hepatobiliary or pancreatic manifestations No/Fatty liver/sclerosing cholangitis | 127/7/1 |
| Other extra intestinal manifestations No/conjunctivitis/arthralgia/both | 109/10/16/25 |
| History of appendicectomy (yes/no) | 18/117 |
| Family history of UC (yes/no) | 2/135 |
| Extent of colitis Proctitis/left side colitis/pancolitis | 25/88/22 |
| Types of intestinal infections detected (total 31) Entameba histolytica/giardiasis/schistosomiasis | 23/5/3 |
M: Male; F: Female; UC: Ulcerative colitis.
Table 3.
Sociodemographic and clinical data of Crohn’s disease patient
| Total number | 22 |
| Gender (M/F) | 16/6 |
| Age at first presentation (mean ± SD) | 29.72 ± 12.13 |
| Resident of cairo (yes/no) | 21/1 |
| Smoking (yes/no) | 2/20 |
| Main presenting symptoms included: diarrhea, colics, fever, WT loss, rectal bleeding, vomiting | 22/18/9/10/6/4 |
| Follow up duration (mean ± SD) | 5.52 ± 2.83 |
| Severity of symptoms (mild/moderate/severe) | 9/11/2 |
| Anoperineal lesions (yes/no) | 8/14 |
| Extra intestinal manifestations (yes/no) No/fatty liver/arthralgia/arthralgia and uveitis | 8/1414/1/7/3 |
| History of appendicectomy (yes/no) | 3/19 |
| Montreal classification: | |
| A1/A2/A3 | 2/14/6 |
| B1/B2/B3 /P>> | 16/5/1/8 |
| L1/L2/L3/L4>> | 6/3/13/2 |
| Endoscopic grading (mild/moderate/severe) | 4/13/5 |
| Follow up endoscopy (stationary/improved/ progressed/did not do) | 2/15/2/3 |
| Localization of sites of CD affection Ileal/ileocolonic/colonic/oesophageal | 6/13/3/2 |
| Types of intestinal infections detected (total 9) Entameba histolytica/schistosomiasis | 7/2 |
M: Male; F: Female; CD: Crohn’s disease.
We studied all of the endoscopic findings (Table 4), and we did not find any statistically significant correlations among the area of residency, the gender, the age of presentation of the UC and active smoking with either the severity of the lesions or with the extent of the disease.
Table 4.
Clinical scoring of the endoscopic findings of ulcerative colitis patients according to the Montreal classification
| Number of patients/extent of colitis (endoscopic grading according to the Montreal classification) | E1 (Proctitis) (25 patient, 18.5%) | E2 Left side colitis (88 patient, 65.2%) | E3 Pancolitis (22 patient, 16.3%) |
| Number of patients/severity of colitis (endoscopic grading according to the Montreal classification) | Mild (25 patient, 18.5%) | Moderate (94 patient, 69.6%) | Severe (16 patient, 11.9%) |
| Follow up endoscopic grading | |||
| Stationary/improved/progressed/did not do | 20/83/6/26 | ||
The pathological interpretation of the examined biopsies taken during endoscopic examinations for all the patients concluded with changes typical of the disease (Tables 5 and 6).
Table 5.
Type of treatment, relapses, histopathological and laboratory findings of ulcerative colitis patients
| Treatment used: (single drug or combinations) Oral 5-ASA ±, 5-ASAenemas or supp., prednisone, azathioprine, corticosteroids enemas. (Infliximab was used in 2 cases) | |
| Surgical interference (yes/no) | 4/131 |
| Relapses (mean ± SD) | 1.514 ± 1.575 |
| Relapses (median/minimum/maximum) | 1/0/10 |
| Malignant transformation | 1 |
| Mean ESR 1st hour (mean ± SD) | 36.37 ± 24.73 |
| CRP (positive/negative/not done) | 39/34/60 |
| ANCA (positive/negative/not done) | 12/28/95 |
| ASCA (positive/negative/not done) | 8/31/96 |
| Pathological findings included: Vascular congestion, Crypt abscesses, Mucin depletion, Cellular infiltrate, Cryptitis, Crypt branching | |
| Pathology (diagnostic/suggestive/non conclusive) | 78/43/14 |
| Mean Hemoglobin concentration, gm/dL (mean ± SD) | 11.2 ± 2.8 |
| Mean PLT count (mean ± SD) | 335428.6 ± 140119.1 |
| Mean TLC (mean ± SD) | 8875 ± 4059.67 |
ASA: Aminosalicylic acid; CRP: C-reactive protein; ANCA: Anti-neutrophil cytoplasmic antibodies; ASCA: Anti-Saccharomyces cerevisiae antibodies.
Table 6.
Type of treatment, relapses, histopathological and laboratory findings of Crohn's disease patients
| CDAI (mean ± SD) | 108.21 ± 53.84 |
| CDAI (median/minimum/maximum) | 103.33/38/259.5 |
| Treatment used: (single drug or combinations) oral 5-ASA ± Metronidazole, ciprofloxacin, azathioprine, prednisone, infliximab, | |
| surgical interference (yes/no) | 7/15 |
| Relapses (mean ± SD) | 1.68 ± 2.21 |
| Relapses (median/minimum/maximum) | 1/0/8 |
| Malignant transformation | None |
| Mean ESR 1st hour (mean ± SD) | 49.52 ± 30.98 |
| CRP (positive/negative) | 17/5 |
| ANCA (positive/negative/not done) | 6/16 |
| ASCA (positive/negative) | 4/18 |
| Pathological findings included: cellular infiltrate, focal inflammation, microfisttulization, non caseating granulomas, cobblestoning, lymphoid hyperplasia | |
| Pathology (diagnostic/suggestive/non conclusive) | 7/8/7 |
| Mean hemoglobin concentration, gm/dL (mean ± SD) | 11.95 ± 2.14 |
| Mean PLT count (mean ± SD) | 296272.72 ± 146308.60 |
| Mean TLC (mean ± SD) | 7868.57 ± 2708.17 |
CDAI: Crohn’s disease activity index; ASA: Aminosalicylic acid; CRP: C-reactive protein; ANCA: Anti-neutrophil cytoplasmic antibodies; ASCA: Anti-Saccharomyces cerevisiae antibodies.
In our series, only 11 patients had surgical interventions during the years of follow up, which were either a total colectomy (4 UC patients) or an anal surgery (7 CD patients). The type of medical treatment, number of relapses and laboratory findings at the time of diagnosis are shown in Tables 5 and 6.
Only two patients had family history of IBD, and only 18 patients had previous history of appendicectomy prior to the presentation of UC. Neither of these was correlated with the age at presentation, the gender of the patient or the area of residency.
We did not confirm any malignant changes in the series of patients followed in our centre, except for one patient who developed colorectal carcinoma on follow-up. In our series, 40 patients had a non-evolving and non-complicated parasitic infection.
DISCUSSION
The exact aetiology of IBD remains obscure; many epidemiologic studies in different populations have shown an environmental and a genetic role in CD and UC. These studies have reported that the rate of this disease is higher in Scandinavian countries, Great Britain, Canada, and the United States than in Central Europe, Africa, or Asia. Our series included a small number of patients, which could have reflected either a weak incidence or prevalence of the disease in this population or a misdiagnosis of patients presenting with symptoms suggestive of IBD (trying to find another explanation for such a presentation).
The mean age of diagnosis for UC patients residing in Cairo was 27.9, while the mean age for those living outside Cairo was 25.9, with no significant difference. However, these findings cannot allow us to conclude that differences exist in the incidence of the disease between the urban and rural populations of Egypt.
The male: female ratio was 1:1.15 for UC, which is similar to most other studies[20-22] (with a slight increase of the female prevalence, denoting an increased number of affected females compared to other parts of the world). However, for CD, the male: female ratio was 2.6:1, denoting a male predominance similar to the results of a study from Tunisia, which showed a male predominance in CD[23], and similar to the results of epidemiological studies from Japan and China[24]. In contrast, studies from North America, Sweden and Northern France showed a female predominance in CD[24], and a recent study from Kuwait concluded that CD is equally common in males and females[25]. Several reasons may explain these differences, including the possibility that the gender ratios in CD are highly dependent on age, as well as geographic region[24].
In our study, the ratio of patients diagnosed with UC to patients diagnosed with CD was approximately 6:1. This was similar to results observed from different parts of the world, where UC is much more common than CD. In northeastern Poland, this ratio was approximately 15:1, and an increase in the total number of cases diagnosed with IBD has been reported[26]. Additionally, reports from Greece, Hungary, China and Lebanon confirm that CD is diagnosed less frequently[27].
Lifestyles may contribute to the expression of UC. Such factors as smoking, drinking tea and adhering to vegetarian diets have a protective effect against UC, while ex-smoking, psychological stress and family history of UC are shown to be risks for an increased incidence of IBD[20,28-31].
In our study, we did not find any correlation between smoking habits and the occurrence or severity of UC because most of our patients in this study did not have a history of smoking at the time of diagnosis. Additionally, there was no correlation between smoking and the occurrence of extra-intestinal manifestations.
It is generally thought that a family history of UC increases an individual’s risk of developing UC. We failed to find this in our study, as a family history was reported in only two patients.
This may be explained by the under diagnosis of these diseases due to a low disease awareness; to the confusion of IBD with the causes of infectious diarrhoea, which is common in our country; and to limited access to diagnostic tools as a result of the limited resources available in community health centres.
The clinical characteristics of the disease in our study showed that most of our patients with UC had mild distal or left-sided colitis (approximately 85%), confirmed clinically and endoscopically, and that most responded well to medical treatment. Only a few patients were referred for surgical intervention. Throughout the follow up period for UC, 83 patients (61.5%) showed an improvement in the disease activity; 20 patients retained the same activity score (14.8%); the disease activity progressed in 6 patients (4.4%); and 26 (19.2%) patients did not submit to a follow up endoscopy but also did not show any clinical relapse. The total colectomy rate in our study was 2.9% after 5-15 years, which is far lower than the total colectomy rates in studies from Western countries, which ranged from 24% to 34% after 10 years[32-37]. On the other hand, the total colectomy rate after 5-15 years in a study from South Korea was 3.3%[38]. These data indicate that the behaviour of UC in our Egyptian patients is milder than its behaviour in Western countries, more closely approximating that in the Asian population.
Although a recent study from the Middle East showed a different pattern of clinical characteristics, with more patients having pancolitis 45.5%[27], most of the data from the Asian and European regions reflected a very similar population description[26,39-41].
We also noted in our series that the rectal form of UC represented approximately 30% of cases-not far from 40%-which may indicate a good recruitment of all cases of UC, including those with the early forms of the disease (not only the advanced or complicated cases).
Importantly, our data showed that a marked increase in the diagnoses of both UC and CD occurred in the past 5 years, resulting in an incidence greater than the previous 5 years and much greater than that of the 5 years before that. These data are shown in Figure 1, which indicates the increasing incidence of IBD in Egypt. This increasing incidence is also supported by a recent study in 2012 by Molodecky et al[42], who concluded that the incidence and prevalence of IBD have been increasing with time in different regions around the world, even in developing countries as they became more industrialised. The increased awareness of IBD and improvements in the necessary diagnostic tools, especially endoscopes, over the last 10 years in Egypt may be an additional factor affecting the increased frequency of IBD diagnoses.
Ruyssers et al[43] discussed in depth the hygiene hypothesis, which proposes a converse relationship between parasitic infections and the incidence of IBD. Epidemiological, experimental, and clinical data corroborate the knowledge that helminthes provide protection against IBD. Therefore, the use of helminth-derived molecules may result in a protective effect[43].
In our study, the parasites identified among the IBD patients (157) were Entameba histolytica (19.1%), Giardiasis (3.2%), and Schistosomiasis (3.2%), while the parasites identified in patients without IBD (23998) were Entameba histolytica (20.1%), Enterobius vermicularis (8.3%), Giardiasis (7.2%), Schistosomiasis (5.4%), Ascaris lumbricoides (2.1%), Ancylostoma duodenale (2.1%), Trichuris trichiura (1.1%), Hymenolepis nana (1%) and mixed infections (3%). These data support the hygiene hypothesis, as the exposure to helminthes in patients without IBD was much higher than in those with IBD. The findings of several recent studies provide evidence for the role of helminthes in protecting against IBD[44-51].
In conclusions, in our experience, we found that the epidemiological characteristics of IBD in Egyptian population closely resembled those of the Asian and African patterns of IBD. UC was more common than CD, and the mean age at presentation was in the late twenties. UC was more common in females, while CD was more common in males. No correlation with active smoking was found at the time of presentation. We noticed a marked increase in the frequency of IBD diagnoses IBD in the last 10 years. We observed a ratio of 6:1 for UC to CD in our series, although the global natural course of IBDs in other countries may predict a future rise in CD in Egypt and other Middle Eastern countries, such that its incidence equals that of UC. We believe that the present moment is critical in assessing the pattern of IBD spreading in Egypt, and the current status should be further studied by more exhaustive database and registry documentations of IBD patients and their characteristics.
We believe that this work should direct the further identification of factors correlating with the severity and extent of UC in African, Arab and Asian populations. Exposure to pollutants in the environment, parasitic infestations, and changes of the life styles and food habits have all been proposed to theoretically account for the observed increase in the incidence of IBD in those communities; these hypotheses require further study and focus.
COMMENTS
Background
Inflammatory bowel diseases (IBD) include ulcerative colitis (UC) and Crohn’s disease (CD), both are chronic inflammations of the gastrointestinal tract. UC involve only the colon. On the other hand CD can involve any part of the gastrointestinal tract from the mouth to the anus. They mainly affect young populations with major effect on the quality of life and increased morbidity compared to general population. IBD have been considered a disease of Western countries with a pathogenesis related to the Western lifestyle. Studies showed that the incidence of IBD is now increasing in developing countries.
Research frontiers
The available data in the literature regarding IBD in North Africa are limited; therefore, the aim was to study the natural history of IBD in Egypt and to compare its clinical pattern with those in other regions including Western countries.
Innovations and breakthroughs
In the present study, the authors showed a marked increase in the frequency of diagnosis of both UC and CD in the last 10 years. These results suggests that the characteristics of IBD in Egyptian population are near to Asian and African pattern of IBD and the behavior of the disease is milder than in Western countries.
Applications
The study results suggest that the incidence of IBD seems to be rising in Egypt and may predict a future rise in IBD incidence in Egypt and other Middle East countries. This work should direct further studies with more exhaustive database and registry documentation of IBD patients and their characteristics in Egyptian, Arab and Asian population.
Terminology
True love classification: Classify the severity of UC according to the severity of symptoms to mild, moderate, and severe. Montreal classification: Classify disease phenotypes in CD based on the age at presentation, disease location and disease behavior.
Peer review
It is a very informative epidemiology study, worth of being published.
Footnotes
P- Reviewers: Castiglione F, Yan Y S- Editor: Song XX L- Editor: A E- Editor: Wang CH
References
- 1.Button LA, Roberts SE, Goldacre MJ, Akbari A, Rodgers SE, Williams JG. Hospitalized prevalence and 5-year mortality for IBD: record linkage study. World J Gastroenterol. 2010;16:431–438. doi: 10.3748/wjg.v16.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikhailov TA, Furner SE. Breastfeeding and genetic factors in the etiology of inflammatory bowel disease in children. World J Gastroenterol. 2009;15:270–279. doi: 10.3748/wjg.15.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DT, Osterman MT, Bewtra M, Lewis JD. Passive smoking and inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2382–2393. doi: 10.1111/j.1572-0241.2008.01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6:339–346. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 9.Ehlin AG, Montgomery SM, Ekbom A, Pounder RE, Wakefield AJ. Prevalence of gastrointestinal diseases in two British national birth cohorts. Gut. 2003;52:1117–1121. doi: 10.1136/gut.52.8.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan RF. Inflammatory bowel disease incidence: up, down or unchanged? Gut. 1998;42:309–311. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trallori G, Palli D, Saieva C, Bardazzi G, Bonanomi AG, d’Albasio G, Galli M, Vannozzi G, Milla M, Tarantino O, et al. A population-based study of inflammatory bowel disease in Florence over 15 years (1978-92) Scand J Gastroenterol. 1996;31:892–899. doi: 10.3109/00365529609051998. [DOI] [PubMed] [Google Scholar]
- 12.Tezel A, Dökmeci G, Eskiocak M, Umit H, Soylu AR. Epidemiological features of ulcerative colitis in Trakya, Turkey. J Int Med Res. 2003;31:141–148. doi: 10.1177/147323000303100211. [DOI] [PubMed] [Google Scholar]
- 13.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Lowe AM, Roy PO, B-Poulin M, Michel P, Bitton A, St-Onge L, Brassard P. Epidemiology of Crohn’s disease in Québec, Canada. Inflamm Bowel Dis. 2009;15:429–435. doi: 10.1002/ibd.20756. [DOI] [PubMed] [Google Scholar]
- 15.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 16.Yang SK, Hong WS, Min YI, Kim HY, Yoo JY, Rhee PL, Rhee JC, Chang DK, Song IS, Jung SA, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol. 2000;15:1037–1042. doi: 10.1046/j.1440-1746.2000.02252.x. [DOI] [PubMed] [Google Scholar]
- 17.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edouard A, Paillaud M, Merle S, Orhan C, Chenayer-Panelatti Dagger M. Incidence of inflammatory bowel disease in the French West Indies (1997-1999) Gastroenterol Clin Biol. 2005;29:779–783. doi: 10.1016/s0399-8320(05)86347-x. [DOI] [PubMed] [Google Scholar]
- 19.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 21.Gower-Rousseau C, Salomez JL, Dupas JL, Marti R, Nuttens MC, Votte A, Lemahieu M, Lemaire B, Colombel JF, Cortot A. Incidence of inflammatory bowel disease in northern France (1988-1990) Gut. 1994;35:1433–1438. doi: 10.1136/gut.35.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin GP, Hungin AP, Kelly PJ, Ling J. Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther. 2000;14:1553–1559. doi: 10.1046/j.1365-2036.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- 23.Ouakaa-Kchaou A, Gargouri D, Bibani N, Elloumi H, Kochlef A, Kharrat J. Epidemiological evolution of epidemiology of the inflammatory bowel diseases in a hospital of Tunis. Tunis Med. 2013;91:70–73. [PubMed] [Google Scholar]
- 24.Brant SR, Nguyen GC. Is there a gender difference in the prevalence of Crohn’s disease or ulcerative colitis? Inflamm Bowel Dis. 2008;14 Suppl 2:S2–S3. doi: 10.1002/ibd.20540. [DOI] [PubMed] [Google Scholar]
- 25.Siddique I, Alazmi W, Al-Ali J, Al-Fadli A, Alateeqi N, Memon A, Hasan F. Clinical epidemiology of Crohn’s disease in Arabs based on the Montreal Classification. Inflamm Bowel Dis. 2012;18:1689–1697. doi: 10.1002/ibd.21890. [DOI] [PubMed] [Google Scholar]
- 26.Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, Prokopowicz D. Epidemiological characteristics of inflammatory bowel disease in North-Eastern Poland. World J Gastroenterol. 2005;11:2630–2633. doi: 10.3748/wjg.v11.i17.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdul-Baki H, ElHajj I, El-Zahabi LM, Azar C, Aoun E, Zantout H, Nasreddine W, Ayyach B, Mourad FH, Soweid A, et al. Clinical epidemiology of inflammatory bowel disease in Lebanon. Inflamm Bowel Dis. 2007;13:475–480. doi: 10.1002/ibd.20022. [DOI] [PubMed] [Google Scholar]
- 28.Oliva-Hemker M, Fiocchi C. Etiopathogenesis of inflammatory bowel disease: the importance of the pediatric perspective. Inflamm Bowel Dis. 2002;8:112–128. doi: 10.1097/00054725-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Tsujii M, Iijima H, Nishida T, Takehara T. Smoking and alimentary diseases. Nihon Rinsho. 2013;71:436–442. [PubMed] [Google Scholar]
- 30.Frolkis A, Dieleman LA, Barkema H, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18–e24. doi: 10.1155/2013/102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakatos PL, Vegh Z, Lovasz BD, David G, Pandur T, Erdelyi Z, Szita I, Mester G, Balogh M, Szipocs I, et al. Is current smoking still an important environmental factor in inflammatory bowel diseases? Results from a population-based incident cohort. Inflamm Bowel Dis. 2013;19:1010–1017. doi: 10.1097/MIB.0b013e3182802b3e. [DOI] [PubMed] [Google Scholar]
- 32.Leijonmarck CE, Persson PG, Hellers G. Factors affecting colectomy rate in ulcerative colitis: an epidemiologic study. Gut. 1990;31:329–333. doi: 10.1136/gut.31.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 34.Hendriksen C, Kreiner S, Binder V. Long term prognosis in ulcerative colitis--based on results from a regional patient group from the county of Copenhagen. Gut. 1985;26:158–163. doi: 10.1136/gut.26.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 36.Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Ekbom A, Rönnblom A. Incidence and natural history of ulcerative colitis in the Uppsala Region of Sweden 2005-2009 - results from the IBD cohort of the Uppsala Region (ICURE) J Crohns Colitis. 2013;7:e351–e357. doi: 10.1016/j.crohns.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573–583. doi: 10.1002/ibd.21815. [DOI] [PubMed] [Google Scholar]
- 38.Park SH, Kim YM, Yang SK, Kim SH, Byeon JS, Myung SJ, Cho YK, Yu CS, Choi KS, Chung JW, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007;13:278–283. doi: 10.1002/ibd.20015. [DOI] [PubMed] [Google Scholar]
- 39.Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 40.Hilmi I, Singh R, Ganesananthan S, Yatim I, Radzi M, Chua AB, Tan HJ, Huang S, Chin KS, Menon J, et al. Demography and clinical course of ulcerative colitis in a multiracial Asian population: a nationwide study from Malaysia. J Dig Dis. 2009;10:15–20. doi: 10.1111/j.1751-2980.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 41.Niriella MA, De Silva AP, Dayaratne AH, Ariyasinghe MH, Navarathne MM, Peiris RS, Samarasekara DN, Satharasinghe RL, Rajindrajith S, Dassanayake AS, et al. Prevalence of inflammatory bowel disease in two districts of Sri Lanka: a hospital based survey. BMC Gastroenterol. 2010;10:32. doi: 10.1186/1471-230X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, Moreels TG. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol. 2008;2008:567314. doi: 10.1155/2008/567314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donskow-Łysoniewska K, Bien J, Brodaczewska K, Krawczak K, Doligalska M. Colitis promotes adaptation of an intestinal nematode: a Heligmosomoides polygyrus mouse model system. PLoS One. 2013;8:e78034. doi: 10.1371/journal.pone.0078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu KM, Watermeyer G, Shelly L, Janssen J, May TD, Brink K, Benefeld G, Li X. Childhood helminth exposure is protective against inflammatory bowel disease: a case control study in South Africa. Inflamm Bowel Dis. 2013;19:614–620. doi: 10.1097/MIB.0b013e31827f27f4. [DOI] [PubMed] [Google Scholar]
- 46.Kron MA, Metwali A, Vodanovic-Jankovic S, Elliott D. Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis. Clin Vaccine Immunol. 2013;20:276–281. doi: 10.1128/CVI.00594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinstock JV, Elliott DE. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelan RA, Hartmann S, Rausch S. Nematode modulation of inflammatory bowel disease. Protoplasma. 2012;249:871–886. doi: 10.1007/s00709-011-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun S, Wang X, Wu X, Zhao Y, Wang F, Liu X, Song Y, Wu Z, Liu M. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors. 2011;4:186. doi: 10.1186/1756-3305-4-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin J, Hackam DJ. Worms, flies and four-legged friends: the applicability of biological models to the understanding of intestinal inflammatory diseases. Dis Model Mech. 2011;4:447–456. doi: 10.1242/dmm.007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, Van den Bossche RM, Martinet W, Pelckmans PA, Moreels TG. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:491–500. doi: 10.1002/ibd.20787. [DOI] [PubMed] [Google Scholar]


