Abstract
AIM: To review the effectiveness of distance management methods in the management of adult inflammatory bowel disease (IBD) patients.
METHODS: A systematic review and meta-analysis of randomized controlled trials comparing distance management and standard clinic follow-up in the management of adult IBD patients. Distance management intervention was defined as any remote management method in which there is a patient self-management component whereby the patient interacts remotely via a self-guided management program, electronic interface, or self-directs open access to clinic follow up. The search strategy included electronic databases (Medline, PubMed, CINAHL, The Cochrane Central Register of Controlled Trials, EMBASE, KTPlus, Web of Science, and SCOPUS), conference proceedings, and internet search for web publications. The primary outcome was the mean difference in quality of life, and the secondary outcomes included mean difference in relapse rate, clinic visit rate, and hospital admission rate. Study selection, data extraction, and risk of bias assessment were completed by two independent reviewers.
RESULTS: The search strategy identified a total of 4061 articles, but only 6 randomized controlled trials met the inclusion and exclusion criteria for the systematic review and meta-analysis. Three trials involved telemanagement, and three trials involved directed patient self-management and open access clinics. The total sample size was 1463 patients. There was a trend towards improved quality of life in distance management patients with an end IBDQ quality of life score being 7.28 (95%CI: -3.25-17.81) points higher than standard clinic follow-up. There was a significant decrease in the clinic visit rate among distance management patients mean difference -1.08 (95%CI: -1.60--0.55), but no significant change in relapse rate or hospital admission rate.
CONCLUSION: Distance management of IBD significantly decreases clinic visit utilization, but does not significantly affect relapse rates or hospital admission rates.
Keywords: Telemanagement, Telehealth, Inflammatory bowel disease, Distance management, Self-management
Core tip: Distance management of inflammatory bowel disease (IBD) involves the use of telemedicine, web-based intervention, telephone clinics, patient directed open access clinics, and other methods that incorporate patient self-management strategies to manage patients remotely. This systematic review and meta-analysis of six randomized controlled trials shows that distance management of IBD significantly decreases clinic visit utilization, and can improve quality of life in certain groups. Consideration should be made in tailoring distance management approaches to select IBD patient populations.
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic intestinal diseases that adversely affects quality of life and societal interaction and functioning. They are associated with significant morbidity and mortality. Patients have intermittent flares requiring adjustments in medications as well as frequent clinic visits, hospitalizations, and surgeries. In Canada, approximately 233000 Canadians have a diagnosis of IBD, and about 10200 Canadians are diagnosed each year[1]. The total direct costs of IBD are estimated at C$ 1.2 billion, with 76% being comprised of drug costs and inpatient hospitalizations. Total indirect costs are estimated to be C$ 1.6 billion, mainly due to lower labour participation rates[1]. With the increasing incidence rate of IBD over the past decade[1,2], there are longer wait lists to see specialists in clinics and increased health care resource utilization. The Canadian Association of Gastroenterology guidelines have recommended no longer than 2 wk waiting time for patients presenting with symptoms of active inflammatory bowel disease[3,4], yet in 2012 the average waiting time to see a consultant was 72 d[5]. This delay in medical assessment can adversely affect patients’ quality of life[6]. This increasing burden of IBD on patients’ quality of life and functioning and on national health care resources has been reported globally[7-10]. Earlier and more aggressive optimization of therapy could alter disease course and reduce hospitalization and health care resource costs[10,11].
Clinicians have focused on techniques to improve the out-patient management of IBD patients. Strategies to improve patient education alone increase IBD-related knowledge, but do not consistently improve clinical outcomes or decrease health care resource use[12-15]. Focusing on improving self-management behaviour, however, may be effective[16,17]. A previous systematic review on patient education and self-management reported that self-management strategies demonstrated improved outcomes of symptoms, psychological well-being, and health-care resource use[15].
In the past decade, clinicians have investigated using self-management as a component of distance management of chronic diseases. Telemedicine generally refers to “medicine practised at a distance”[18] and has been used to remotely manage several chronic diseases such as diabetes, heart failure, hypertension, chronic obstructive pulmonary disease with reported variable outcomes[18-20]. The main concept of medicine practised at a distance is that it incorporates a component of patient self-management where patients relay information about their state of health to a program or health care team, which gives them feedback. Patients can then adjust their therapy based on pre-determined algorithms or seek medical assessments. Researchers have recently investigated the use of telemedicine for IBD patients[21-24] with a recent review suggesting a potential role for telemedicine in the management of IBD patients[21].
Two prior reviews have looked at patient self-management and telemedicine management of IBD patients separately, and more recently studies of this topic have been published; therefore, the objective of this systematic review and meta-analysis is to provide an updated and comprehensive analysis of the efficacy of distance management methods vs standard clinic follow-up in the management of IBD patients. The primary outcome is the mean difference in quality of life, and secondary outcomes include the mean difference in relapse rate, clinic visit rate, and hospital admission rate.
MATERIALS AND METHODS
Registration of protocol
The protocol for this review was registered with Prospero, the international prospective register of systematic reviews in health and social care (registration number CRD42013004286) and can be found at the http://www.crd.york.ac.uk/prospero/ website.
Eligibility criteria
Randomized controlled trials (RCTs) comparing the efficacy of distance self-management vs standard clinic follow-up in the management of adult (> 16 years old) IBD patients were included. We had initially included cohort studies, before-after, and pilot studies in our search strategies, but as we aimed to present an analysis of the most stringent methodology, and there were sufficient RCTs, we excluded them from this review and meta-analysis.
Distance management intervention was defined as any remote management method in which there is a patient self-management component whereby the patient interacts remotely via a self-guided management program or electronic interface, and actively adjusts medications or self-directs open access to clinic follow up. Studies that included interventions that only involved improving patient education or managing stress and lifestyle, but had no self-management and no distance management component were excluded. The comparator group was standard clinic follow-up for the institution at the time of the study. Included studies had to implement the intervention and continue to follow the patient for at least 6 mo. Included studies had to report at least one of the outcomes of interest: quality of life (QoL), relapse rate, clinic visit rate, and hospital admission rate. Studies that did not report any of these outcomes were excluded from this review.
Search methods for identification of studies
A systematic search of the following electronic databases was performed in January 2013: Medline (1950-2013), PubMed (1950-2013), CINAHL (1937-2013), The Cochrane Central Register of Controlled Trials, EMBASE (1974-2013), KTPlus, Web of Science (1990-2013), and SCOPUS (1960-2013). We used the MeSH subject headings and text-words including “Inflammatory bowel disease”, “crohn’s”, “ulcerative colitis”, “patient education”, “self-care”, “self-management”, “telehealth”, “telemedicine”, “ehealth”, and similar keywords (Appendix A). If the database allowed, we exploded terms to be more inclusive. We also hand searched the conference proceedings of the major Gastroenterology and Inflammatory Bowel Disease conferences from 2008-2013 (Canadian Digestive Diseases Week, Digestive Diseases Week, Advances in IBD, and European Crohn’s and Colitis Organisation congress). We searched for Internet publications using www.google.ca with the same search terms as for the electronic databases; and we also reviewed the reference lists of review articles and related studies. Searches were updated on a regular basis, and the last search completed on March 16, 2013.
Study selection
One reviewer (VH) completed the electronic search of the above listed databases, the hand searching of conference proceedings, and the internet search. Duplicate articles were removed using RefWorks 2.0 manager. VH screened the remaining titles to remove irrelevant articles, review articles, case series, and case reports. Using pre-determined inclusion criteria, two reviewers (VH and KR) independently screened the remaining abstracts as “definitely include” (meeting all of the inclusion criteria, reported at least one of the outcomes), “maybe” (meeting some of the inclusion criteria, but unclear outcomes), and “definitely not” (did not meet any of the inclusion criteria). VH and KR then independently screened the full text manuscripts of the “definitely include” and “maybe articles”, and excluded those that did not report any of the a priori outcomes. The decision on the final list of included articles was reached by discussion and consensus, with consensus on questionable inclusion or exclusion confirmed by a third reviewer (RF).
Data extraction
A data extraction form was developed based on the Cochrane data extraction form[25], and pilot tested for understanding and consistency among the two reviewers (VH and KR). Data regarding first author, publication date, study design, patient characteristics, intervention and control, and funding sources was extracted by VH and checked for accuracy by KR. VH and KR independently extracted outcomes data into the data extraction form. Disagreements were resolved by discussion and consensus. Attempts were made to contact study authors for data values and clarification of results.
Cross et al[26] expressed the method of analysis as intention to treat (ITT), yet the authors reported change from baseline IBDQ scores; the authors confirmed their results were calculated based on the final number of patients who completed the study [n = 14 Ulcerative colitis (UC) HAT and n = 18] best available care (BAC). The article by Elkjaer et al[27] reported on two parallel studies, one in Denmark and one in Ireland. Since each study population had separately randomized intervention and control groups, we felt it reasonable to treat these two studies as separate RCTs. They did not report values for the QoL score or the hospital admission rate. They also did not report SD for clinic visits, so SD was imputed from the P values. The articles by Kennedy et al[28] and Richardson et al[29] reported on different outcomes from the same RCT. The Robinson et al[30] study reported baseline IBDQ and end of study IBDQ but did not report SD, so we decided to use the largest SD from the Cross study, as both studies looked at change from baseline results. The Robinson et al[30] study did not report SD or P values for the relapse rate, so the largest SD of the studies (2.5 from Kennedy control group) was used. They also did not report SD values for clinic visits, so the SD was imputed from the p-value reported.
Quality and risk of bias assessment
Two reviewers (VH and KR) independently assessed each study for quality and risk of bias using the Cochrane Collaboration tool for risk of bias for RCT[31]. The final decision on overall risk of bias was reached through discussion and consensus.
Statistical analysis
All data was analyzed using Review Manager (RevMan) Version 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Data was analyzed on an intention-to-treat basis, unless otherwise specified in the results of the articles, or in communications with the study’s authors. Outcomes were all recorded as continuous variables, and the effect size was reported as mean difference with 95%CI. Pooled meta-analyses were completed on studies that reported the same outcomes. I2 statistics were used to test for heterogeneity, and if there was significant heterogeneity, the random effects model was used. The source of heterogeneity was investigated by completion of subgroup analysis by type of distance method and disease type [UC vs UC and Crohn’s disease (CD); there were no CD studies eligible for inclusion]. Sensitivity analysis was not done because of the significant heterogeneity among studies. Publication bias was planned if we had more than 10 studies, but it was not assessed because of the small number of studies.
RESULTS
Study selection
A total of 4061 articles were identified by the electronic search strategy (Figure 1), and 15 abstracts identified by hand searching conference proceedings. No additional articles were identified by the internet web search. After exact duplications were removed, 2884 articles remained. Screening by title excluded 2701 articles. Two reviewers independently screened the remaining 183 articles, resulting in exclusion of 115 articles. Thus, 68 full text manuscripts were independently reviewed for eligibility. Of these, 62 were excluded for the follow reasons: (1) patient education/counseling only, no self-management via distance method[12-14,32-40]; (2) stress or lifestyle management, no self-management via distance method[41-50]; (3) audit or retrospective cohort study[51-58]; (4) review or commentary[17,59-63]; (5) feasibility or pilot studies with no comparator group[64-78]; (6) pilot study reporting on same study population as included study[24,79-81]; (7) pediatric patients[82,83]; (8) no a priori outcomes reported[84]; (9) duplicate studies[26,84-86]; and (10) smart phone method of symptom assessment, but no distance management or self-management[87]. Six full text articles reporting on six randomized controlled trials met the inclusion criteria for this systematic review[26-30,88]. An updated literature search was completed in March 2013, and did not reveal any new randomized controlled studies for this review.
Figure 1.

Flow chart of literature search results.
Study characteristics
Table 1 summarizes the patient characteristics and interventions of the included RCTs. The Cross et al[26] RCT included UC patients from the University of Maryland, Baltimore and the Veterans Affairs, Maryland Health Care System, Baltimore. Patients were recruited through invitation by letter and also at the time of their clinic visits. Randomization by permuted block design with randomly varied block sizes was stratified by baseline disease activity strata, and assignment was concealed. However, post hoc analysis revealed that patients in the intervention group may have had higher disease activity, as they reported higher immunosuppressant use and lower baseline IBDQ scores. Research staff at study visits was blinded to treatment allocation. Patients answered questions weekly about symptoms, side effects, adherence, and received disease-specific education and customized action plans using the home unit, which then transmitted results to the decision support server. E-mail alerts were sent to the nurse coordinator if the patient met certain clinical conditions. The patients could also send electronic messages to the nurse coordinator, who then made management changes through consultation with the medical provider. The control group was managed by BAC.
Table 1.
Summary of included studies on distance management of inflammatory bowel disease in adults
| Author, yr | Patients randomized/baseline (N) intervention vs control | DiseaseDisease severity | Inclusion/exclusion | Mean age (yr) Control vs intervention | Male (%) Control vs intervention | Intervention | Control | Duration (mo) |
| Cross et al[26],2012 | 47 pts rand.14 web vs 18 BAC | UC | Not specified | 40.3 vs 41.7 | 32 vs 40 | UC HAT (Home telemanagement: - a home unit (laptop and electronic weight scale) a decision support server, -a web-based clinician portal | Best Available Care (educational material, action plan, clinics visits) | 12 |
| Elkjaer et al[27],2010 | 233 pts rand.105 web vs 106 control | UC mild/mod | Inclusion: age 18-69 yr, mild/moderate UC, treated with 5- ASAExclusion: acute phase of co-morbid conditions, drug dependence or substance abuse, use of immunomodulators, frequent treatment with high dose systemic corticosteroids, likely requirement of IBD surgery, previous IBD surgery | 40 vs 44 (P = 0.03) | 49.5 vs 31.1(P = 0.008) | Web-intervention (Educational training then www.constant-care.dk) | Conventional treatment and follow up in the IBD out-patient clinic | 12 |
| Elkjaer et al[27], 2010 | 100 pts rand.51 web vs 41 control | UC | Same as above | 41 vs 46 | 60.8 vs 41.5 | Web-intervention (Educational training then www.constant-care.dk) | Conventional treatment and follow up in the IBD out-patient clinic | 12 |
| Kennedy et al[28], 2004Richardson et al[29],2006 | 700 pts rand.270 interv.365 control | Mild/modCD (n = 231)UC or ID (n = 404) | Inclusion: UC or CD, over age of 16 yr, able to write English, attending a follow-up clinicExclusion: Not specified | 46.3 vs 44.4 | 43 vs 41.5 | Guided self-management- patient guidebook- self-management plan- patient centered approach to care by a trained clinician- direct access to services for patients to self-refer | Management process deemed appropriate by the hospital specialist-6 sites follow long term- 2 sites discharge quiescent IBD-1 site no consistent follow up | 12 |
| Robinson et al[30],2001 | 203 pts101 interv.102 control | UC | Inclusion: newly diagnosedExclusion: require hospital outpatient follow-up for other illnesses, unable to read informed consent or follow written instructions | 48 vs 49 | 48 vs 49 | Personalised guided self-management regimen with direct access to outpatient care on request | Clinician’s normal treatment and follow-up | Until 11 mo after last pt recruited |
| Williams et al[88], 2000 | 180 pts 88 interv.92 control | CD (n = 78) UC or ID (n = 77)Proctitis (n = 25)Inactive or mildly active | Inclusion: over 18 yr, inactive or mildly active but stable IBDExclusion: active disease requiring treatment, stoma, other disease requiring regular follow up, unable to comply with data collection | N/A (no significant difference reported) | N/A (no significant difference reported) | Open access follow up | Routine follow up | 24 |
IBDQ: Inflammatory bowel disease questionnaire; SIBDQ: Short-IBDQ; NS: Not significant; ID: Indeterminate colitis; CD: Crohn’s disease; UC: Ulcerative colitis.
The Elkjaer et al[27] article reported on two separate RCTs conducted on mild to moderate ulcerative colitis patients in Denmark (Herlev and Amager Hospital, Copenhagen) and Ireland (Adelaide and Meath Hopital in Dublin). Eligible patients who consented were randomized by a web-based randomization program, and assignments were concealed using closed, consecutive, numbered envelopes. They also included a historical control group from a separate hospital in Denmark, who were unaware of this trial, and prospectively from Adelaide hospital in Ireland. The intervention group received a 1.5 h disease specific presentation, and then a 1.5 h practical training session on the web-program http://www.constant-care.dk. Patients were instructed on how to recognize a relapse, and in case of relapse, would log on daily, complete the disease activity score, and follow management instructions, until they entered a green zone of quiescent disease. They then logged on weekly until 4 weeks after initiation of the relapse. Once in remission, they reported monthly. Patients could also email, call, or text the web-doctor. The control group continued to receive conventional treatment and follow up in the out-patient clinic.
The Kennedy et al[28]/Richardson et al[29] trial was a cluster-randomized multicentre trial conducted in the North West of England out of 7 teaching hospitals and 12 non-teaching hospitals. Both UC and CD patients were included, but specific inclusion and exclusion criteria were not reported. The first 38 eligible patients who consented were recruited at each site. The intervention consisted of four components (see Table 1). Those in the control group received management deemed appropriate by the hospitalist specialist.
The Robinson et al[30] RCT was conducted in four hospitals in the Greater Manchester area of the United Kingdom (Hope Hospital, Salford, Burgy General Hospital, Trafford General Hospital, and the Royal Oldham Hospital). Patients with ulcerative colitis were first assessed for suitability for inclusion by their normal clinician, and then interviewed by an investigator and invited to participate. Only patients in clinical remission were included in this study. The first 20 eligible patients who consented in each centre were included. Randomization was done by random number tables, and allocation was concealed by an assistant not involved in the study. The intervention consisted of a personalized guided self-management regimen, developed during a 15-30 min consultation by a clinician. Those in the control group received routine clinic follow-up.
The Williams et al[88] RCT was conducted out of two urban district general hospitals in Swansea (Morriston Hospital) and Neath (Neath Hospital), Wales. Patients with inactive or mildly active but stable disease were invited to participate. Patients with Crohn’s disease, ulcerative colitis, indeterminate colitis, and proctitis were included. Those with ulcerative colitis or indeterminate colitis were analyzed as one group. The open access group contacted their general practitioner or the hospital about problems and were offered an early appointment.
Risk of bias within studies
The RCTs were of moderate to high risk of bias (Table 2). All of the studies reported on randomization and concealment, except for the Kennedy et al[28] and Richardson et al[29] trial. All of the trials were deemed high risk for performance bias because blinding could not be controlled for. Due to the nature of the intervention involving patient self-management, it was not possible to blind participants to the intervention. The outcomes were all affected by patient subjectivity, and therefore were prone to performance bias. There may have been changes to patient or physician behaviours depending on which group the patient was randomized to. The QoL outcome depended on self-reporting of symptoms. The relapse rate was somewhat dependent on the patient reporting symptoms compatible with a priori defined relapse/flare criteria. The number of clinic visits in the intervention group was dependent on the patient’s responses to the self-management criteria, and their initiation to contact the health care team. Hospital admission rate was less likely prone to bias, as one would expect that admission to hospital was unlikely to be biased on group allocation.
Table 2.
Risk of bias of included studies on distance management compared with standard clinic follow-up for adult inflammatory bowel disease patients
| Author, yr | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Source of funding |
| Cross et al[26],2012 | Low (random permuted block design; concealed) | High | Low (research staff blinded to treatment allocation) | High (more discontinued in intervention group 8/25 vs control 1/22) | Low | Broad Medical Research Program, University of Maryland General Clinical Research Center Grant, General Clinical Research Centers Program, NCRR, NIH, Baltimore Education and Research Foundation |
| Elkjaer et al[27],2010 | Low (randomisation program; closed envelope) | High | High | High (LTF higher in the web group 24% vs control 17%) | Low | Colitis Crohn Patient Organization, Moran’s Foundation, Vibeke Binder and Povl Riis’ Foundation, Bayer Health Care Funding, Augustinus Foundtaion, Munkholms Foundation, Tillotts Funding, Scientific Councel at Herlev Hospital, Prof. Fagerhol Research Foundation, Aase and Einar Danielsen Foundation, Ole Trock-Jansen and Hustrus Foundation, and European Crohn Colitis Organization |
| Elkjaer et al[27], 2010 | Unclear (cluster randomization; no mention of concealment) | High | High | High (LTF higher in control group) | Low | |
| Kennedy et al[28], 2004Richardson et al[29], 2006 | Low (random number tables; concealed) | High | High | High (LTF higher in control group 24% vs intervention 13%) | Low | Health Technology Assessment Programme of the United Kingdom NHS (MS) Career Scientist Award in Public Health, NHS R and D(GS) Researcher Development Award, NHS R and D |
| Robinson et al[30], 2001 | Low (computer generated lists, concealed) | High | High | Low | Low | (AR) United Kingdom Medical Research Council Training Fellowship |
| Williams et al[88], 2000 | Low (computer generated lists, concealed) | High | High | Low | Low | NHS research and development primary/secondary care interface programme, West Wales and Swansea Group of the National Association for Colitis and Crohn’s Disease. |
LTF: Lost to follow-up; NCCR: National Center for Research Resources; NIH: National Institute of Health; NHS R and D: National Health Services Research and Development.
There was attrition bias in the Cross et al[26] and Elkjaer et al[27] (Denmark) RCTs (see Table 2) with higher discontinuation or loss to follow up (LTF) in the intervention groups. In the Cross et al[26] study, trial completers had less extensive colitis than those who did not complete the trial. In the Kennedy et al[28]/Richardson et al[29] RCT, there was a bias towards greater loss to follow up in the control group; however the number of patients who did not complete the study was higher (12 vs 4) in the Morriston hospital.
There may also have been bias in terms of differences in patient characteristics. In the Cross et al[26] RCT, a significantly higher percentage (56%) of the UC HAT group were on immune suppressants compared to the BAC group (27%)[26]. In the Elkjaer et al[27] DenmarkRCT, there were significantly more males (49.5% vs 31.1%; P = 0.008) and younger patients (40-year-old vs 44-year-old; P = 0.03) in the web intervention group. There were no reported significant differences in baseline demographics in the Kennedy et al[28] and Richardson et al[29] trial, but they did not report specific inclusion/exclusion criteria. The Robinson et al[30] trial matched controls for age, sex, time since diagnosis, extent of disease, and numbers within hospitals. In the Williams et al[88] trial, there were no reported differences in baseline age, sex, diagnostic group, or quality of life. There may have been bias due to differences in standard clinic follow-up policies, as hospitals and clinicians have different follow-up protocols, as shown in Table 1.
Primary outcome: Quality of life
All five studies reported on QoL. There was a significant improvement in QoL (P = 0.04) in the Elkjaer et al[27] Denmark web group, but no significant difference in QoL in the Ireland web group. They did not report the actual QoL score values, thus their results could not be pooled. The Williams et al[88] trial reported the various components of the UK Inflammatory Bowel Disease questionnaire and found no significant change in mean health related QOL scores; although, there was some deterioration in both groups on most subscales. They did not report summary values that could be pooled. Three trials[26,28,30] (n = 338) reported changes in IBDQ scores after one year of intervention and were included in the meta-analysis. The baseline IBDQ scores in the Cross et al[26] and Robinson et al[30] trials differed between groups; thus for the meta-analysis, we used their published change in IBDQ scores, adjusted for baseline IBDQ scores. The Kennedy et al[28] trial presented data that was already adjusted for baseline scores.
Effect sizes varied, and there was significant heterogeneity (I2 = 96%; P < 0.00001). Subgroup analysis by type of distance management (Figure 2A) decreased the heterogeneity, and also showed that the electronic telemanagement system significantly improved QoL mean difference 16.30; 95%CI: 12.36-20.24). Subgroup analysis by disease type (UC vs both) did not decrease heterogeneity within groups and did not result in significant mean differences (Figure 2B).
Figure 2.

Mean change in quality of life between distance management and standard clinic follow up subgroup analysis by intervention and disease. A: Intervention; B: Disease.
Secondary outcome: Relapse rate
Four RCTs[27,28,30] (n = 450) reported relapse rate. Effect sizes varied, however, there was significant heterogeneity (I2 = 75%; P = 0.0007). Subgroup analysis by type of distance management (Figure 3A) decreased heterogeneity. It also showed that the Elkjaer et al[27] trials, which used the electronic, web-based distance management, favoured the control group mean difference 0.33; 95%CI: 0.15-0.51, while the patient directed open access clinic management studies favoured the distance management group mean difference -0.40; 95%CI: -0.77--0.03. Heterogeneity may also be explained by differences in disease type (Figure 3B), as four RCTs[26,27,30] included only UC patients, while the Kennedy et al[28]/Richardson et al[29] and the Williams et al[88] RCT included UC and CD. The UC studies were in favour of control mean difference 0.25; 95%CI: -0.04-0.54), while the UC and CD study was in favour of distance management mean difference -0.40; 95%CI: (-0.83-0.03); neither of the effect sizes were statistically significant (Figure 3B).
Figure 3.
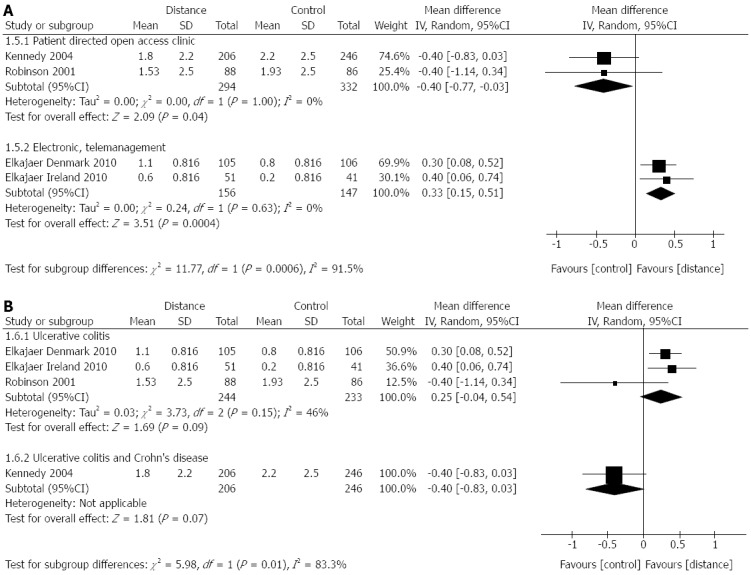
Difference in relapse rate between distance management and standard clinic follow-up subgroup analysis by intervention and disease. A: Intervention; B: Disease.
Secondary outcome: Number of clinic visits/patient/year
Five RCTs[27,29,30,88] reported on the number of outpatient clinic visits. The Williams et al[88] data could not be pooled with the others, as they reported clinic visits over 24 mo; however results favoured the distance management group 4.12 (SD 3.41) visits per patient vs 4.64 (SD 2.38) visits per patient in the control group). Effect sizes varied, and there was significant heterogeneity (I2 = 89%; P < 0.00001), but even with subgroup analysis by intervention type (Figure 4A) and disease (Figure 4B), the results favoured distance management.
Figure 4.
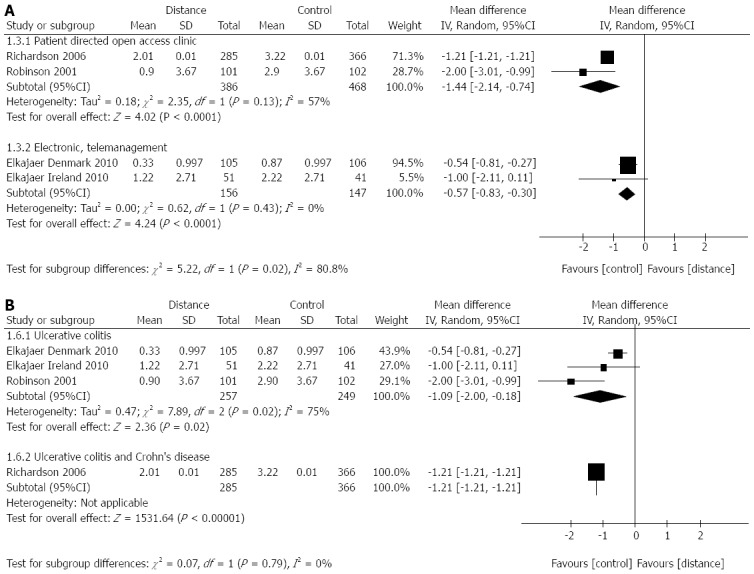
Difference in number of clinic visits per patient per year between distance management and standard clinic follow-up subgroup analysis by intervention and disease. A: Intervention; B: Disease.
Secondary outcome: Hospital admission rate
We had a priori planned to analyze the number of hospital admissions/patient/year, but only the Elkjaer et al[27] trials reported non-significant differences but did not provide actual values for this outcome. Two trials[29,88] reported mean number of hospital inpatient days but could not be pooled as one trial duration was 24 mo compared to 12 mo. However, there were no significant differences in the number of inpatient days per patient over 24 months reported in the Williams et al[88] open-access intervention trial [open access 0.83 (SD 3.53) vs control 0.41 (SD 1.74); P = 0.71] or over 12 mo reported in the Kennedy et al[28]/Richardson et al[29] trial [self-care 1.01 (SE 0.36) vs control 1.18 (SE 0.28); NS]. Thus, although actual data values are not available, there was no significant difference reported in hospital admission rate between distance management and standard clinic follow-up.
Publication bias
Our search strategy was a comprehensive search which included conference proceedings and internet searches for unpublished studies. We were unable to do a funnel plot to assess for publication bias, due to the small number of eligible studies. However, the results reported from the included RCTs were equivocal in favouring distance management vs standard clinic follow-up, so it does not appear that there is publication bias in this field of interest.
DISCUSSION
This review included six randomized controlled trials comparing distance management and standard clinic follow-up of inflammatory bowel disease patients for a total 1463 randomized IBD patients[26-30,88]. Three trials used electronic telemanagement or web-based systems, and three trials used patient directed open-access clinics. Distance management of a chronic disease such as IBD ideally would maintain or improve QoL, maintain or decrease relapse rates, and decrease health care utilization. This review shows that distance management intervention resulted in variable improvements in QoL, clinic visits, relapse rates, and hospitalization rates. Overall, the results support the rationale of using distance management in the management of IBD patients.
The six RCTs showed a trend toward an improvement in QoL scores overall[26-30,88]. Subgroup analysis showed that the UC Home telemanagement system resulted in significantly improved QoL scores (mean difference 16.30) while the patient-directed open-access clinic intervention resulted in a non-statistically significant improvement in QoL. One potential reason for this difference is there is more interaction with the home telemanagement system. The patient answers specific questions, receives instructions, and is able to email the clinicians. On the contrary, in the open-access clinic approach, the patient is left to self-direct their own management based on their symptoms or a pre-determined management plan. Another potential reason may be that patients in the Cross et al[26] UC home telemanagement system group had higher immunosuppressant use and lower baseline IBDQ scores, and thus may have had higher disease activity than the control group. This may have resulted in significance in even small improvements in QoL. However, any small, even if not statistically significant, improvement in QoL may be clinically important and beneficial to IBD patients. Thus, even the open-access clinic approach may be useful in improving IBD patient QoL.
All six RCTs showed a significant decrease in clinic utilization in the intervention group, regardless of type of intervention or disease type[26-30,88]. On average, the interventions decreased the number of clinic visits from 2 to 3 visits to 1 visit per patient per year. This might allow these clinic visit times to become available for other patients or urgent cases. This may help consultants to achieve the target CAG waiting time of 2 wk for patients presenting with symptoms of active inflammatory bowel disease. However, telemedicine still requires time from the nursing staff or the physician, and exchange of clinic visits for telemedicine contact and follow up may still result in equivalent use of health care resources.
Since decreased clinic visit utilization may theoretically affect relapse rates and hospitalization rates, this meta-analysis also looked at these two outcomes. The Elkjaer et al[27] RCTs reported increased relapse rates in the web-based group, thus favouring the control group. On the other hand, the patient directed open access studies favoured the intervention group. This difference could be explained by a difference in the definition of relapse. The Elkjaer et al[27] trials used an objective measure of SCCAI (Simple Clinical Colitis Activity Index) score > 5, and the Kennedy et al[28]/Richardson et al[29] and Robinson et al[30] trials used patient self-reported relapses. However, the absolute difference in relapse rate was small -0.40 (patient directed open access clinic) vs 0.33 (web-based) relapse per year per patient. This difference in relapse rate may not be clinically significant, since there was no difference in hospital admission rate between intervention and control groups, and there was actually a decrease in the number of clinic visits per patient per year.
There are several limitations of this review. The overall risk of bias of the included studies was moderate to high when assessed using the Cochrane Collaboration risk of bias tool. Reasons included inability to blind participants due to the nature of the intervention of interest and unequal loss to follow-up and study completion rates between groups. Since some studies did not fully report inclusion/exclusion criteria, there may have been subtle differences in the study populations that may have led to some of the reported differences in effect size. There may be differences in the personalities of patients who consented to participate, in terms of their perceptions of self-reported disease relapse[89] or their beliefs about personal control and self-management[90]. In addition, some of the studies did not clarify specific inclusion and exclusion data, and therefore there may have been differences in disease activity or severity between groups. This may have affected quality of life scores or number of clinic visits and presentations to hospital for admission. In addition, these types of management methods for any chronic disease may be more beneficial for select patients at certain disease stages[17] or patients living farther away from urban centres. Further studies comparing different management strategies for patients with different disease severities would be useful, as patients with more severe or active disease may require more intensive management.
Another limitation was heterogeneity in the type of distance management and in the reported standard clinic follow-up policies between different hospitals. This made it difficult to pool results from different studies; however, sub-group analysis showed some differences between web-based and patient directed open access interventions. Finally, there was an issue with variable and incomplete data-reporting. Some results could not be pooled as raw data was unavailable. Standard deviation had to be imputed for many of the variables. However, overall, subgroup analysis by intervention or disease type showed consistent mean differences.
This review only included detailed analysis of randomized controlled trials of distance management of IBD. However, non-randomized studies have shown benefit with telephone clinics[32,53,54,91], nurse specialist management[37,38,55,58], e-mail[75,92], smart-phone programs[76,77,89,93], chronic care models[47-51], and virtual clinics[68]. Incorporating these distance management methods may also be useful in improving standard clinical care and should be considered for future randomized controlled trials.
In conclusion, distance management of IBD decreases clinic visit utilization, but it does not significantly improve patients’ quality of life, relapse rates, or hospital admission rates. Consideration should be made in tailoring these approaches to select patient populations. Perhaps a combined web-based and patient directed open access clinic distance management program, whereby patients interact with an electronic web-based management program and are able to initiate self-treatment strategies and self-referral to clinic assessments, may be the solution. Further studies are needed to determine the best type and the cost effectiveness of distance management of inflammatory bowel disease patients. Future randomized controlled trials comparing different types of distance management with different groups of IBD patients may help to determine which type of distance management is the optimal method for specific groups of patients.
ACKNOWLEDGMENTS
Special thanks to Karen Kroeker (Assistant Professor, Department of Medicine, University of Alberta) for reviewing the manuscript, to Dagmar Chojecki (Institute of Health Economics Research Librarian, University of Alberta) for assistance in the development of the search strategy, to Donna Dryden (Associate Director, University of Alberta Evidence-based Practice Center and Associate Professor, Department of Paediatrics, University of Alberta) for advice on systematic review methods, and to Ben Vandermeer (Biostatiscian, University of Alberta Evidence-based Practice Center) for statistical advice.
COMMENTS
Background
Inflammatory bowel disease is a group of chronic bowel diseases that affect patients of all ages, and all locations. The health care utilization of inflammatory bowel disease patients is increasing over time. Distance management of patients using remote methods that involve self-management strategies and patient-health care provider interaction, may be a mechanism to improve management of inflammatory bowel disease. Distance management of inflammatory bowel disease includes methods such as internet and web-based programs, telephone clinics, digital phones, patient self-management via pre-determined action plans, patient self-directed open access clinics.
Research frontiers
Prior reviews have looked at self-management and telemedicine strategies separately. This systematic review and meta-analysis examines the effectiveness of distance management using patient self-management techniques in improving quality of life, clinic visit utilization, relapse rate, and hospitalization rate in IBD patients.
Innovations and breakthroughs
This review and meta-analysis presents a unique comparison of distance management methods to standard clinical care of IBD patients. The authors have found that distance management methods significantly decreases clinic visit rates, and slightly improves quality of life in inflammatory bowel disease (IBD) patients, but does not significantly affect the relapse rate or hospital admission rate.
Applications
Distance management of IBD can be an important part of the management of IBD patients, but may require tailoring of these approaches to select patient populations. A combined web-based and patient directed open access clinic distance management program, whereby patients interact with an electronic web-based management program and are able to initiate self-treatment strategies and self-referral to clinic assessments, may be a solution.
Terminology
The main concept of distance medicine is that it incorporates a component of patient self-management where patients relay information about their state of health to a program or health care team, which gives them feedback. Patients can then adjust their therapy based on pre-determined algorithms or seek medical assessments.
Peer review
This is an interesting and well-written systematic review and meta-analysis on a relevant and current topic. The question of whether IBD patient self-management can be optimized through “distance” techniques is a worthy topic for consideration. This paper is well written and the methodology for the most part was spot-on.
Footnotes
P- Reviewers: Bailey MT, Femia AN, Keefer L S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, Glasgow KW, Fernandes A, Ghosh S. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein CN, Longobardi T, Finlayson G, Blanchard JF. Direct medical cost of managing IBD patients: a Canadian population-based study. Inflamm Bowel Dis. 2012;18:1498–1508. doi: 10.1002/ibd.21878. [DOI] [PubMed] [Google Scholar]
- 3.Leddin D, Armstrong D, Barkun AN, Chen Y, Daniels S, Hollingworth R, Hunt RH, Paterson WG. Access to specialist gastroenterology care in Canada: comparison of wait times and consensus targets. Can J Gastroenterol. 2008;22:161–167. doi: 10.1155/2008/479684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leddin D, Bridges RJ, Morgan DG, Fallone C, Render C, Plourde V, Gray J, Switzer C, McHattie J, Singh H, et al. Survey of access to gastroenterology in Canada: the SAGE wait times program. Can J Gastroenterol. 2010;24:20–25. doi: 10.1155/2010/246492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leddin D, Armstrong D, Borgaonkar M, Bridges RJ, Fallone CA, Telford JJ, Chen Y, Colacino P, Sinclair P. The 2012 SAGE wait times program: Survey of Access to GastroEnterology in Canada. Can J Gastroenterol. 2013;27:83–89. doi: 10.1155/2013/143018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson WG, Barkun AN, Hopman WM, Leddin DJ, Paré P, Petrunia DM, Sewitch MJ, Switzer C, van Zanten SV. Wait times for gastroenterology consultation in Canada: the patients’ perspective. Can J Gastroenterol. 2010;24:28–32. doi: 10.1155/2010/912970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Lofland JH. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–3091. doi: 10.1007/s10620-012-2289-y. [DOI] [PubMed] [Google Scholar]
- 9.Kappelman MD, Porter CQ, Galanko JA, Rifas-Shiman SL, Ollendorf DA, Sandler RS, Finkelstein JA. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:62–68. doi: 10.1002/ibd.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis. 2011;17:1603–1609. doi: 10.1002/ibd.21488. [DOI] [PubMed] [Google Scholar]
- 11.van Langenberg DR, Simon SB, Holtmann GJ, Andrews JM. The burden of inpatient costs in inflammatory bowel disease and opportunities to optimize care: a single metropolitan Australian center experience. J Crohns Colitis. 2010;4:413–421. doi: 10.1016/j.crohns.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Borgaonkar M, Moody G, Donnelly M, Irvine EJ. Patient education does not improve health related quality of life (HRQOL) in inflammatory bowel disease (IBD) Gastroenterology. 1999;116:A671–A671. [Google Scholar]
- 13.Borgaonkar MR, Townson G, Donnelly M, Irvine EJ. Providing disease-related information worsens health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:264–269. doi: 10.1097/00054725-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bregenzer N, Lange A, Fürst A, Gross V, Schölmerich J, Andus T. Patient education in inflammatory bowel disease does not influence patients knowledge and long-term psychosocial well-being. Z Gastroenterol. 2005;43:367–371. doi: 10.1055/s-2004-813867. [DOI] [PubMed] [Google Scholar]
- 15.Barlow C, Cooke D, Mulligan K, Beck E, Newman S. A critical review of self-management and educational interventions in inflammatory bowel disease. Gastroenterol Nurs. 2010;33:11–18. doi: 10.1097/SGA.0b013e3181ca03cc. [DOI] [PubMed] [Google Scholar]
- 16.Saibil F, Lai E, Hayward A, Yip J, Gilbert C. Self-management for people with inflammatory bowel disease. Can J Gastroenterol. 2008;22:281–287. doi: 10.1155/2008/428967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Protheroe J, Rogers A, Kennedy AP, Macdonald W, Lee V. Promoting patient engagement with self-management support information: a qualitative meta-synthesis of processes influencing uptake. Implement Sci. 2008;3:44. doi: 10.1186/1748-5908-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wootton R. Twenty years of telemedicine in chronic disease management--an evidence synthesis. J Telemed Telecare. 2012;18:211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378:731–739. doi: 10.1016/S0140-6736(11)61229-4. [DOI] [PubMed] [Google Scholar]
- 20.Gaikwad R, Warren J. The role of home-based information and communications technology interventions in chronic disease management: a systematic literature review. Health Informatics J. 2009;15:122–146. doi: 10.1177/1460458209102973. [DOI] [PubMed] [Google Scholar]
- 21.Torrejón Herrera A, Masachs Peracaula M, Borruel Sainz N, Castells Carner I, Castillejo Badía N, Malagelada Benaprés JR, Casellas Jordá F. [Application of a model of continued attention in inflammatory bowel disease: the Crohn-colitis care unit] Gastroenterol Hepatol. 2009;32:77–82. doi: 10.1016/j.gastrohep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Patil S, Cross R. Where we’re going, we don’t need appointments: the future of telemedicine in IBD. Inflamm Bowel Dis. 2012;18:2199–2200. doi: 10.1002/ibd.23014. [DOI] [PubMed] [Google Scholar]
- 23.Cross RK, Arora M, Finkelstein J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006;40:200–208. doi: 10.1097/00004836-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cross RK, Cheevers N, Finkelstein J. Home telemanagement for patients with ulcerative colitis (UC HAT) Dig Dis Sci. 2009;54:2463–2472. doi: 10.1007/s10620-008-0640-0. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Green S. The Cochrane Handbook for Systematic Review of Interventions. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 26.Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT) Inflamm Bowel Dis. 2012;18:1018–1025. doi: 10.1002/ibd.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, Avnstrøm S, Langholz E, O’Morain C, Lynge E, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59:1652–1661. doi: 10.1136/gut.2010.220160. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy AP, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, Rogers AE, Sculpher M, Thompson DG. A randomised controlled trial to assess the effectiveness and cost of a patient orientated self management approach to chronic inflammatory bowel disease. Gut. 2004;53:1639–1645. doi: 10.1136/gut.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson G, Sculpher M, Kennedy A, Nelson E, Reeves D, Roberts C, Robinson A, Rogers A, Thompson D. Is self-care a cost-effective use of resources? Evidence from a randomized trial in inflammatory bowel disease. J Health Serv Res Policy. 2006;11:225–230. doi: 10.1258/135581906778476508. [DOI] [PubMed] [Google Scholar]
- 30.Robinson A, Thompson DG, Wilkin D, Roberts C. Guided self-management and patient-directed follow-up of ulcerative colitis: a randomised trial. Lancet. 2001;358:976–981. doi: 10.1016/S0140-6736(01)06105-0. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J, Altman D, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 32.Cook PF, Emiliozzi S, El-Hajj D, McCabe MM. Telephone nurse counseling for medication adherence in ulcerative colitis: a preliminary study. Patient Educ Couns. 2010;81:182–186. doi: 10.1016/j.pec.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Corbett S, Welfare M, McColl E Lecouturier J, Devine Z. Implementing a colitis education and support programme. Gastrointest Nurs. 2006;4:12–20. [Google Scholar]
- 34.Jäghult S, Larson J, Wredling R, Kapraali M. A multiprofessional education programme for patients with inflammatory bowel disease: a randomized controlled trial. Scand J Gastroenterol. 2007;42:1452–1459. doi: 10.1080/00365520701439685. [DOI] [PubMed] [Google Scholar]
- 35.Larsson K, Sundberg Hjelm M, Karlbom U, Nordin K, Anderberg UM, Lööf L. A group-based patient education programme for high-anxiety patients with Crohn disease or ulcerative colitis. Scand J Gastroenterol. 2003;38:763–769. doi: 10.1080/00365520310003309. [DOI] [PubMed] [Google Scholar]
- 36.Misra T, Waters B, Jensen L, Fedorak RN. The effect of education on health care utilization in patients with inflammatory bowel disease. Gastroenterol. 2005;128:A17–A18. [Google Scholar]
- 37.Smith GD, Watson R, Roger D, McRorie E, Hurst N, Luman W, Palmer KR. Impact of a nurse-led counselling service on quality of life in patients with inflammatory bowel disease. J Adv Nurs. 2002;38:152–160. doi: 10.1046/j.1365-2648.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- 38.Stewart M, MacIntosh D, Phalen-Kelly K, Stewart J. Disease specific teaching by a nurse educator: A randomized trial. Can J Gastroenterol. 2009:23. [Google Scholar]
- 39.Verma S, Tsai HH, Giaffer MH. Does better disease-related education improve quality of life? A survey of IBD patients. Dig Dis Sci. 2001;46:865–869. doi: 10.1023/a:1010725106411. [DOI] [PubMed] [Google Scholar]
- 40.Waters BM, Jensen L, Fedorak RN. Effects of formal education for patients with inflammatory bowel disease: a randomized controlled trial. Can J Gastroenterol. 2005;19:235–244. doi: 10.1155/2005/250504. [DOI] [PubMed] [Google Scholar]
- 41.Elsenbruch S, Langhorst J, Popkirowa K, Müller T, Luedtke R, Franken U, Paul A, Spahn G, Michalsen A, Janssen OE, et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom. 2005;74:277–287. doi: 10.1159/000086318. [DOI] [PubMed] [Google Scholar]
- 42.García-Vega E, Fernandez-Rodriguez C. A stress management programme for Crohn’s disease. Behav Res Ther. 2004;42:367–383. doi: 10.1016/S0005-7967(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 43.Keefe B. Getting on our nerves: a look at group therapy interventions in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:64–65. doi: 10.1097/00054725-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Keefer L, Kiebles JL, Kwiatek MA, Palsson O, Taft TH, Martinovich Z, Barrett TA. The potential role of a self-management intervention for ulcerative colitis: a brief report from the ulcerative colitis hypnotherapy trial. Biol Res Nurs. 2012;14:71–77. doi: 10.1177/1099800410397629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keefer L, Kiebles JL, Martinovich Z, Cohen E, Van Denburg A, Barrett TA. Behavioral interventions may prolong remission in patients with inflammatory bowel disease. Behav Res Ther. 2011;49:145–150. doi: 10.1016/j.brat.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keefer L, Kiebles JL, Taft TH. The role of self-efficacy in inflammatory bowel disease management: preliminary validation of a disease-specific measure. Inflamm Bowel Dis. 2011;17:614–620. doi: 10.1002/ibd.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langhorst J, Mueller T, Luedtke R, Franken U, Paul A, Michalsen A, Schedlowski M, Dobos GJ, Elsenbruch S. Effects of a comprehensive lifestyle modification program on quality-of-life in patients with ulcerative colitis: a twelve-month follow-up. Scand J Gastroenterol. 2007;42:734–745. doi: 10.1080/00365520601101682. [DOI] [PubMed] [Google Scholar]
- 48.Mikocka-Walus AA, Turnbull D, Holtmann G, Andrews JM. An integrated model of care for inflammatory bowel disease sufferers in Australia: development and the effects of its implementation. Inflamm Bowel Dis. 2012;18:1573–1581. doi: 10.1002/ibd.22850. [DOI] [PubMed] [Google Scholar]
- 49.Mikocka-Walus AA, Turnbull DA, Holtmann G, Andrews JM. Coping with the unmet needs of gastroenterology and hepatology outpatients: A systematic approach towards an integrated model of care in South Australia. Gastroenterol. 2011;140:S208. [Google Scholar]
- 50.Oxelmark L, Magnusson A, Löfberg R, Hillerås P. Group-based intervention program in inflammatory bowel disease patients: effects on quality of life. Inflamm Bowel Dis. 2007;13:182–190. doi: 10.1002/ibd.20061. [DOI] [PubMed] [Google Scholar]
- 51.Casellas-Jordá F, Borruel-Sainz N, Torrejón-Herrera A, Castells I. Effect upon hospital activity of the application of a continued care model centered on patients with inflammatory bowel disease. Rev Esp Enferm Dig. 2012;104:16–20. doi: 10.4321/s1130-01082012000100004. [DOI] [PubMed] [Google Scholar]
- 52.Forry M, Godwin N, Kelly O, Harewood G, Murray F, Patchett S. From motivated and compliant to empowered: Do we need an IBD nurse led self-management programme? J Crohns Colitis. 2012;6:S198. [Google Scholar]
- 53.Greveson K. Auditing telephone advice for IBD patients in a district general hospital. Gastrointest Nurs. 2006;4:32–34. [Google Scholar]
- 54.Miller L, Caton S, Lynch D. Telephone clinic improves quality of follow-up care for chronic bowel disease. Nurs Times. 2002;98:36–38. [PubMed] [Google Scholar]
- 55.Nightingale AJ, Middleton W, Middleton SJ, Hunter JO. Evaluation of the effectiveness of a specialist nurse in the management of inflammatory bowel disease (IBD) Eur J Gastroenterol Hepatol. 2000;12:967–973. doi: 10.1097/00042737-200012090-00001. [DOI] [PubMed] [Google Scholar]
- 56.Rejler M, Spångéus A, Tholstrup J, Andersson-Gäre B. Improved population-based care: Implementing patient-and demand-directed care for inflammatory bowel disease and evaluating the redesign with a population-based registry. Qual Manag Health Care. 2007;16:38–50. doi: 10.1097/00019514-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Sack C, Phan VA, Grafton R, Holtmann G, van Langenberg DR, Brett K, Clark M, Andrews JM. A chronic care model significantly decreases costs and healthcare utilisation in patients with inflammatory bowel disease. J Crohns Colitis. 2012;6:302–310. doi: 10.1016/j.crohns.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Stansfield C, Robinson A. Implementation of an IBD nurse-led self-management programme. Gastrointest Nurs. 2008;6:12–18. [Google Scholar]
- 59.Elkjaer M. E-health: Web-guided therapy and disease self-management in ulcerative colitis. Impact on disease outcome, quality of life and compliance. Dan Med J. 2012;59:B4478. [PubMed] [Google Scholar]
- 60.Noeker M. Towards a general theory of patient education: Common treatment modules among specific chronic conditions. Pravention und Rehabilitation. 2008;20:2–11. [Google Scholar]
- 61.Kennedy A, Gask L, Rogers A. Training professionals to engage with and promote self-management. Health Educ Res. 2005;20:567–578. doi: 10.1093/her/cyh018. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy AP, Rogers AE. Improving patient involvement in chronic disease management: the views of patients, GPs and specialists on a guidebook for ulcerative colitis. Patient Educ Couns. 2002;47:257–263. doi: 10.1016/s0738-3991(01)00228-2. [DOI] [PubMed] [Google Scholar]
- 63.Siegel CA. Shared decision making in inflammatory bowel disease: helping patients understand the tradeoffs between treatment options. Gut. 2012;61:459–465. doi: 10.1136/gutjnl-2011-300988. [DOI] [PubMed] [Google Scholar]
- 64.Gethins S, Duckett T, Shatford C, Robinson R. Self-management programme for patients with long-term inflammatory bowel disease. Gastrointest Nurs. 2011;9:33–37. [Google Scholar]
- 65.Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, Rogers A, Sculpher M, Thompson D. A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self-help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technol Assess. 2003;7:iii, 1–113. doi: 10.3310/hta7280. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy A, Robinson A, Hann M, Thompson D, Wilkin D. A cluster-randomised controlled trial of a patient-centred guidebook for patients with ulcerative colitis: effect on knowledge, anxiety and quality of life. Health Soc Care Community. 2003;11:64–72. doi: 10.1046/j.1365-2524.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 67.Gethins S, Duckett T, Shatford C, Robinson RRJ. Guided self-management for patients with ulcerative colitis and Crohn’s disease. Gut. 2009;58:A153. [Google Scholar]
- 68.Hunter J, Claridge A, James S, Chan D, Stacey B, Stroud M, Patel P, Fine D, Cummings JR. Improving outpatient services: the Southampton IBD virtual clinic. Postgrad Med J. 2012;88:487–491. doi: 10.1136/postgradmedj-2012-100123rep. [DOI] [PubMed] [Google Scholar]
- 69.Keefer L, Doerfler B, Artz C. Optimizing management of Crohn’s disease within a project management framework: results of a pilot study. Inflamm Bowel Dis. 2012;18:254–260. doi: 10.1002/ibd.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krier M, Kaltenbach T, McQuaid K, Soetikno R. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol. 2011;106:2063–2067. doi: 10.1038/ajg.2011.329. [DOI] [PubMed] [Google Scholar]
- 71.Krier MJ, Kaltenbach TR, McQuaid KR, Soetikno R. Broadening the access to specialized IBD care using a consumer grade affordable telemedicine system. Gastroenterology. 2010;138:S473. [Google Scholar]
- 72.Pearson C. Demonstrating the impact of an inflammatory bowel disease nurse specialist. CME J Gastroenterol Hepat and Nutr. 2005;7:15–19. [Google Scholar]
- 73.Pedersen N, Ekjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S, et al. Ehealth: Optimization of infliximab treatment and disease course via self-initiated web-based solution in Crohn’s disease. J Crohns Colitis. 2011;5:S84. doi: 10.1111/apt.12043. [DOI] [PubMed] [Google Scholar]
- 74.Pedersen N, Elkjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S, et al. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:840–849. doi: 10.1111/apt.12043. [DOI] [PubMed] [Google Scholar]
- 75.Plener I, Morgan M, Garbens A, Seth R, Saibil F. Effectiveness of e-mail management in patients with IBD; A component of self-management. Inflamm Bowel Dis. 2011;17:S57. [Google Scholar]
- 76.Shafran I, Burgunder P, Shafran A, Chew E. Barriers to the use of a mobile and web-based application for tracking inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:S63. [Google Scholar]
- 77.Shafran I, Burgunder P, Shamosh B. Mobile and web-based application for IBD tracking. Inflamm Bowel Dis. 2009;15:S40. [Google Scholar]
- 78.Strid H, Bjork J, Grip O. IBD care- the new Swedish web-based clinical decision support system provides opportunity for benchmarking and longitudinal clinical research. Scand J Gastroenterol. 2012;47:S69. [Google Scholar]
- 79.Castro HK, Cross RK, Finkelstein J. Using a Home Automated Telemanagement (HAT) system: experiences and perceptions of patients with inflammatory bowel diseaseh. AMIA Annual Symposium Proceedings/AMIA Symposium. 2006 [PMC free article] [PubMed] [Google Scholar]
- 80.Cross RK, Finkelstein J. Challenges in the design of a Home Telemanagement trial for patients with ulcerative colitis. Clin Trials. 2009;6:649–657. doi: 10.1177/1740774509346978. [DOI] [PubMed] [Google Scholar]
- 81.Elkjaer M, Burisch J, Avnstrøm S, Lynge E, Munkholm P. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22:695–704. doi: 10.1097/MEG.0b013e32832e0a18. [DOI] [PubMed] [Google Scholar]
- 82.Kurbegow AC, Ferry GD. Guided self-management and patient-directed follow-up of ulcerative colitis: a randomised trial. J Pediatr Gastroenterol Nutr. 2002;34:428–429. doi: 10.1097/00005176-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 83.Hommel KA, Hente E, Herzer M, Ingerski LM, Denson LA. Telehealth behavioral treatment for medication nonadherence: a pilot and feasibility study. Eur J Gastroenterol Hepatol. 2013;25:469–473. doi: 10.1097/MEG.0b013e32835c2a1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moshkovska T, Stone MA, Smith RM, Bankart J, Baker R, Mayberry JF. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17:1874–1881. doi: 10.1002/ibd.21570. [DOI] [PubMed] [Google Scholar]
- 85.Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. A Randomized, Controlled Trial of Home Telemanagement in Patients With Ulcerative Colitis (UC HAT) Gastroentero. 2011;140:S264–S265. doi: 10.1002/ibd.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duricova D, Pedersen N, Burisch J, Elkjaer M, Dobrzanski C, Anderssen NN, Bendtsen F, Nordgaard-Lassen I, Leotta S, Langholz E, et al. Ehealth: Impact of web-based treatment optimization solution (traffic light) on the quality of life in Crohn’s disease patients treated with infliximab. Gastroenterol. 2011;140:S203–S204. [Google Scholar]
- 87.Stunkel L, Karia K, Okoji O, Warren R, Jean T, Jacob V, Swaminath A, Scherl E, Bosworth B. Impact on quality of life of a smart device mobile application in patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:S635–S636. [Google Scholar]
- 88.Williams JG, Cheung WY, Russell IT, Cohen DR, Longo M, Lervy B. Open access follow up for inflammatory bowel disease: pragmatic randomised trial and cost effectiveness study. BMJ. 2000;320:544–548. doi: 10.1136/bmj.320.7234.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolge SC, Waters H, Piech CT. Self-reported frequency and severity of disease flares, disease perception, and flare treatments in patients with ulcerative colitis: results of a national internet-based survey. Clin Ther. 2010;32:238–245. doi: 10.1016/j.clinthera.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Cooper JM, Collier J, James V, Hawkey CJ. Beliefs about personal control and self-management in 30-40 year olds living with Inflammatory Bowel Disease: a qualitative study. Int J Nurs Stud. 2010;47:1500–1509. doi: 10.1016/j.ijnurstu.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Gethins S, Robinson R, de Caestecker J, Stewart J. Impact of a nurse-led telephone clinic on quality of IBD care. Gastrointest Nurs. 2007;5:34–39. [Google Scholar]
- 92.Tully MA, Parker-Hartigan L. E-mails from college. Managing inflammatory bowel disease in college. Gastroent Nurs. 2008;31:147–148. doi: 10.1097/01.SGA.0000316535.61861.26. [DOI] [PubMed] [Google Scholar]
- 93.Elkjaer M, Burisch J, Voxen Hansen V, Deibjerg Kristensen B, Slott Jensen JK, Munkholm P. A new rapid home test for faecal calprotectin in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:323–330. doi: 10.1111/j.1365-2036.2009.04164.x. [DOI] [PubMed] [Google Scholar]


