Abstract
We herein report a case of anaplastic carcinoma of the pancreas with remarkable intraductal tumor growth into the main pancreatic duct. A 76-year-old male was referred to our hospital for treatment of a pancreatic tumor. Preoperative examinations revealed a poorly defined tumor in the main pancreatic duct in the body of the pancreas, accompanied with severe dilatation of the main pancreatic duct, which was diagnosed as an intraductal papillary-mucinous neoplasm. We performed distal pancreatectomy and splenectomy. The pathological examination revealed that the tumor consisted of a mixture of anaplastic carcinoma (giant cell type) and adenocarcinoma in the pancreas. There was a papillary projecting tumor composed of anaplastic carcinoma in the dilated main pancreatic duct. The patient is now receiving chemotherapy because liver metastasis was detected 12 mo after surgery. In this case, we could observe a remarkable intraductal tumor growth into the main pancreatic duct. We also discuss the pathogenesis and characteristics of this rare tumor with specific tumor growth.
Keywords: Anaplastic carcinoma, Giant cell carcinoma, Intraductal tumor growth, Papillary projecting tumor
Core tip: A very rare case of anaplastic carcinoma with remarkable intraductal tumor growth into the main pancreatic duct is reported. This case presented unique features and might help us to better understand the pathogenesis of this rare entity.
INTRODUCTION
Anaplastic carcinoma of the pancreas is an aggressive tumor. The incidence of anaplastic carcinoma varies from 2.1% to 6.8% among reported case series[1-5]. It was first reported by Sommers and Meissner as pleomorphic carcinoma[6]. Pathologically, it is classified into spindle cell, giant cell and pleomorphic types. When detected, patients usually have huge tumors showing rapid growth, which are associated with a very poor prognosis. It usually presents as a large cystic pancreatic tumor with areas of hemorrhage and necrosis. En-bloc surgical resection is the only appropriate treatment for the tumors[5,7]. We herein report the clinicopathological features of a case of anaplastic carcinoma of the pancreas with remarkable intraductal tumor growth into the main pancreatic duct.
CASE REPORT
A 76-year-old male was admitted to our hospital for further investigation of a pancreatic tumor. He had received treatment for type 2 diabetes mellitus, essential hypertension and severe arteriosclerosis obliterans. He received ultrasonography as a screening examination, which showed a cystic tumor in the body and tail of the pancreas. He had no significant symptoms. The laboratory findings revealed that the serum carbohydrate antigen (CA19-9) was elevated to 57 U/mL, but the other tumor markers, carcinoembryonic antigen and DUPAN-II, were all within the normal ranges. Abdominal CT revealed a tumor measuring 6.0 cm × 3.5 cm in the body and tail of the pancreas. It consisted of a multilocular cystic component and a solid tumor in the lumen of the dilated main pancreatic duct (MPD) (Figure 1). No distant metastasis or lymph node swelling were detected. Endoscopic ultrasonography showed a heterogeneous hypoechoic tumor with a cystic component. On abdominal magnetic resonance imaging, the solid tumor presented with low intensity on T1-weighted images and relatively high intensity on T2-weighted images. MR cholangiopancreatography showed a segmental defect of the MPD by the solid tumor associated with the multilocular cystic lesion in the body and tail of the pancreas (Figure 2). Endoscopic retrograde cholangiopancreatography showed significant dilatation of the MPD from the body to the tail of the pancreas, and an elliptic filling defect in the MPD, suggesting the presence of an intraductal tumor in the lumen of the MPD (Figure 3A). Intraductal ultrasonography demonstrated a solid tumor filling the main pancreatic duct and a cystic lesion in the body of the pancreas (Figure 3B). The diagnosis of the exfoliative cytology was adenocarcinoma. We therefore diagnosed the tumor as an intraductal papillary mucinous neoplasm (IPMN) of the pancreas with obvious dilatation of the MPD. At laparotomy, the tumor had invaded into the transverse mesocolon, but dissemination and distant metastasis were not found. We performed distal pancreatectomy with splenectomy. We decided to make the transecting line of the pancreas by conforming it to the tumor extension using intra-operative ultrasonography. When we transected the pancreas, a reddish tumor protruded from the lumen of the MPD in the resected pancreas (Figure 4A). The main tumor, which replaced the body and tail of the pancreas, was dark reddish-brown and white on the surface, and had infiltrated into the adjacent MPD (Figure 4B). There was a papillary tumor associated with the main tumor in the lumen of the MPD. The MPD was significantly dilated by this protruded tumor. Hematoxylin and eosin staining showed that the part of the tumor presenting a white surface consisted of an adenocarcinoma (tub1, tub2) component, and the part of the tumor presenting the dark reddish-brown surface consisted of anaplastic carcinoma (giant cell type). The projecting tumor in the MPD was composed of anaplastic carcinoma (Figure 5). There was moderate dysplasia with no malignancy (pancreatic intraepithelial neoplasm-2) in the epithelium of dilated main pancreatic duct. The pathological diagnosis was a mixture of anaplastic carcinoma (giant cell type) and adenocarcinoma of the pancreas. The postoperative course was uneventful. The patient is now receiving chemotherapy because liver metastasis was detected 12 mo after surgery.
Figure 1.
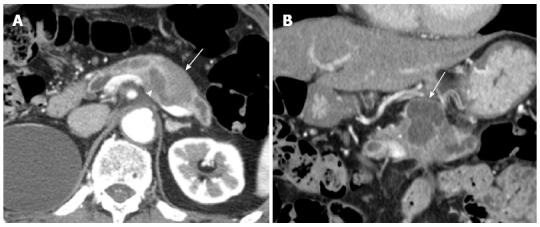
Abdominal computed tomography. A: An axial section image of an abdominal computed tomography showed dilatation of the main pancreatic duct (arrow head) and the adjacent solid tumor (arrow); B: The coronal section image revealed a cystic lesion measuring 6.0 cm × 3.5 cm in the body and tail of the pancreas (arrow).
Figure 2.
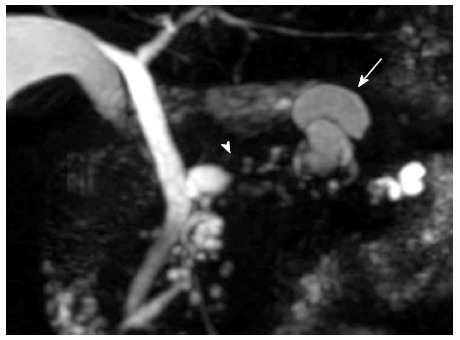
Magnetic resonance cholangiopancreatography revealed a segmental defect of the main pancreatic duct by the solid tumor (arrow head) associated with the multilocular cystic lesion (arrow) in the body and tail of the pancreas.
Figure 3.
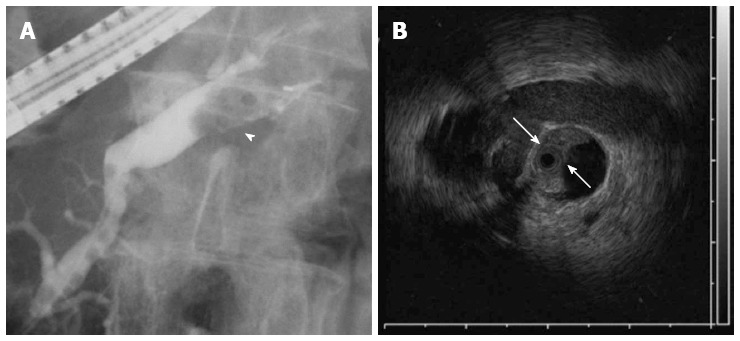
Endoscopic retrograde cholangiopancreatography showed the dilatation of the main pancreatic duct. A: A filling defect in the main pancreatic duct (MPD) (arrow head); B: Intraductal ultrasonography demonstrated a filling lesion in the MPD (arrow).
Figure 4.
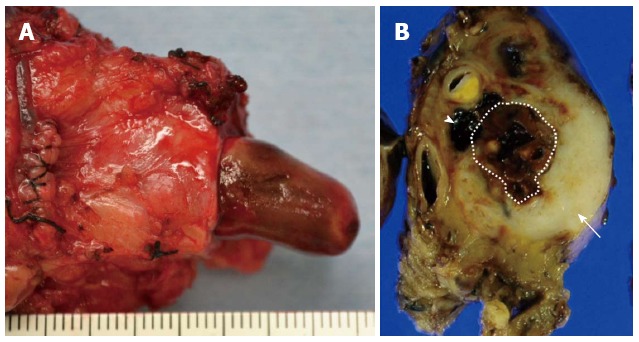
Pathological findings. A: The dilated main pancreatic duct was filled with a papillary projecting tumor; B: The main tumor was dark reddish-brown (arrow head) and white (arrow) on the surface, and showed significant dilatation of the main pancreatic duct (dotted line region).
Figure 5.
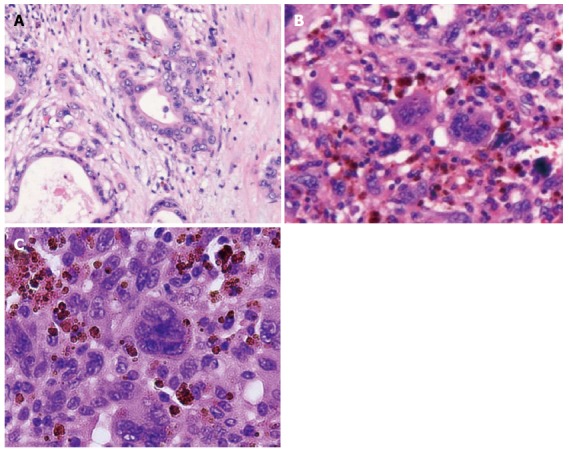
Microscopic appearance of the tumor. Hematoxylin and eosin staining showed adenocarcinoma (tub1, tub2) in the white component, bizarre mono-and multi-nucleated giant cells in the dark reddish-brown component and a projecting tumor into the main pancreatic duct. A: the white component; B: the dark red-brown component; C: the projecting tumor.
DISCUSSION
Anaplastic carcinoma of the pancreas is a rare and aggressive tumor. Several terms have been used for this tumor, including pleomorphic carcinoma, pleomorphic giant cell carcinoma, pleomorphic large cell carcinoma, sarcomatoid carcinoma and undifferentiated carcinoma[2,3,8]. Three histological variants of anaplastic carcinoma, namely the spindle cell type, pleomorphic cell type and giant cell type, have been described[9]. According to the guideline of the World Health Organization (WHO) in 2010[10], these carcinomas were classified as undifferentiated (anaplastic) carcinomas. On the other hand, anaplastic carcinoma with osteoclast-like giant cells is classified as a subtype of invasive ductal carcinoma.
The clinical features of anaplastic carcinoma have been reported in the literature. The clinical symptoms induced by this tumor are non-specific, such as abdominal pain, fatigue, jaundice, body weight loss, anorexia and back pain[3], which resemble those induced by adenocarcinoma of the pancreas. In the imaging study, anaplastic carcinomas are usually detected as large, moderately hypervascular and exophytic tumors with large areas of necrosis[2].
The preoperative diagnosis of anaplastic carcinoma of the pancreas is difficult. The preoperative diagnosis for the tumor in our case was IPMN, because it appeared to be a multilocular cystic lesion with obvious dilatation of the MPD. The tumor of our case showed a very characteristic appearance because it consisted of a mixture of components of adenocarcinoma and anaplastic carcinoma. The components of adenocarcinoma presented as a solid tumor. A part of the anaplastic carcinoma component developed cystic degeneration, and the other part penetrated and proliferated into the MPD.
Anaplastic carcinoma with intraductal growth into the MPD is so rare that only 9 cases, including our case, have been reported in the English literature[11-18] (Table 1). Only our case was the giant cell type of the anaplastic carcinoma. All cases except for the cases with the osteoclastic giant cell type were associated with a poor prognosis.
Table 1.
Reported cases of anaplastic carcinoma with intraductal growth in the main pancreatic duct
| Case | Author | Year | Age (yr) | Sex | Location | Size (mm) | Subtype | Treatment | Outcome (mo) |
| 1 | Higuchi et al[11] | 2004 | 65 | F | Pbt | 110 | spindle | DP | dead (4) |
| 2 | Tezuka et al[12] | 2006 | 68 | F | Ph | 42 | osteoclastic | PD | alive (22) |
| 3 | Suzuki et al[13] | 2007 | 71 | M | Ph | 35 | plemorphic | PPPD | dead (1) |
| 4 | Kuroda et al[14] | 2007 | 59 | M | Ph | 100 | plemorphic | PD | dead (2) |
| 5 | Nara et al[15] | 2009 | 79 | F | Phb | 116 | osteoclastic | PPPD | alive (14) |
| 6 | Maksymov et al[16] | 2011 | 68 | F | Ph | 30 | osteoclastic | PD | alive (36) |
| 7 | Ishii et al[17] | 2012 | 61 | M | Ph | 32 | osteoclastic | PD | alive (14) |
| 8 | Yamano et al[18] | 2013 | 63 | F | Pb | 850 | plemorphic | DP | dead (4) |
| 9 | Our case | 2013 | 76 | M | Pbt | 600 | giant cell | DP | alive (12) |
M: Male; F: Female; Ph: Pancreatic head; Pb: Pancreatic body; Pt: Pancreatic tail; Phb: Pancreatic head and body; Pbt: Pancreatic body and tail; DP: Distal pancreatectomy; PD: Pancreatoduodenectomy; PPPD: Pylorus-preserving pancreatoduodenectomy.
The prognosis of anaplastic carcinoma of the pancreas is worse than that of poorly differentiated ductal adenocarcinoma of the pancreas[3,8,19]. Reyes et al[20] reported that the median survival time of pleomorphic giant cell carcinoma patients was three months. Strobel et al[4]suggested that the median duration of survival was significantly prolonged after R0/R1 resection, as compared with palliative surgery (7.1 mo vs 2.3 mo). We recommend that patients with anaplastic carcinomas of the pancreas should be offered pancreatic resection whenever possible. Due to its aggressive nature and ability to rapidly recur, the benefits of radiotherapy and chemotherapy have not yet been demonstrated[21,22]. Sporadic case reports have demonstrated a reduction of the tumor mass and prolongation of survival by treatments with 5-fluorouracil, gemcitabine, paclitaxel and radiation[23-25]. However, there is insufficient evidence to recommend any particular treatment, except for surgical resection.
In conclusion, we herein reported a very rare case of anaplastic carcinoma with remarkable intraductal tumor growth into the MPD. This case presented unique features and might help us to better understand the pathogenesis of this rare entity.
COMMENTS
Case characteristics
A 76-year-old male was admitted to our hospital for further investigation of a pancreatic tumor, but he had no significant symptoms.
Clinical diagnosis
This case was diagnosed as an intraductal papillary mucinous neoplasm of the pancreas.
Differential diagnosis
Adenocarcinoma of pancreatic was mentioned in the differential diagnosis.
Laboratory diagnosis
The laboratory findings revealed that the serum carbohydrate antigen was elevated to 57 U/mL, but the other tumor markers, carcinoembryonic antigen and DUPAN-II, were all within the normal ranges.
Imaging diagnosis
Computed tomography, endoscopic ultrasonography, magnetic resonance imaging, endoscopic retrograde cholangiopancreatography and intraductal ultrasonography showed significant dilatation of the main pancreatic duct (MPD) from the body to the tail of the pancreas, and an elliptic filling defect in the MPD.
Pathological diagnosis
Hematoxylin and eosin staining showed that the part of the tumor presenting a white surface consisted of an adenocarcinoma (tub1, tub2) component, and the part of the tumor presenting the dark reddish-brown surface consisted of anaplastic carcinoma (giant cell type).
Treatment
Distal pancreatectomy with splenectomy was performed in this case.
Related reports
Only 9 cases of anaplastic carcinoma with intraductal growth into the MPD, including our case, have been reported in the English literature.
Term explanation
Anaplastic carcinoma with intraductal growth into the MPD is rare.
Experiences and lessons
Anaplastic carcinomas are highly malignant tumors, and the authors will follow up the patient closely and continuously.
Peer review
This case presented unique features and might help us to better understand the pathogenesis of this rare entity.
Footnotes
P- Reviewer: Vetvicka V S- Editor: Qi Y L- Editor: Ma JY E- Editor: Liu XM
References
- 1.Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol. 1985;146:17–29. doi: 10.1002/path.1711460103. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa T, Federle MP, Ohba S, Ohtomo K, Sugiyama A, Fujimoto H, Haradome H, Araki T. Atypical exocrine and endocrine pancreatic tumors (anaplastic, small cell, and giant cell types): CT and pathologic features in 14 patients. Abdom Imaging. 2000;25:409–419. doi: 10.1007/s002610000058. [DOI] [PubMed] [Google Scholar]
- 3.Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 4.Strobel O, Hartwig W, Bergmann F, Hinz U, Hackert T, Grenacher L, Schneider L, Fritz S, Gaida MM, Büchler MW, et al. Anaplastic pancreatic cancer: Presentation, surgical management, and outcome. Surgery. 2011;149:200–208. doi: 10.1016/j.surg.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Clark CJ, Graham RP, Arun JS, Harmsen WS, Reid-Lombardo KM. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg. 2012;215:627–634. doi: 10.1016/j.jamcollsurg.2012.06.418. [DOI] [PubMed] [Google Scholar]
- 6.Sommers SC, MEISSNER WA. Unusual carcinomas of the pancreas. AMA Arch Pathol. 1954;58:101–111. [PubMed] [Google Scholar]
- 7.Moore JC, Bentz JS, Hilden K, Adler DG. Osteoclastic and pleomorphic giant cell tumors of the pancreas: A review of clinical, endoscopic, and pathologic features. World J Gastrointest Endosc. 2010;2:15–19. doi: 10.4253/wjge.v2.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tschang TP, Garza-Garza R, Kissane JM. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer. 1977;39:2114–2126. doi: 10.1002/1097-0142(197705)39:5<2114::aid-cncr2820390528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Pan ZG, Wang B. Anaplastic carcinoma of the pancreas associated with a mucinous cystic adenocarcinoma. A case report and review of the literature. JOP. 2007;8:775–782. [PubMed] [Google Scholar]
- 10.Bosman FT, Carneiro F, Hruban RH, Theise N. WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010. [Google Scholar]
- 11.Higuchi R, Hatori H, Fukuda A, Imaizumi T, Takasaki K, Itabashi M. A case of spindle cell type anaplastic carcinoma of the pancreas. Suizo. 2004;19:516–521. [Google Scholar]
- 12.Tezuka K, Yamakawa M, Jingu A, Ikeda Y, Kimura W. An unusual case of undifferentiated carcinoma in situ with osteoclast-like giant cells of the pancreas. Pancreas. 2006;33:304–310. doi: 10.1097/01.mpa.0000235303.11734.2a. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Harada N, Suzuki M, Hanyu F. A case of pleomorphic carcinoma of the pancreas. Suizo. 2007;22:137–142. [Google Scholar]
- 14.Kuroda N, Iwamura S, Fujishima N, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, Lee GH. Anaplastic carcinoma of the pancreas with rhabdoid features and hyaline globule-like structures. Med Mol Morphol. 2007;40:168–171. doi: 10.1007/s00795-006-0349-0. [DOI] [PubMed] [Google Scholar]
- 15.Nara S. A case of anaplastic carcinoma of the pancreas with portal vein tumor thrombus. Jpn J Clin Oncol. 2010;40:96–97. doi: 10.1093/jjco/hyp189. [DOI] [PubMed] [Google Scholar]
- 16.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 17.Ishii S, Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Koshita S, Kanno Y, Ogawa T, Masu K, et al. Undifferentiated carcinoma of the pancreas involving intraductal pedunculated polypoid growth. Intern Med. 2012;51:3373–3377. doi: 10.2169/internalmedicine.51.8779. [DOI] [PubMed] [Google Scholar]
- 18.Yamano T, Hirai R, Kuroda M, Takagi S, Ikeda E, Tsuji H. A case of anaplastic ductal carcinoma with tumor thrombus in the main pancreatic duct. J Japan Surg Assoc. 2013;74:1053–1059. [Google Scholar]
- 19.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Reyes CV, Crain S, Wang T. Pleomorphic giant cell carcinoma of the pancreas: a review of nine cases. J Surg Oncol. 1980;15:345–348. doi: 10.1002/jso.2930150407. [DOI] [PubMed] [Google Scholar]
- 21.Singhal A, Shrago SS, Li SF, Huang Y, Kohli V. Giant cell tumor of the pancreas: a pathological diagnosis with poor prognosis. Hepatobiliary Pancreat Dis Int. 2010;9:433–437. [PubMed] [Google Scholar]
- 22.Leighton CC, Shum DT. Osteoclastic giant cell tumor of the pancreas: case report and literature review. Am J Clin Oncol. 2001;24:77–80. doi: 10.1097/00000421-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka M, Uchinami H, Watanabe G, Takahashi T, Nakagawa Y, Andoh H, Yoshioka T, Nanjo H, Yamamoto Y. Effective use of gemcitabine in the treatment of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thrombus. Intern Med. 2012;51:2145–2150. doi: 10.2169/internalmedicine.51.7670. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzawa G, Shirabe K, Gion T, Tsujita E, Ooya M, Kajiyama K, Nagaie T. Surgically resected undifferentiated carcinoma with osteoclast-like giant cells of the periampullary region involving the orifice of the papilla of Vater: Report of a case. Surg Today. 2010;40:376–379. doi: 10.1007/s00595-009-4078-6. [DOI] [PubMed] [Google Scholar]
- 25.Wakatsuki T, Irisawa A, Imamura H, Terashima M, Shibukawa G, Takagi T, Takahashi Y, Sato A, Sato M, Ikeda T, et al. Complete response of anaplastic pancreatic carcinoma to paclitaxel treatment selected by chemosensitivity testing. Int J Clin Oncol. 2010;15:310–313. doi: 10.1007/s10147-010-0038-9. [DOI] [PubMed] [Google Scholar]


