Abstract
Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors that arise from the gastrointestinal tract. In rare cases, these tumors are found in intra-abdominal sites unrelated to the gastrointestinal tract, such as the mesentery, omentum and retroperitoneum. However, pancreatic extra-gastrointestinal stromal tumors are extremely rare, with only 14 previous cases reported. A 61-year-old man with no clinical symptoms had a routine check-up, during which an abdominal mass located in the pancreas tail was detected. Abdominal surgery was performed with resection of the pancreas tail and the spleen, and he was diagnosed with low-risk GISTs. Another 60-year-old man with no clinical symptoms underwent Computed tomography which revealed a well-demarcated tumor, 6 cm in diameter, in the head of the pancreas. He was diagnosed with pancreatic GISTs. Here, we describe two rare cases of pancreatic GISTs and review the cases previously reported in the literature.
Keywords: Gastrointestinal Stromal tumors, Extra-gastrointestinal Stromal Tumors, Pancreatic gastrointestinal stromal tumors
Core tip: Gastrointestinal stromal tumors (GISTs) tend to arise with a higher frequency in the stomach and the small bowel. In fewer than 5% of cases, they originate primarily from EGISTs. Among them, pancreatic GIST is very rare, with only 14 previous cases reported. Here, we report two cases of malignant pancreatic GIST and review the cases previously reported in the literature.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms of gastrointestinal tract, which may occur along the entire length of gastrointestinal tract from the mouth cavity to the anus, and sometimes in the omentum, mesentery, and retroperitoneum[1-4]. They are characterized by high expression of CD-117, a protein encode by c-kit gene. C-kit gene is expressed in 95% of cases; and 60%-70% of tumors are CD34-positive. Pancreatic GISTs are extremely rare, and only 11 cases have been reported in English literature[5-14], two in France literature[15], and only one in Chinese literature[16]. We report two cases of malignant pancreatic GIST and review the cases previously reported in the literature.
CASE REPORT
Case 1
A 61-year-old man with no clinical symptoms had a routine physical examination, during which an abdominal mass was detected by ultrasound. Abdominal computed tomography (CT) revealed a 3 cm × 5 cm mass which is located in the pancreas tail next to the splenic artery (Figure 1A). We did not see any metastasis in the abdominal and pelvic cavity. The tumor was located in pancreatic body-tail,measured 6 cm × 8 cm,and infiltrated the pancreatic tissues. As a result of the unclear nature of the tumor and its close relationship to the splenic artery, a distal pancreatectomy with splenectomy was performed. Inoperative cytology fine needle aspirations found no tumor cells. Microscopically, the tumor was composed of spindle cells (Figure 1B). The mitotic count was minor 5 mitoses/50 high-power fields. Immunohistochemical staining showed immunoreactivity for CD117 (c-Kit) (Figure 1C), CD34 (Figure 1D), and s-100 protein, whereas cells were completely negative for DOG-1, Desmin, NF, synaptophysin, chromogranin A and cytokeratin. The patient was diagnosed with GISTs (low-risk tumor). The patient had no evidence of recurrence or metastasis during the follow-up > 36 mo after operation.
Figure 1.
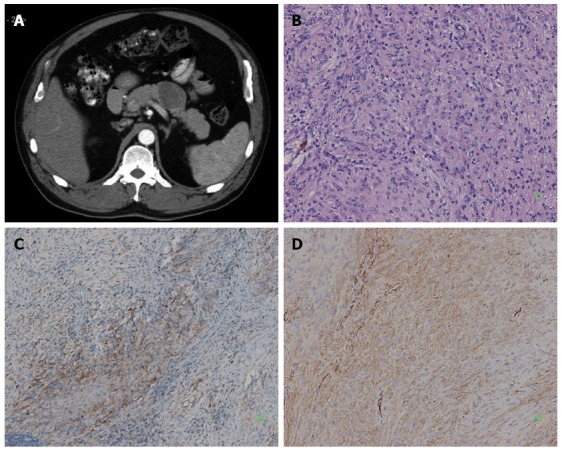
An abdominal mass was detected by ultrasound in a 61-year-old man with no clinical symptoms. A: Enhanced abdominal computed tomography scan showed a solid mass of the pancreatic body, and the tumor located at pancreas tail next to splenic artery; B: The tumor was composed of spindle cell (HE, × 200); C: Immunoreactivity of the tumor cells for CD117 was positive (+) (SP × 100); D: Immunoreactivity of the tumor cells for CD34 was positive (++) (SP × 200).
Case 2
A 60-year-old-man with no clinical symptoms underwent CT (Figure 2A), which revealed a well-demarcated tumor of 3 cm × 5 cm × 5 cm, in the head of the pancreas. A classic Whipple pancreaticoduodenectomy was considered. However, the tumor was found in the uncinate process of pancreas during the operation, sized 4 cm × 5 cm × 6 cm, with part of capsule, and no swollen lymph node was found around the tumor. Benign pancreatic islet cell tumor or duodenal leiomyoma was considered during the operation, and cytology fine needle aspiration did not find any tumor cells. As such, local resection of the tumor was performed. On intraoperative examination, the surgical margin was negative. The patient recovered without any complications. Microscopically, the tumor was composed of spindle cells (Figure 2B). The mitotic count was more 5 mitoses/50 high-power fields. Immunohistochemical staining showed immunoreactivity for CD117 (c-Kit) (Figure 2C), and S100 protein, whereas cells were completely negative for smooth muscle actin, synaptophysin, and cytokeratin. Immunohistochemical staining showed that DOG-1 (Figure 2D) was positive. The patient was diagnosed with GISTs (high-risk tumor). Unfortunately, the patient refused to take imatinib because of poor economic status. One year later, multiple nodules about 0.3-2.0 cm were found on the liver surface. The biggest liver lesion of approximately 2 cm was found in an abdominal CT scan. An excision biopsy showed spindle-shaped tumor cells with atypia, and pancreatic stromal tumor with liver metastasis was considered. The patient was started on imatinib (400 mg bid). The last follow-up on radiology three years after the detection of the metastasis, the patient was found with no disease progression.
Figure 2.
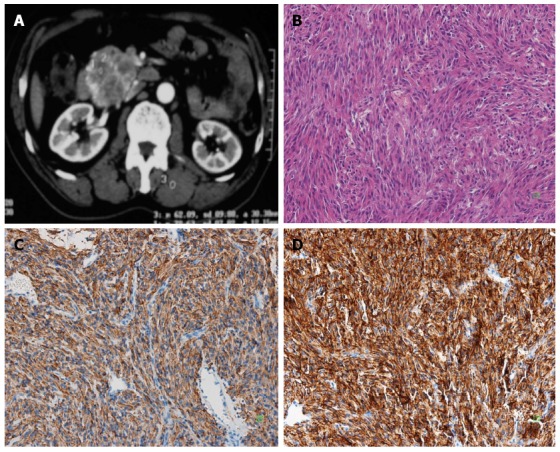
A 60-year-old man with no clinical symptoms underwent computed tomography. A: Enhanced abdominal computed tomography scan showed a solid mass in the pancreatic head; B: The tumor was composed of spindle cell (HE, × 200); C: Immunoreactivity of the tumor cells for CD117 was positive (+) (SP × 200); D: Immunoreactivity of the tumor cells for DOG-1 was positive (+++) (SP × 200).
DISCUSSION
GISTs tend to arise with a higher frequency in the stomach and the small bowel. In fewer than 5% of cases, they originate primarily from extra-gastrointestinal tumors (EGISTs). Among them, pancreatic GIST is very rare, with only 14 previous cases reported (Table 1).
Table 1.
Previous cases report of Pancreatic gastrointestinal stromal tumor
| Ref. | Presentation | Case No. | Location in pancreas | Pathological and immunohistochemical findings | Therapy | Follow-up |
| Boyer et al[15]2001 | Abdominal pain | 2 | Both of two cases were in the head | A 5.0 cm solid mass with central necrosis; positive for CD117 and CD34; negative for S100 | Biopsy of liver lesion and partial duodenopancreatectomy; | NA |
| Neto et al[6] 2004 | Epigastric pain, bloating, weight loss | 1 | Body | A 20.0 cm solid cystic mass with necrotic foci; mitotic count (120/50 HPF); positive for CD117 and CD34; negative for cytokeratins 7 and 20, desmin and synaptophysin | Distal pancreatectomy and splenectomy; treated with imatinib mesylate | Recurrence with peritoneal and retroperitoneal nodal disease 1 mo after surgery |
| Yamaura et al[7]2004 | Incidental finding | 1 | Tail | A 14.0 cm solid mass with cystic degeneration; few mitoses; positive for CD34 and vimentin; negative for SMA and S100 | Distal pancreatectomy splenectomy, and partial gastrectomy | No evidence of disease recurrence at 30 mo |
| Krska et al[8] 2005 | Abdominal pain | 1 | Body and head | A 17.0 cm mass; mitotic count: 1/50 HPF; positive for CD34 and vimentin; negative for S100, chromogranin, actin, and CD117 | Partial pancreatectomy | No evidence of disease recurrence at 30 mo |
| Pauser et al[11]2005 | Incidental finding/abdominal discomfort | 2 | Tail and body | A 3.0 cm solid mass with positive for CD117 and CD34, cell negative for SMA;A 2.0 cm solid mass with positive for CD117 and CD34, cell negative for SMA | Distal pancreatectomy splenectomy, and partial gastrectomy | No evidence of disease at 24 and 48 mo |
| Daum et al[9]2005 | Incidental finding | 1 | Head | A 10.0 cm mass with central hemorrhage mitotic count: 2/50 HPF; negative for CD117, vimentin, actin, S100, CD34, desmin, and cytokeratins | Whipple procedure; imatinib | No evidence of disease recurrence at 6 mo |
| Showalter et al[10] 2008 | Incidental finding on workup for back pain | 1 | Tail | A 7.0 cm solid mass; mitotic count: 3/50 HPF; positive for CD117; negative for S100 protein and SMA | Laparoscopic distal pancreatectomy and splenectomy | No evidence of disease recurrence at 27 mo |
| Yan et al[13]2008 | Nausea and vomiting | 1 | Uncinate process | A 2.4 cm pancreatic mass with spindle cells and mild atypia; positive for CD117; negative for desmin | NA | NA |
| Harindhanavudhi et al[14]2009 | Fatigue and weakness | 1 | Body | A 16.0 cm × 11.0 cm solid cystic mass; positive for CD117, CD34 | Exploratory laparotomy with cystojejunostomy and biopsy of cyst wall | NA |
| Trabelsi et al[12] 2009 | Abdominal pain | 1 | Head | A 10.5 cm × 8.0 cm × 3.0 cm mass with spindle and epithelioid cells Mitotic: 6/50 HPF. Positive for CD117 (c-Kit) and CD34, negative for SMA, S100, synaptophysin and cytokeratins | Hemipancreaticoduodenectomy with antrectomy and partial colectomy | No evidence of disease recurrence at 10 mo |
| Vij et al[5] 2011 | Weakness, postprandial | 1 | Head | A 6.5 cm × 6.0 cm solid mass with pleomorphic Spindle cells; mitosis: 12-15/50 HPF; positive for CD117; negative for CD34, SMA, desmin, S100 and cytokeratins | Whipple procedure; Imatinib on protocol | Developed liver metastasis 24 mo after surgery |
| Rubin et al[16]2011 | fullness and painabdominal pain | 1 | Body and tail | A 25.0 cm × 30.0 cm solid mass positive for CD117, CD34 and VIM; negative for S100, NF, DES, SMA, and CK | Distal pancreatectomy splenectomy | NA |
| Present case 1 | Incidental finding | 1 | Tail | A 6.0 cm × 8.0 cm solid mass with spindle cells mitotic: < 5/50 HPF; positive for CD117, CD34, and s-100 protein, negative for DOG-1, Desmin, NF, synaptophysin, Chromogranin A and cytokeratin | Distal pancreatectomy splenectomy | No evidence of disease recurrence at 36 mo |
| Present case 2 | Incidental finding | 1 | Head | A 4.0 cm × 5.0 cm × 6.0 cm solid mass with spindle cells mitosis: > 5/50 HPF. Positive for CD117, S100 protein and DOG-1, negative for SMA, synaptophysin, and cytokeratin | Pancreatic head tumor resection | Developed liver metastasis 12 mo after surgery progression-free survival for 36 mo after two times of TACE and oral imatinib |
The 14 patients with pancreatic GISTs previously reported aged from 35 to 70 years, averaging 54.2 years, and nine were female. CA19-9 and CEA were mostly normal, which is different from pancreas cancer.
The origin of GISTs currently remains controversial. Most scholars believe that it may originate in Cajal interstitial cells (the gastrointestinal tract pacemaker cells). The expression of tyrosine kinase transmembrane receptor protein c-kit (CD117) is positive in almost all cases of GIST, and this also applies to the pancreas; and 60%-70% of tumors are CD34-positive[8]. Among the 14 cases reported, only one case was CD117 negative in immunohistochemical staining[9], and CD34 was also negative in our cases.
The clinical presentation of pancreatic GISTs varies greatly depending on their size and the presence of mucosal ulceration. The clinical symptoms include abdominal pain, early satiety, flatulence, ileus, bleeding, anemia, and weight loss. Among the previous 14 cases, seven patients experienced epigastric pain, two weaknesses or fatigue, and one nausea and vomiting, whereas four patients had no obvious symptoms, and the tumors were found incidentally. Our two cases had no clinical symptoms, but both underwent CT scanning. Additionally, the patients with tumors located in pancreas head or uncinate process did not have jaundice[5,9,12,13,15], even the tumor size reached nearly 10 cm[9]. Therefore, we conclude that pancreatic GISTs’ presentations are concealed, and it is hard to find the tumors in the early stage.
The manifestations of pancreatic GIST are the same as soft tissue tumors. Benign tumors are more definite, their boundaries are clearer and smoother; small tumors typically appear as homogeneous soft tissue masses, while larger tumors often have necrotic centers. Stromal tumors are hypervascular and have no regional lymph node metastasis. These two characteristics are different from those of pancreatic cancer. According to the literature, most pancreatic GISTs cases were diagnosed by surgical biopsy. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is an accurate method of diagnosis. Yan et al[13] and Harindhanavudhi et al[14] had performed an EUS-FNA biopsy on a pancreatic GIST patient, respectively, which showed that tumor was composed of spindle cells, and immunohistochemistry showed that it was strongly positive for CD117, resulting in a preoperative diagnosis of pancreatic stromal tumor. The result was consistent with the postoperative pathological diagnosis.
In terms of the pathology of pancreatic stromal tumors, most undergo expansive growth, resulting in clearly isolated round or oval lumps. Tumors have a diameter of 2-30 cm, averaging about 10 cm according to the previous literature and are encapsulated with a smooth surface or adhesion with the surrounding tissues that are rich in blood vessels. The tumor section was flat, gray or gray-red because of collagen in blood vessels, and hemorrhage. Other changes after dissolution, such as granular surface or presence of small indentation, are different from the smooth muscle tumors; it does not show outward protrusion or swirling, but is soft and delicate, some are even fish-like. GISTs may have hemorrhage, necrosis, cystic degeneration, and other secondary changes, especially when the tumor is large. But it does not lead to malignancy.
Pancreatic GISTs are mainly composed of spindle cells. Malignant GISTs cells have different degrees of atypia, and mitotic figures are visible. Pancreatic GISTs are different from typical smooth muscle and nerve sheath tumors. Immune markers are expressed at low levels, such as smooth muscle actin (30%-40%), desmin (< 5%), neuron specific enolase and S-100 (< 5%). C-Kit gene is expressed in 95% of cases, making it a characteristic of GISTs. And 60%-70% of tumors are CD34-positive[16].
Pancreatic GISTs can be divided into very low, low, intermediate and high risk of metastases according to the tumor size and mitotic counts on histology and immunohistochemistry. Tumors larger than 5 cm with more than 5 mitoses per 50 HPF and tumors larger than 10 cm, regardless of mitotic count, should be considered as lesions with high risk of malignancy[17]. It was assumed that most tumors reported were of high risk (9/14), which may be related to the symptom concealing and expansive growth. The biggest pancreatic GIST reported was nearly 30 cm[15]. However, the patient only presented with a symptom of abdominal uncomfortableness. Moreover, pancreatic GISTs easily develop liver metastasis. In our second case, multiple metastatic nodules were found one year after surgery.
Surgery remains the preferred treatment for pancreatic stromal tumors[9]. Pancreaticoduodenectomy is feasible when there are stromal tumors of the pancreatic head[15]. If the tumor is small with clear boundaries or the patient cannot tolerate pancreaticoduodenectomy, duodenum-preserving pancreatic head resection or simple tumor excision can also be applied. GISTs and EGISTs regional lymph node metastases are rare; thus, regional lymph node dissection is not required generally in surgery[15].
Imatinib (Gleevec), which is an inhibitor of the tyrosine kinase activity of C-Kit, has revolutionized the treatment of this disease and the overall median survival now reaches 5 years[18]. For large GISTs, Gleevec before surgery can reduce tumor burden, increase the rate of complete resection of the tumor, and help improve prognosis[19]. For metastatic or unresectable GISTs, imatinib treatment can significantly reduce the tumor size as well as improve survival rate[20]. Three cases of pancreatic GISTs had been reported taking imatinib after liver metastasis was found, and all these patients had at least a 2-year progression-free survival. Our second case took imatinib (400 mg/d) for only two months because of poor economic status, while the patient also had a progression-free survival for 3 years.
Many factors can affect the prognosis of pancreatic stromal tumors, such as age, location, cell differentiation, C-Kit gene mutations, DNA factors, histological grade, and p53. The prognosis of pancreatic stromal tumors is closely related to the biological behavior of tumors. Four of the 14 studies did not give the information of follow-up[13-16]. Eight cases were found no evidence of recurrence during follow-up (the minimum period of follow-up was six months)[7-12]. Neto et al[6] reported a case of peritoneal and retroperitoneal nodal disease with recurrence one month after surgery. Vij et al[5] reported one case with liver metastasis 24 mo after surgery. In the present study, in the second case, liver metastases occurred after 1 year; but intervention and treatment with imatinib led to a good survival. The median survival of patients with liver metastasis from pancreatic cancer is only about 6 mo. It is obvious that the prognosis of this tumor is significantly better than that of pancreatic cancer.
In short, pancreatic stromal tumor is rare. The lack of specific clinical manifestations, hypervascular tumor, and regional lymph node metastases, is slightly different from the pancreatic tumor in the imaging characteristics of the two cases. Surgical resection resulted in a significantly better prognosis than that of pancreatic cancer.
COMMENTS
Case characteristics
The two cases presented no clinical symptoms, but both of them underwent computed tomography (CT).
Clinical diagnosis
Both of the two cases were diagnosed with pancreatic tumor.
Differential diagnosis
The two pancreatic Gastrointestinal stromal tumors (GISTs) were diagnosed using immunohistochemical staining.
Imaging diagnosis
Case 1: Abdominal CT revealed a 3 cm × 5 cm mass which is located in the pancreas tail next to the splenic artery; Case 2: Abdominal CT revealed a well-demarcated tumor, 3 cm × 5 cm × 5 cm, in the head of the pancreas.
Pathological diagnosis
Case 1: The patient was diagnosed with a low-risk GIST; Case 2: The patient was diagnosed with a high-risk GIST.
Treatment
Case 1: underwent distal pancreatectomy with splenectomy; Case 2: underwent a tumor local resection; the patient was started on imatinib (400 mg bid) when multiple liver metastases were found one year after surgery.
Term explanation
GISTs are mesenchymal tumors that arise from the gastrointestinal tract.
Experiences and lessons
Pancreatic GIST is very rare, with only 14 previous cases reported. Here, we report two cases of malignant pancreatic GIST and review the cases previously reported in the literature.
Peer review
This case report is very interesting. In rare GIST cases, these tumors are found in intra-abdominal sites unrelated to the gastrointestinal tract, such as the mesentery, omentum and retroperitoneum, but pancreatic extra-GIST are extremely rare. In this manuscript, Tian et al reported two cases of pancreatic GISTs. The cases are well presented, and the discussion is good. It can be accepted for publication after some minor language correction.
Footnotes
Supported by The Beijing Hope Run Special Fund, LC2012A09; and Beijing Municipal Science and Technology Commission, Z131107002213164
P- Reviewers: Mello EL, Matsubara N, Straus HG S- Editor: Qi Y L- Editor: Ma JY E- Editor: Ma S
References
- 1.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 2.Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Vij M, Agrawal V, Pandey R. Malignant extra-gastrointestinal stromal tumor of the pancreas. A case report and review of literature. JOP. 2011;12:200–204. [PubMed] [Google Scholar]
- 6.Neto MR, Machuca TN, Pinho RV, Yuasa LD, Bleggi-Torres LF. Gastrointestinal stromal tumor: report of two unusual cases. Virchows Arch. 2004;444:594–596. doi: 10.1007/s00428-004-1009-1. [DOI] [PubMed] [Google Scholar]
- 7.Yamaura K, Kato K, Miyazawa M, Haba Y, Muramatsu A, Miyata K, Koide N. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol. 2004;19:467–470. doi: 10.1111/j.1440-1746.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 8.Krska Z, Pesková M, Povýsil C, Horejs J, Sedlácková E, Kudrnová Z. GIST of pancreas. Prague Med Rep. 2005;106:201–208. [PubMed] [Google Scholar]
- 9.Daum O, Klecka J, Ferda J, Treska V, Vanecek T, Sima R, Mukensnabl P, Michal M. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch. 2005;446:470–472. doi: 10.1007/s00428-004-1200-4. [DOI] [PubMed] [Google Scholar]
- 10.Showalter SL, Lloyd JM, Glassman DT, Berger AC. Extra-gastrointestinal stromal tumor of the pancreas: case report and a review of the literature. Arch Surg. 2008;143:305–308. doi: 10.1001/archsurg.2007.68. [DOI] [PubMed] [Google Scholar]
- 11.Pauser U, da Silva MT, Placke J, Klimstra DS, Klöppel G. Cellular hamartoma resembling gastrointestinal stromal tumor: a solid tumor of the pancreas expressing c-kit (CD117) Mod Pathol. 2005;18:1211–1216. doi: 10.1038/modpathol.3800406. [DOI] [PubMed] [Google Scholar]
- 12.Trabelsi A, Yacoub-Abid LB, Mtimet A, Abdelkrim SB, Hammedi F, Ali AB, Mokni M. Gastrointestinal stromal tumor of the pancreas: A case report and review of the literature. N Am J Med Sci. 2009;1:324–326. [PMC free article] [PubMed] [Google Scholar]
- 13.Yan BM, Pai RK, Van Dam J. Diagnosis of pancreatic gastrointestinal stromal tumor by EUS guided FNA. JOP. 2008;9:192–196. [PubMed] [Google Scholar]
- 14.Harindhanavudhi T, Tanawuttiwat T, Pyle J, Silva R. Extra-gastrointestinal stromal tumor presenting as hemorrhagic pancreatic cyst diagnosed by EUS-FNA. JOP. 2009;10:189–191. [PubMed] [Google Scholar]
- 15.Boyer C, Duvet S, Wacrenier A, Toursel H, Ernst O, L’herminé C. [Leiomyosarcoma and stromal tumor of the pancreas] J Radiol. 2001;82:1723–1725. [PubMed] [Google Scholar]
- 16.Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48:83–96. doi: 10.1111/j.1365-2559.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 18.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 19.Mearadji A, den Bakker MA, van Geel AN, Eggermont AM, Sleijfer S, Verweij J, de Wilt JH, Verhoef C. Decrease of CD117 expression as possible prognostic marker for recurrence in the resected specimen after imatinib treatment in patients with initially unresectable gastrointestinal stromal tumors: a clinicopathological analysis. Anticancer Drugs. 2008;19:607–612. doi: 10.1097/CAD.0b013e32830138f9. [DOI] [PubMed] [Google Scholar]
- 20.Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, Peake D. Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess. 2005;9:1–142. doi: 10.3310/hta9250. [DOI] [PubMed] [Google Scholar]


