Abstract
Endoscopic and clinical recurrence of Crohn’s disease (CD) is a common occurrence after surgical resection. Smokers, those with perforating disease, and those with myenteric plexitis are all at higher risk of recurrence. A number of medical therapies have been shown to reduce this risk in clinical trials. Metronidazole, thiopurines and anti-tumour necrosis factors (TNFs) are all effective in reducing the risk of endoscopic or clinical recurrence of CD. Since these are preventative agents, the benefits of prophylaxis need to be weighed-against the risk of adverse events from, and costs of, therapy. Patients who are high risk for post-operative recurrence should be considered for early medical prophylaxis with an anti-TNF. Patients who have few to no risk factors are likely best served by a three-month course of antibiotics followed by tailored therapy based on endoscopy at one year. Clinical recurrence rates are variable, and methods to stratify patients into high and low risk populations combined with prophylaxis tailored to endoscopic recurrence would be an effective strategy in treating these patients.
Keywords: Inflammatory bowel disease, Crohn’s disease, Postoperative recurrence, Medical treatment, Biologics
Core tip: This review summarizes and updates the current state of the field of post-operative prevention of Crohn’s disease (CD) after surgery. This review starts by discussing the natural history of postoperative recurrence followed by a summary of the most consistent and evidence based risk factors. We then discuss the evidence for medical prophylaxis of CD highlighting new data regarding biologics. Finally we discuss cost effectiveness and provide a potential novel treatment algorithm for a clinician to use practically when caring for a patient with CD after surgery.
INTRODUCTION
Patients with Crohn’s disease (CD) have a high likelihood of requiring surgery over their lifetime. Reasons for surgical intervention include failure of medical therapy, or a complication of stricturing or penetrating disease[1]. It is estimated that half of patients will require surgery within 10 years of diagnosis[2,3] and around 80% of patients will require surgery at some time in their life[4-6]. Unfortunately, surgery for CD is not curative and recurrence is typically the rule. Symptomatic recurrence varies from 40%-80%[5,7]. Endoscopic recurrence is much higher with up to 90% having lesions at 5 years[4,7,8].
While the overall recurrence rates are high, the severity of recurrence varies amongst individuals. Some patients may only develop mild endoscopic recurrence, and have no symptoms, whereas others may present soon after surgery with recurrent inflammation and clinical symptoms. Interventional studies have measured a drugs ability to prevent endoscopically-visible ulcers, or radiologically-apparent luminal narrowing, or symptoms. Assessing for severe endoscopic recurrence is useful, although still a surrogate predictor of future clinical recurrence. The wide variation of patients and outcomes used in clinical trials makes this topic difficult for the treating physician to apply to a given patient. The aim of this review is to summarize the literature regarding the medications available for prevention of post-operative recurrence as well as to discuss the different strategies for treating postoperative recurrence.
NATURAL HISTORY OF POST-OPERATIVE CROHN’S
The natural history of CD generally follows phenotypic patterns, whether in the pre-operative or post-operative patient. As stated above, recurrence in general is common both endoscopically and symptomatically after surgery. Crohn’s recurrence after ileocecal resection or colectomy with ileorectal anastomosis tends to favor the anastomosis site, specifically the small bowel proximal to the anastomosis[9]. Initially aphthous ulcers develop which then progress to larger ulcers and eventually complications of recurrent CD[7,8]. It was noted in the late 1980s that patients who underwent surgery for a perforating phenotype of disease had a different post-operative course than those who underwent surgery for non-perforating indications[10]. This group examined 770 patients who underwent surgery for CD at Mt. Sinai Hospital in New York. They found that not only did patients had a different course depending on their pre-operative phenotype, but they found that patients who required second and third operations, tended to do so for the same initial indication. Thus, patients who required an initial operation for penetrating disease tended to require a second, and potentially third, operation for penetrating disease. Thus overall the site and prevailing phenotype are thought to be similar before and after resection[11].
An interesting observation into the pathogenesis of post-operative CD is that the fecal stream is needed for recurrence. Rutgeerts et al[8] reported on five patients who underwent ileal resection with ileocolonic anastomosis and additionally had a temporary, diverting ileostomy approximately 25-30 cm from their anastomosis site. At six months time, the anastomoses of these patients were compared to 75 other patients who underwent ileocolonic anastomosis without diverting ileostomy. None of the patients with the diverting ileostomy developed endoscopic or histologic changes consistent with CD compared to 71% of patients in the control group who had endoscopic recurrence at six months. All five patients however, did develop endoscopic recurrence of disease at the anastomosis six months after the diverting ileostomy was taken down[12]. Further study demonstrated that contact with intestinal fluid for only eight days was sufficient to trigger early histologic changes in a previously normal neo-terminal ileum and anastomosis[13]. Within three months after ileocecectomy the neoterminal ileum has been shown to have an increase in colonization with colonic flora, specifically Escherichia coli (E. coli), Enterococci, Bacteroides and Fusobacteria spp[14]. Animal studies have also concluded that the presence of commensal bacteria is required for post-operative recurrence to develop[15]. It remains unclear if these changes have a causative role in post-operative Crohn’s recurrence, but they have been implicated in IBD per se[16]. Thus, post-operative CD is associated with alterations in the local microbiome, development of local ulcers (endoscopic recurrence) and subsequent resumption of clinical recurrence.
RISK FACTORS FOR RECURRENCE: RISK STRATIFICATION
Multiple risk factors have been identified for postoperative recurrence. Risk stratifying patients based on these factors can thus help decide which patients to treat aggressively. Unfortunately, few risk factors have been consistent in the literature for predicting endoscopic, clinical or surgical recurrence. Typically risk factors are divided into patient-related factors, disease-related factors and surgical-related factors. Of the patient related factors, only smoking has consistently been identified as a predictor of postoperative recurrence. The odds ratio for clinical recurrence for current smokers is between 2 and 3[4,17,18]. It is the only modifiable risk factor and smoking cessation appears to decrease the risk of recurrence to that of a non-smoker[19]. Other patient related factors such as age, sex, age at disease onset are inconsistent risk factors in the literature[20,21].
Disease related factors include duration of disease prior to first resection, history of previous resection and penetrating disease[4,22]. Duration of disease prior to first surgery is not a consistent risk factor in the literature[23,24]. Most studies demonstrate that previous surgery for CD is a risk factor for further surgery[11,22,25]. A recent meta-analysis demonstrated a hazard ratio of 1.5 for recurrence of disease for penetrating phenotype compared to non-penetrating[26]. Unfortunately, there was significant heterogeneity in the studies that went into the meta-analysis and thus it is not entirely clear what the relationship between penetrating disease and post-operative recurrence truly is[21]. Further complicating the issue is that penetrating and stricturing disease are not always accurately diagnosed on clinical grounds. Up to a third of patients who clinically have non-penetrating disease may have evidence of penetration at the time of an operation[27].
Surgical factors including length of resection, type of anastomosis and histological disease left in the bowel or granulomas in the resected specimen have not been consistently demonstrated to be risk factors for disease recurrence[28-32]. However, myenteric plexitis in the resection specimen has been found to be predictive of postoperative recurrence[33-35]. One small study found plexitis to be associated with prior intestinal surgery and shorter duration of disease thus potentially accounting for some of the variability of those risk factors as predictors of disease[36]. Select risk factors that have been consistent in the literature are shown in Table 1.
Table 1.
Risk factors for postoperative recurrence
| Risk factor | Strength of association (OR) | Grade of evidence1 |
| Active smoking | 2 (1.3-3.4)[18] | B |
| Penetrating disease | 1.5 (1.2-1.9)[26] | B |
| Prior resection | 1.8 (1.1-2.9)[22] | B |
| Myenteric plexitis | 1.9 (1.0-3.5)[33] | C |
Based on American Gastroenterological Association guidelines. A: Multiple, well-designed randomized controlled trials; B: At least one large well-designed clinical trial with or without randomization; C: Based on clinical experience, expert committees or descriptive studies.
MEDICAL THERAPIES TO PREVENT RECURRENCE
Multiple medications used for the treatment of Crohn’s disease have been studied for the prevention of post-operative recurrence. Table 2 summarizes the endoscopic and clinical recurrences rates vs placebo from various trials.
Table 2.
Recurrence rates with each agent discussed (range) from randomized controlled trials, vs placebo rates
| Agent |
Endoscopic recurrence |
Clinical recurrence |
||
| Drug | Placebo | Drug | Placebo | |
| Mesalamine | 5%-51% | 50% | 11%-16% | 19%-23% |
| Thiopurines | 2%-22% | 50% | 0%-36% | 10%-50% |
| Anti-TNF | 0%-21%1 | 81%-85%1 | 0%-20% | 25%-46% |
| Antibiotics | 13% | 43% | 7%-24% | 25%-38% |
| Probiotics2 | 9%-21% | 15%-16% | 9%-17% | 6%-14% |
| Budesonide | 52% | 57% | 57%3 | 70%3 |
Ranges of reported endoscopic and clinical recurrences for various therapies and the range of placebo reports from those trials. Endoscopic recurrence rates are for severe (≥ i2) recurrence unless otherwise stated. Follow-up time is 12 mo unless otherwise stated.
Any endoscopic recurrence;
Three to twelve months follow-up;
Combined clinical and endoscopic recurrence. TNF: Tumour necrosis factor.
5-ASA
The use of mesalamine in the postoperative setting is appealing given its favorable safety profile, ease of administration, and relatively lower costs to anti-TNFs. However controversy exists over its efficacy. McLeod et al[31] demonstrated a 10% decrease in symptomatic recurrence (P = 0.03) with mesalamine at 3.0 g per day. Others have noted a decrease in endoscopic recurrence with varying mesalamine formulations[37,38]. Despite this, some small prospective and retrospective studies failed demonstrate a difference compared to placebo[39,40]. Interestingly, Hanauer et al[41] found that postoperative mesalamine demonstrated a non-significant trend towards a clinical improvement compared to placebo, but did not have any effect on endoscopic or radiologic recurrence. A meta-analysis of available prospective studies did demonstrate that mesalamine decreased clinical as well as severe endoscopic recurrence at 12 mo with a NNT of 8 (RR vs placebo: 0.76, 95%CI: 0.62-0.94)[42].
Thiopurines
Azathioprine and 6-mercaptopurine are efficacious in maintenance of CD and have been extensively studied in the post-operative setting. Only two studies have compared to a thiopurine to placebo and one was in combination with three months of metronidazole[41,43]. Both trials showed a decrease of endoscopic recurrence in the thiopurine arm at 12 mo. Pooling the data demonstrated a favorable risk ratio for decreasing both clinical (RR vs placebo: 0.59, 95%CI: 0.38-0.92) and severe endoscopic recurrence (RR vs placebo: 0.64, 95%CI: 0.44-0.92) with thiopurines compared to placebo[42].
Other trials have compared thiopurine to mesalamine formulation. However, superiority of a thiopurine over mesalamine was unable to be shown in multiple trials[41,44-47]. It is possible that side effects often prohibit adherence of thiopurines[46]. The meta-analysis by Doherty et al[42] reported a higher risk of treatment discontinuation due to side-effects in patients taking thiopurines. Long term data from a retrospective analysis demonstrated that thiopurine treatment for over 36 mo decreased surgical recurrence compared to treatment less than 36 mo or no treatment at all[48]. Thus long-term maintenance may be beneficial especially in those who can tolerate the treatment, but the side effect profile may be prohibitive especially as mesalamine agents may provide a similar decreased risk of recurrence.
Anti-TNF antibodies
Most recently anti-TNF therapeutic antibodies have been studied in the prevention of post-operative prophylaxis. Infliximab has been studied most, although there is some emerging data for adalimumab. Early non-controlled reports noted a decreased in both endoscopic and clinical recurrence of CD postoperatively[49]. Subsequent prospective controlled studies also demonstrated a benefit compared to placebo[50,51], mesalamine[28,52] and thiopurines[28]. However, there have only been two randomized trials comparing infliximab and both were compared with placebo. Regueiro et al[53] demonstrated that endoscopic lesions were significantly lower at one year in the infliximab group compared to controls (9.1% vs 84.6% respectively, P = 0.0006), but were unable to detect a significant difference in the clinical recurrence rate due to small numbers. A follow up abstract from the same group suggested that patients can have a benefit for up to two years, although they will relapse if infliximab is stopped[54]. Yoshida et al[55] did demonstrate a decrease in the clinical remission rate at 12 mo with infliximab compared to placebo (100% vs 68% respectively, P < 0.03). Additionally adalimumab has demonstrated efficacy if prevention of postoperative recurrence although data remains small with a lack of controls[56-58]. Preliminary data from the POCER study was presented in abstract form demonstrated that at 6 mo 94% of high-risk patients treated with postoperative adalimumab were in endoscopic remission (i0-i1) vs 62% of high risk patients on a thiopurine (P = 0.02)[59]. However, patients on a thiopurine who had recurrence had an escalation of therapy to adalimumab. Thus at 18 mo, there was no difference between endoscopic recurrence in patients who received adalimumab immediately post operatively and those who had tailored therapy based on 6 mo endoscopy[60]. Thus early data from ongoing trials suggests that prophylaxis with anti-TNF antibodies may be highly effective compared to other treatments, although careful patient selection is likely required to identify whom to administer prophylaxis to.
Probiotics
Manipulation of the bacterial flora is an attractive mode of preventing postoperative recurrence, as specific bacteria including bacteroides, fusobacteria and E. coli have been found in increased amount in the neo-terminal ileum[14]. Both individual lactobacillus strains as well as probiotic cocktails have been studied. Lactobacillus johnsonii (LA1) and Lactobacillus rhamnosus GG (LGG), failed to show any benefit in prevention of recurrence[61-63]. The probiotic cocktail Synbiotic 2000 similarly failed to show a benefit compared to placebo in a randomized controlled trial[64]. Similarly the yeast Sacchromyces boulardii did not demonstrate prevention of relapse in a randomized controlled trial of patients in medical remission[65]. However, a small study with VSL#3 did note that patients receiving the probiotic had less severe endoscopic recurrence (lower Rutgeerts score) and reduced levels of pro-inflammatory cytokines[66]. Ultimately, pooled data failed to show any difference with regards to clinical (RR vs placebo: 1.41, 95%CI: 0.59-3.36) or endoscopic recurrence (RR vs placebo: 0.96, 95%CI: 0.58-1.58)[42].
Antibiotics
Nitroimidazole antibiotics including metronidazole and ornidazole have been shown to decrease the risk of clinical and severe endoscopic recurrence[42,67,68]. Further illustrating the usefulness of antibiotics was a trial where all patients received metronidazole for one month and either azathioprine or placebo for one year. The overall rate of recurrence was low which was attributed to the widespread metronidazole use, although in combination with azathioprine endoscopic recurrence was less frequent and less severe[43]. However, nitroimidazole antibiotics are difficult to tolerate and cessation rates are high, up to one third, limiting widespread clinical utility[67,68].
COSTS AND DURATION OF CHEMOPROPHYLAXIS
The goal of therapy for post-operative prophylaxis is to decrease clinical recurrence. Not all patients will develop clinical recurrence and so treating everyone one may not be cost effective or feasible. Two studies examined the cost and feasibility of treating patients with various medical therapies in the postoperative setting. Doherty et al[69] noted that incremental cost-effectiveness ratio (ICER), or costs per QALY gained, was $1.8M for infliximab, when compared with no prophylaxis. In their cost-effectiveness model, azathioprine was the most cost-effective medication strategy over 1 year, when compared to “no prophylaxis”. Ananthakrishnan et al[70] similarly noted that initial therapy with infliximab is not cost effective although suggested that antibiotics (metronidazole or ornidazole) were cost effective when tolerated. Both of these studies made assumptions of a recurrence rate of around 20%-30% with no treatment. As the rate of recurrence increases, the cost-effectiveness of an anti-TNF increases as well.
USING ENDOSCOPIC SCREENING TO TAILOR INTERVENTIONS
Endoscopic recurrence precedes clinical recurrence, but not all endoscopic recurrence leads to clinical recurrence. Endoscopic changes are often noted as early as 2 mo following surgery[71]. Endoscopic recurrence rates vary widely at 1 year without any treatment ranging from 28%-93%[72]. Overall, it is estimated that 20% of patients with endoscopic recurrence will progress to clinic recurrence, although the severity of the endoscopic recurrence can predict the likelihood of clinical recurrence[8,19]. Over a four year follow-up period, 100% of patients with severe endoscopic recurrence (Rutgeerts scope of i2-i4) developed symptomatic recurrence compared to only 9% of patients with a low score (i0-i1)[73]. Thus evaluation of a patient within the first year following resection of CD can effectively stratify patients at a high risk of clinical recurrence. Patients who develop a high Rutgeerts score (≥ i2) who are then treated with infliximab have decreased clinical activity compared to treatment with thiopurines or mesalamine[28]. One study demonstrated that an endoscopically-tailored therapy (with any medical therapy) has a similar time to symptomatic recurrence compared to immediate post operative prophylaxis[74]. Recently, preliminary results of the POCER study were presented, supporting the use of endoscopically tailored therapy. In this trial, all patients received three months of antibiotics and high-risk patients (smoker, penetrating disease, ≥ second operation) received a thiopurine (or every other week adalimumab if thiopurine intolerant). Patients were then randomized to active care with a colonoscopy at 6 mo and step up of therapy if evidence of recurrence (Rutgeerts score ≥ i2) or standard of care. At 18 mo, significantly less endoscopic recurrence was seen in the active care group vs the standard of care group (49% vs 67% respectively, P = 0.028)[75].
An interesting option to explore in the future is immediate prophylaxis followed by endoscopic assessment and cessation of treatment if no endoscopic recurrence or other surrogate marker for inflammations such as serum inflammatory markers or fecal calprotectin. One retrospective cohort demonstrated that patients who achieve clinical remission on infliximab and maintain maintenance therapy for one year have a 69% probability of being disease free at 1 year after infliximab discontinuation[76]. The STORI trial is a prospective cohort following a group of patients who have received > 1 year of therapy with infliximab and an immunomodulator and achieved steroid free remission who then stop infliximab. Over 50% of patients will not relapse at one year and there may be a subgroup of patients in deep remission who do even better[77]. Extrapolating this premise and applying it to patients who achieve surgical remission followed by a year of therapy is appealing and potentially cost effective. One small study has evaluated one year of infliximab for chronic refractory pouchitis following colectomy for ulcerative colitis. Notably the patients who responded completely to infliximab were able to discontinue after one year without recurrence at follow-up[78].
IMPLICATIONS FOR PRACTICE
The questions of if and when to treat in the post-operative setting, as well as the optimal therapeutic regimen, remains incompletely answered. Each patient requires an individual risk assessment as well as careful consideration to prior therapies and what led them to surgery[4]. Overall, tailored therapy is advisable as a general rule given the cost and side effect profile of the majority of treatments. However, in the highest risk patients (i.e., penetrating disease, history of prior resection, current smoking), upfront infliximab use provides the greatest benefit although it is not considered cost effective[70]. As long-term data on infliximab becomes available, the balance of cost and prevention of disease may change. Additionally as data becomes available for other anti-TNFs, this model will need to be updated. Thus for the highest risk patients, the decision for upfront anti-TNF vs tailored therapy remains unclear and must likely be individualized. If the decision for tailored therapy is made, then endoscopic evaluation should take place at 6 mo time as suggested by the recent POCER data[60,75]. In patients who are not high risk, then an empiric course of nitroimidazole antibiotic is likely to be cost effective, if the patient can tolerate the therapy, with or without maintenance thiopurine followed by endoscopic assessment at 6 mo time[4,60,70,75]. A proposed algorithm for post-operative prevention is shown in Figure 1. While this approach is the most efficacious based on the available evidence, it is not the most cost effective and the decision for upfront anti-TNF therapy or tailored therapy in high risk patients will need to be individualized.
Figure 1.
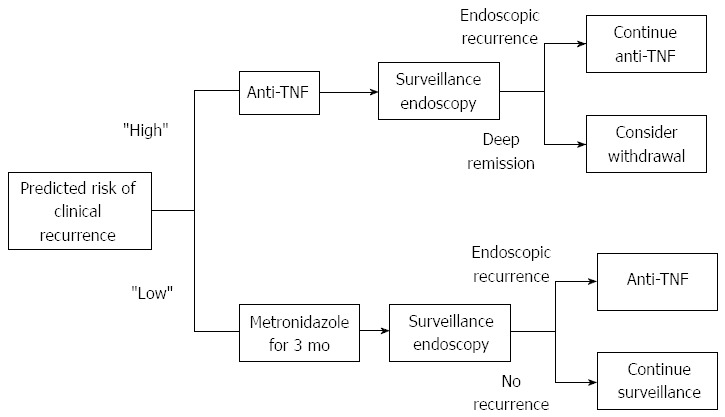
Suggested algorithm for deciding when to administer post-operative prophylaxis based on effectiveness of treatment. Other considerations such as cost and prior success or failure of treatment need to be individualized. Patients at high risk are those who have 2 or more of the following risk factors: smoking, penetrating disease, history of prior resection, and myenteric plexitis. Based on the available evidence, we suggest first surveillance endoscopy be done at 6 mo time for all patients. Treatment escalation at 6 mo should be considered for all patients with evidence of endoscopic recurrence (Rutgeerts ≥ i2). For select patients who achieve and maintain deep remission on therapy (Rutgeerts of i0 with normal histology), consideration can be given to de-escalation of therapy to either thiopurine alone or close monitoring.
The optimal treatment regimen is not known and likely will depend heavily on the patient’s prior treatments. The biologic naïve patient may achieve the best effect with an anti-TNF, although the cost effectiveness of long-term prevention may not be favorable. While the majority of data is with infliximab, it appears that adalimumab will be efficacious as well, necessitating further cost effective analysis. Postoperative prophylaxis in CD does decrease clinical recurrence. However the optimal timing and type of therapy remain unknown. Stratifying patients according to risk of symptomatic recurrence and tailoring therapy is the ideal and most cost effective way to treat patients, however these questions have not been fully answered.
Footnotes
Supported by NIH grant, No. K23DK084338 (to Moss AC); NIH training grant, No. 5T32DK007760-14 (to Vaughn BP)
P- Reviewers: Caprilli R, Deepak P, Tysk C S- Editor: Cui XM L- Editor: A E- Editor: Liu XM
References
- 1.Amiot A, Gornet JM, Baudry C, Munoz-Bongrand N, Auger M, Simon M, Allez M, Cattan P, Sarfati E, Lémann M. Crohn’s disease recurrence after total proctocolectomy with definitive ileostomy. Dig Liver Dis. 2011;43:698–702. doi: 10.1016/j.dld.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 4.Moss AC. Prevention of postoperative recurrence of Crohn’s disease: what does the evidence support? Inflamm Bowel Dis. 2013;19:856–859. doi: 10.1097/MIB.0b013e3182802c21. [DOI] [PubMed] [Google Scholar]
- 5.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87:1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 6.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Dombal FT, Burton I, Goligher JC. Recurrence of Crohn’s disease after primary excisional surgery. Gut. 1971;12:519–527. doi: 10.1136/gut.12.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenstein AJ, Lachman P, Sachar DB, Springhorn J, Heimann T, Janowitz HD, Aufses AH. Perforating and non-perforating indications for repeated operations in Crohn‘s disease: evidence for two clinical forms. Gut. 1988;29:588–592. doi: 10.1136/gut.29.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onali S, Petruzziello C, Calabrese E, Condino G, Zorzi F, Sica GS, Pallone F, Biancone L. Frequency, pattern, and risk factors of postoperative recurrence of Crohn’s disease after resection different from ileo-colonic. J Gastrointest Surg. 2009;13:246–252. doi: 10.1007/s11605-008-0726-1. [DOI] [PubMed] [Google Scholar]
- 12.Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, Aerts R, Kerremans R, Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338:771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 13.D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 14.Neut C, Bulois P, Desreumaux P, Membré JM, Lederman E, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel JF. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol. 2002;97:939–946. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- 15.Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009;58:1104–1112. doi: 10.1136/gut.2008.157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane SV, Flicker M, Katz-Nelson F. Tobacco use is associated with accelerated clinical recurrence of Crohn’s disease after surgically induced remission. J Clin Gastroenterol. 2005;39:32–35. [PubMed] [Google Scholar]
- 18.Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213–1221. doi: 10.1007/s00384-008-0542-9. [DOI] [PubMed] [Google Scholar]
- 19.De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758–777. doi: 10.1002/ibd.21825. [DOI] [PubMed] [Google Scholar]
- 20.Moskovitz D, McLeod RS, Greenberg GR, Cohen Z. Operative and environmental risk factors for recurrence of Crohn’s disease. Int J Colorectal Dis. 1999;14:224–226. doi: 10.1007/s003840050215. [DOI] [PubMed] [Google Scholar]
- 21.Borowiec AM, Fedorak RN. Predicting, treating and preventing postoperative recurrence of Crohn’s disease: the state of the field. Can J Gastroenterol. 2011;25:140–146. doi: 10.1155/2011/591347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M. Recurrence of Crohn’s disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum. 2009;52:919–927. doi: 10.1007/DCR.0b013e3181a4fa58. [DOI] [PubMed] [Google Scholar]
- 23.Poggioli G, Laureti S, Selleri S, Brignola C, Grazi GL, Stocchi L, Marra C, Magalotti C, Grigioni WF, Cavallari A. Factors affecting recurrence in Crohn’s disease. Results of a prospective audit. Int J Colorectal Dis. 1996;11:294–298. doi: 10.1007/s003840050065. [DOI] [PubMed] [Google Scholar]
- 24.Wolff BG. Factors determining recurrence following surgery for Crohn’s disease. World J Surg. 1998;22:364–369. doi: 10.1007/s002689900398. [DOI] [PubMed] [Google Scholar]
- 25.Ng SC, Lied GA, Arebi N, Phillips RK, Kamm MA. Clinical and surgical recurrence of Crohn‘s disease after ileocolonic resection in a specialist unit. Eur J Gastroenterol Hepatol. 2009;21:551–557. doi: 10.1097/MEG.0b013e328326a01e. [DOI] [PubMed] [Google Scholar]
- 26.Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, Tekkis PP. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008;103:196–205. doi: 10.1111/j.1572-0241.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 27.Sachar DB, Lemmer E, Ibrahim C, Edden Y, Ullman T, Ciardulo J, Roth E, Greenstein AJ, Bauer JJ. Recurrence patterns after first resection for stricturing or penetrating Crohn’s disease. Inflamm Bowel Dis. 2009;15:1071–1075. doi: 10.1002/ibd.20872. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn’s disease: a prospective pilot study. Inflamm Bowel Dis. 2009;15:1460–1466. doi: 10.1002/ibd.20915. [DOI] [PubMed] [Google Scholar]
- 29.Ikeuchi H, Kusunoki M, Yamamura T. Long-term results of stapled and hand-sewn anastomoses in patients with Crohn’s disease. Dig Surg. 2000;17:493–496. doi: 10.1159/000051946. [DOI] [PubMed] [Google Scholar]
- 30.Tersigni R, Alessandroni L, Barreca M, Piovanello P, Prantera C. Does stapled functional end-to-end anastomosis affect recurrence of Crohn’s disease after ileocolonic resection? Hepatogastroenterology. 2003;50:1422–1425. [PubMed] [Google Scholar]
- 31.McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O’Rourke K, Andrews DF, Blair JE, Cangemi JR, Cohen Z, Cullen JB. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn’s disease. Gastroenterology. 1995;109:404–413. doi: 10.1016/0016-5085(95)90327-5. [DOI] [PubMed] [Google Scholar]
- 32.Trnka YM, Glotzer DJ, Kasdon EJ, Goldman H, Steer ML, Goldman LD. The long-term outcome of restorative operation in Crohn’s disease: influence of location, prognostic factors and surgical guidelines. Ann Surg. 1982;196:345–355. doi: 10.1097/00000658-198209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokol H, Polin V, Lavergne-Slove A, Panis Y, Treton X, Dray X, Bouhnik Y, Valleur P, Marteau P. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn’s disease. Gut. 2009;58:1218–1225. doi: 10.1136/gut.2009.177782. [DOI] [PubMed] [Google Scholar]
- 34.Bressenot A, Chevaux JB, Williet N, Oussalah A, Germain A, Gauchotte G, Wissler MP, Vignaud JM, Bresler L, Bigard MA, et al. Submucosal plexitis as a predictor of postoperative surgical recurrence in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1654–1661. doi: 10.1097/MIB.0b013e318281f336. [DOI] [PubMed] [Google Scholar]
- 35.Ferrante M, de Hertogh G, Hlavaty T, D’Haens G, Penninckx F, D’Hoore A, Vermeire S, Rutgeerts P, Geboes K, van Assche G. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology. 2006;130:1595–1606. doi: 10.1053/j.gastro.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Ng SC, Lied GA, Kamm MA, Sandhu F, Guenther T, Arebi N. Predictive value and clinical significance of myenteric plexitis in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1499–1507. doi: 10.1002/ibd.20932. [DOI] [PubMed] [Google Scholar]
- 37.Caprilli R, Cottone M, Tonelli F, Sturniolo G, Castiglione F, Annese V, Papi C, Viscido A, Cammà C, Corrao G, et al. Two mesalazine regimens in the prevention of the post-operative recurrence of Crohn’s disease: a pragmatic, double-blind, randomized controlled trial. Aliment Pharmacol Ther. 2003;17:517–523. doi: 10.1046/j.1365-2036.2003.01462.x. [DOI] [PubMed] [Google Scholar]
- 38.Brignola C, Cottone M, Pera A, Ardizzone S, Scribano ML, De Franchis R, D’Arienzo A, D’Albasio G, Pennestri D. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn’s disease. Italian Cooperative Study Group. Gastroenterology. 1995;108:345–349. doi: 10.1016/0016-5085(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 39.Florent C, Cortot A, Quandale P, Sahmound T, Modigliani R, Sarfaty E, Valleur P, Dupas JL, Daurat M, Faucheron JL, et al. Placebo-controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn’s disease. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Eur J Gastroenterol Hepatol. 1996;8:229–233. doi: 10.1097/00042737-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Papi C, Aratari A, Tornatore V, Koch M, Capurso L, Caprilli R. Long-term prevention of post-operative recurrence in Crohn’s disease cannot be affected by mesalazine. J Crohns Colitis. 2009;3:109–114. doi: 10.1016/j.crohns.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, Present DH. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723–729. doi: 10.1053/j.gastro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst Rev. 2009;(4):CD006873. doi: 10.1002/14651858.CD006873.pub2. [DOI] [PubMed] [Google Scholar]
- 43.D'Haens GR, Vermeire S, Van Assche G, Noman M, Aerden I, Van Olmen G, Rutgeerts P. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology. 2008;135:1123–1129. doi: 10.1053/j.gastro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar-Meir S, Teml A, Schaeffeler E, Schwab M, Dilger K, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn‘s disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut. 2010;59:752–759. doi: 10.1136/gut.2009.194159. [DOI] [PubMed] [Google Scholar]
- 45.Nos P, Hinojosa J, Aguilera V, Molés JR, Pastor M, Ponce J, Berenguer J. [Azathioprine and 5-ASA in the prevention of postoperative recurrence of Crohn’s disease] Gastroenterol Hepatol. 2000;23:374–378. [PubMed] [Google Scholar]
- 46.Herfarth H, Tjaden C, Lukas M, Obermeier F, Dilger K, Müller R, Schölmerich J. Adverse events in clinical trials with azathioprine and mesalamine for prevention of postoperative recurrence of Crohn’s disease. Gut. 2006;55:1525–1526. [PMC free article] [PubMed] [Google Scholar]
- 47.Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, Imbesi V, Molteni M, Danelli PG, Taschieri AM, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology. 2004;127:730–740. doi: 10.1053/j.gastro.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Papay P, Reinisch W, Ho E, Gratzer C, Lissner D, Herkner H, Riss S, Dejaco C, Miehsler W, Vogelsang H, et al. The impact of thiopurines on the risk of surgical recurrence in patients with Crohn’s disease after first intestinal surgery. Am J Gastroenterol. 2010;105:1158–1164. doi: 10.1038/ajg.2009.673. [DOI] [PubMed] [Google Scholar]
- 49.Sorrentino D, Terrosu G, Avellini C, Maiero S. Infliximab with low-dose methotrexate for prevention of postsurgical recurrence of ileocolonic Crohn disease. Arch Intern Med. 2007;167:1804–1807. doi: 10.1001/archinte.167.16.1804. [DOI] [PubMed] [Google Scholar]
- 50.Araki T, Uchida K, Okita Y, Fujikawa H, Inoue M, Ohi M, Tanaka K, Inoue Y, Mohri Y, Kusunoki M. The impact of postoperative infliximab maintenance therapy on preventing the surgical recurrence of Crohn's disease: a single-center paired case-control study. Surg Today. 2013:Epub ahead of print. doi: 10.1007/s00595-013-0538-0. [DOI] [PubMed] [Google Scholar]
- 51.Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8:591–599.e1; quiz e78-79. doi: 10.1016/j.cgh.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Sorrentino D, Terrosu G, Paviotti A, Geraci M, Avellini C, Zoli G, Fries W, Danese S, Occhipinti P, Croatto T, et al. Early diagnosis and treatment of postoperative endoscopic recurrence of Crohn’s disease: partial benefit by infliximab--a pilot study. Dig Dis Sci. 2012;57:1341–1348. doi: 10.1007/s10620-011-2025-z. [DOI] [PubMed] [Google Scholar]
- 53.Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441–450.e1; quiz 716. doi: 10.1053/j.gastro.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 54.Regueiro M, Schraut WH, Baidoo L, Kip K, Plevy SE, El-Hachem S, Harrison J, Pesci M, Watson AR, Binion DG. Two Year Postoperative Follow-Up of Patients Enrolled in the Randomized Controlled Trial (RCT) of Infliximab (IFX) for Prevention of Recurrent Crohn's Disease (CD). Presented at Digestive Diseases Week; 2009 May 30 to June 4. Chicago IL, USA. Gastroenterology. 2009;136:A–522. [Google Scholar]
- 55.Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, Yokoyama Y, Iimuro M, Takeda N, Kato K, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18:1617–1623. doi: 10.1002/ibd.21928. [DOI] [PubMed] [Google Scholar]
- 56.Aguas M, Bastida G, Cerrillo E, Beltrán B, Iborra M, Sánchez-Montes C, Muñoz F, Barrio J, Riestra S, Nos P. Adalimumab in prevention of postoperative recurrence of Crohn’s disease in high-risk patients. World J Gastroenterol. 2012;18:4391–4398. doi: 10.3748/wjg.v18.i32.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papamichael K, Archavlis E, Lariou C, Mantzaris GJ. Adalimumab for the prevention and/or treatment of post-operative recurrence of Crohn’s disease: a prospective, two-year, single center, pilot study. J Crohns Colitis. 2012;6:924–931. doi: 10.1016/j.crohns.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Savarino E, Dulbecco P, Bodini G, Assandri L, Savarino V. Prevention of postoperative recurrence of Crohn’s disease by Adalimumab: a case series. Eur J Gastroenterol Hepatol. 2012;24:468–470. doi: 10.1097/MEG.0b013e3283500849. [DOI] [PubMed] [Google Scholar]
- 59.De Cruz P, Kamm M, Hamilton AL, Ritchie K, Gorelik A, Liew D, Prideaux L, Lawrance I, Andrews JM, Bampton P, Sparrow M, Jakobovits S, editors. Florin T, Gibson P, Debinski H, Gearry R, Macrae F, Leong R, Kronborg I, Connor S, Pavli P, Radford-Smith G, Selby W, Johnston M, Brouwer R, Keck JO, Woods R, Connell W, Brown SJ, Bell SJ, Lust M, Elliot R, Desmond PV. Adalimumab prevents post-operative Crohn’s disease recurrence, and is superior to thioprines: Early results from the prospective POCER study. Presented at the 25th Conference of the European Chapter on Combinatorial Optimization; Antalya, Turkey. 2012. [Google Scholar]
- 60.De Cruz P, Kamm M, Hamilton A, Ritchie K, Gearry R, editors. Strategic timing of anti-TNF therapy in postoperative Crohn’s disease: Comparison of routine use immediately postoperatively with selective use after demonstrated recurrence at 6 month endoscopy. Results from POCER. 2013. Presented at 21st United European Gastroenterology Week; Berlin, Germany. 2012. [Google Scholar]
- 61.Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, Cadiot G, Soulé JC, Bourreille A, Metman E, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–847. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, DeVos M, Enslen M, Paintin M, Franchimont D. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after lleo-caecal resection. Inflamm Bowel Dis. 2007;13:135–142. doi: 10.1002/ibd.20063. [DOI] [PubMed] [Google Scholar]
- 63.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–409. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, Gelber M, Halpern Z, Bengmark S, Eliakim R. Failure of Synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci. 2007;52:385–389. doi: 10.1007/s10620-006-9549-7. [DOI] [PubMed] [Google Scholar]
- 65.Bourreille A, Cadiot G, Le Dreau G, Laharie D, Beaugerie L, Dupas JL, Marteau P, Rampal P, Moyse D, Saleh A, et al. Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin Gastroenterol Hepatol. 2013;11:982–987. doi: 10.1016/j.cgh.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Madsen K, Backer JL, Leddin D, Dieleman LA, Bitton A, Feagan B, Petrunia DM, Chiba N, Enns RA, Fedorak R. M1207 A Randomized Controlled Trial of VSL#3 for the Prevention of Endoscopic Recurrence Following Surgery for Crohn's Disease. Gastroenterology. 2008;134:A–361. [Google Scholar]
- 67.Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 68.Rutgeerts P, Van Assche G, Vermeire S, D’Haens G, Baert F, Noman M, Aerden I, De Hertogh G, Geboes K, Hiele M, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128:856–861. doi: 10.1053/j.gastro.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Doherty GA, Miksad RA, Cheifetz AS, Moss AC. Comparative cost-effectiveness of strategies to prevent postoperative clinical recurrence of Crohn’s disease. Inflamm Bowel Dis. 2012;18:1608–1616. doi: 10.1002/ibd.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ananthakrishnan AN, Hur C, Juillerat P, Korzenik JR. Strategies for the prevention of postoperative recurrence in Crohn‘s disease: results of a decision analysis. Am J Gastroenterol. 2011;106:2009–2017. doi: 10.1038/ajg.2011.237. [DOI] [PubMed] [Google Scholar]
- 71.Tytgat GN, Mulder CJ, Brummelkamp WH. Endoscopic lesions in Crohn‘s disease early after ileocecal resection. Endoscopy. 1988;20:260–262. doi: 10.1055/s-2007-1018188. [DOI] [PubMed] [Google Scholar]
- 72.Achkar JP. Endoscopic evaluation of postoperative recurrence in the patient with Crohn’s disease. Tech Gastrointest Endosc. 2004;6:165–168. [Google Scholar]
- 73.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 74.Bordeianou L, Stein SL, Ho VP, Dursun A, Sands BE, Korzenik JR, Hodin RA. Immediate versus tailored prophylaxis to prevent symptomatic recurrences after surgery for ileocecal Crohn’s disease? Surgery. 2011;149:72–78. doi: 10.1016/j.surg.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 75.De Cruz P, Kamm M, Hamilton A, Ritchie K, Krejany S, editors. Gorelik A, Liew D, Prideaux L, Lawrance I, Andrews J, Bampton P, Sparrow M, Florin T, Gibson P, Debinski H, Gearry R, Macrae F, Leong R, Kronborg I, Radford-Smith G, Selby W, Johnston M, Woods R, Elliot R, Bell S. Optimizing post-operative Crohn’s disease management: Best drug therapy alone versus endoscopic monitoring with treatment step-up. The POCER study. 2013. Presented at 21st United European Gastroenterology Week; Berlin, Germany. 2012. [Google Scholar]
- 76.Domènech E, Hinojosa J, Nos P, Garcia-Planella E, Cabré E, Bernal I, Gassull MA. Clinical evolution of luminal and perianal Crohn’s disease after inducing remission with infliximab: how long should patients be treated? Aliment Pharmacol Ther. 2005;22:1107–1113. doi: 10.1111/j.1365-2036.2005.02670.x. [DOI] [PubMed] [Google Scholar]
- 77.Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70.e5; quiz e31. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 78.Viazis N, Giakoumis M, Koukouratos T, Anastasiou J, Katopodi K, Kechagias G, Anastasopoulos E, Saprikis E, Chanias M, Tribonias G, et al. Long term benefit of one year infliximab administration for the treatment of chronic refractory pouchitis. J Crohns Colitis. 2013;7:e457–e460. doi: 10.1016/j.crohns.2013.02.018. [DOI] [PubMed] [Google Scholar]


