Abstract
Videocapsule endoscopy (VCE) has revolutionized our ability to visualize the small bowel mucosa. This modality is a valuable tool for the diagnosis of obscure small bowel Crohn’s disease (CD), and can also be used for monitoring of disease activity in patients with established small-bowel CD, detection of complications such as obscure bleeding and neoplasms, evaluation of response to anti-inflammatory treatment and postoperative recurrence following small bowel resection. VCE could also be an important tool in the management of patients with unclassified inflammatory bowel disease, potentially resulting in reclassification of these patients as having CD. Reports on postoperative monitoring and evaluation of patients with ileal pouch-anal anastomosis who have developed pouchitis have recenty been published. Monitoring of colonic inflammatory activity in patients with ulcerative colitis using the recently developed colonic capsule has also been reported. Capsule endoscopy is associated with an excellent safety profile. Although retention risk is increased in patients with small bowel CD, this risk can be significanty decreased by a routine utilization of a dissolvable patency capsule preceding the ingestion of the diagnostic capsule. This paper contains an overview of the current and future clinical applications of capsule endoscopy in inflammatory bowel disease.
Keywords: Small bowel videocapsule endoscopy, Crohn’s disease, Pouchitis, Indeterminate inflammatory bowel disease, Ileal pouch-anal anastomosis, Patency capsule
Core tip: Videocapsule endoscopy has revolutionized our ability to visualize the small bowel mucosa. This modality is a valuable tool for the diagnosis of obscure small bowel Crohn’s disease (CD), and can also be used for monitoring of disease activity, detection of complications, evaluation of therapeutic response and postoperative recurrence in established CD, evaluation of the small bowel in patients with unclassified inflammatory bowel disease and pouchitis. Monitoring of colonic inflammation in patients with ulcerative colitis has also been reported. This manuscript contains an overview of the current and future clinical applications of capsule endoscopy in inflammatory bowel disease.
INTRODUCTION
In the past, the small bowel has largely been inaccessible to direct endoscopic examination, with only the duodenum, proximal jejunum and terminal ileum being subject to direct visualization by a conventional endoscope. This paradigm changed dramatically with the invention and introduction of small bowel videocapsule endoscopy (VCE) in 2000[1]. The first wireless capsule, manufactured by Given Imaging (Yokneam, Israel) was approved for clinical use in United States and Europe in 2001[2]. Several other manufacturers subsequently released their own versions of VCE. This technology has been extensively used for the diagnosis and monitoring of patients with inflammatory bowel disease (IBD), mostly Crohn’s disease (CD). About 30% of the patients with CD have exclusive small bowel involvement[3], and their diagnosis will frequently be missed if based solely on ileocolonoscopic findings. VCE is now also considered an important technique for monitoring small bowel CD, and has also been employed in management of patients with unclassified IBD and ulcerative colitis.
The aim of the current review is to outline the diagnostic role of VCE in the diagnosis and monitoring of inflammatory bowel disease, in particular small-bowel CD.
DIAGNOSIS OF CD
Characteristic endoscopic findings
Several VCE findings are frequently associated with CD: ulcerations, erythema, mucosal edema, loss of villi, strictures and mucosal fissures (Figure 1)[4]. Unfortunately, none of these findings is specific for CD. In fact, minor small bowel lesions maybe present in up to 10% of normal subjects[5]. As VCE lacks tissue-sampling capabilities, it cannot confirm the etiology of the observed lesions. The most common mimicker of CD in the small bowel is non-steroidal anti-inflammatory medication (NSAID)-induced enteropathy that may manifest with lesions indistinguishable from those of CD. Such lesions, appearing as early as 2 wk from the onset of NSAID therapy, can be demonstrated in 70% of chronic NSAID users[6,7]. Thus, VCE should be reserved for patients with high clinical index of suspicion for CD. Patients who are candidates for VCE should be instructed to avoid NSAIDs for at least 1 mo before the examination. Similar bowel mucosal lesions may result from multitude of other pathologies, such as lymphoma, radiation enteritis, HIV with opportunistic infection, intestinal tuberculosis and Behcet’s disease[5].
Figure 1.
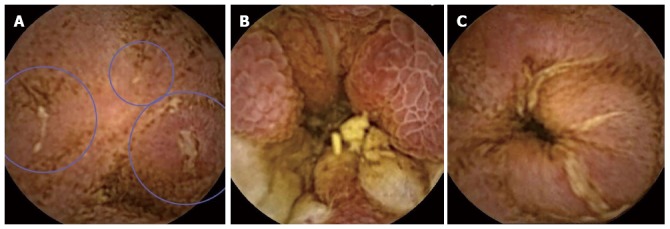
Videocapsule endoscopy findings. A: Small ulcers (encircled); B: Edematous mucosa; C: Ulcerated stenosis (SBII capsule, RAPID and Imaging software, Given Imaging, Yokneam, Israel).
Diagnostic scores
The criteria for diagnosis of CD using VCE have not been well established. The most commonly used validated diagnostic index is the Lewis score[8]. This score divides the small bowel into 3 tertiles (dividing the small bowel transit time in 3) and uses an algorithm that assigns points to various findings (mucosal edema, ulcers, strictures) characteristic for CD in each of the tertiles, taking in account the severity and the reproducibility of each finding. The final score represents the number of points accumulated by the most significantly involved tertile. The Lewis score is incorporated in the software used for decoding, reading and interpretation of VCE images obtained by PillCAM (RAPID). A score < 135 is designated as normal or clinically insignificant mucosal inflammatory changes, a score between 135 and 790 indicates mild, and a score ≥ 790 moderate to severe inflammation, respectively. An additional score known as capsule endoscopy CD activity index (CECDAI or Niv score), was recently proposed (Table 1)[9]. This score incorporates three main characteristics of CD: inflammation, extent of disease, and strictures, in both the proximal and distal segments of the small bowel. It should be noted that while these scores attempt to quantify the severity and extent of small bowel (SB) CD, the lesions are not pathognomonic and may represent other causes of bowel inflammation.
Table 1.
A comparison of 2 capsule endoscopy scoring indices for quantification of mucosal inflammation
| Parameter |
Lewis score[8] |
CECDAI[80] |
|||
| Number/quality | Longitudinal extent | Descriptors | Parameter | Descriptors | |
| Villous appearance | Normal/edematous | Short segment/long segment/whole tertile | Single/patchy/diffuse | Inflammation score | None to large ulcer (> 2 cm) |
| Ulceration | Non/single/few/multiple | Short segment/long segment/whole tertile | < 25%, 25%-50%, > 50% | Extent of disease | No disease to diffuse (3 segments) |
| Stricture | Non/single/few/multiple | Ulcerated/non-ulcerated | Traversed/non-traversed | Stricture score | None to complete obstruction |
| Small bowel segmentation | Tertiles (strictures for the entire length of the examination) | Proximal to distal small bowel | |||
| Score | < 135: Normal or clinically insignificant inflammation | 0 (normal examination)-26 (severe inflammation) | |||
| 135-790: Mild inflammation | |||||
| 790: Moderate to severe. | |||||
CECDAI: Capsule endoscopy Crohn’s disease activity score.
VCE was also utilized for diagnosis of SB CD in patients primarily presenting with extraintestinal manifestations of IBD. Arhtropathy is the most common extraintestinal manifestation in IBD, occurring in 6%-46% of the patients[10], and frequently manifesting even before the onset of bowel disease. Capsule endoscopy may be a valuable tool in evaluation of these patients, especially if conventional ileocolonoscopy is unappealing to the patient[11]. Spondyloarthroathy can be diagnosed in up to 30% of IBD patients[12,13]. Capsule endoscopy can demonstrate small bowel lesions consistent with CD in 33% of these patients (twice as many as conventional ileocolonoscopy[14]).
VCE vs other modalities for the diagnosis of CD
The yield of VCE for the diagnosis of CD was compared to that of cross-sectional imaging modalities such as small bowel follow-through (SBFT), computer tomography enterography (CTE) and magnetic resonance enterography (MRE) in multiple studies (Table 2). Patients with suspected small bowel stenosis were excluded from VCE evaluation in these studies. The superiority of VCE over small bowel follow-through and enteroclysis has been repeatedly demonstrated in multiple studies[15-18].
Table 2.
Key studies evaluating the diagnostic yield of capsule endoscopy for Crohn’s disease
| Modality | Ref. | Number of patients | Diagnostic yield of VCE | Diagnostic yield of the compared modality | IY | P value |
| CTE | Eliakim et al[81] | 35 | 77% | 20% | 47% | < 0.05 |
| Hara et al[82] | 17 | 71% | 53% | 18% | NA | |
| Voderholzer et al[83] | 41 | 61% | 49% (CT enteroclysis) | 12% | < 0.04 | |
| Solem et al[84] | 40 | 83% | 83% | 0 | NS | |
| MRE | Albert et al[85] | 27 | 93% | 78% | 15% | NS |
| Crook et al[22] | 19 | 93% | 71% | 18% | NS | |
| Jensen et al[20] | 93 | 100% | 86% | 14% | NS | |
| Ileocolonoscopy | Hara et al[82] | 17 | 71% | 65% | 6% | NS |
| Solem et al[84] | 40 | 83% | 74% | 9% | NS | |
| Leighton et al[86] | 80 | 55% | 25% | 30% | NA |
VCE: Videocapsule endoscopy; CTE: Computed tomography enterography; MRE: Magnetic resonance enterography; IY: Incremental yield; NA: Not available; NS: Non significant.
A recent meta-analysis demonstrated an incremental diagnostic yield (IY) of VCE in comparison to CTE in both suspected and established CD patients (IY, 47%; 95%CI: 31%-63%, P < 0.00001; and 32%; 95%CI: 16%-47%, P < 0.0001), respectively[19]. A prospective trial evaluated the diagnostic accuracy of VCE, MRE and CTE in 93 patients with suspected CD as compared to ileocolonoscopy. The sensitivity and specificity for diagnosis of CD of the terminal ileum was 100% and 91% by CE, 81% and 86% by MRE, and 76% and 85% by CTE, respectively. There was statistical difference in sensitivity compared with CTE, but only a trend in comparison with MRE. Specificity was not significantly different between the modalities. Proximal small bowel CD was detected in 18 patients by using CE, compared with 2 and 6 patients using MRE or CTE, respectively (P < 0.05)[20]. In earlier studies, VCE and MRE were reported to have comparable accuracy. Overall, VCE is more accurate in diagnosing subtle small bowel lesions and MRE in diagnosing intramural inflammation, stricturing complications and extra-intestinal manifestations[19,21,22]. The superior sensitivity of VCE clinical for proximal small bowel disease is a potentially important diagnostic advantage, as proximal small bowel disease has recently been demonstrated to be a significant negative prognostic factor[23].
Importantly, data acquired by different endoscopic and imaging modalities can be combined to improve the diagnostic accuracy, utilizing the specific advantages and strengths of each modality.
VCE in established CD
VCE is a potentially important but currently underutilized tool for monitoring of SB CD. In the latter years, the leading treatment paradigm in IBD has shifted form merely controlling symptoms to reversing the underlying inflammation, as expressed by objective surrogate markers such as laboratory inflammatory markers and endoscopic evidence of mucosal healing[24]. Capsule endoscopy provides meaningful information on the inflammatory burden in the small bowel mucosa, similarly to the role of conventional ileocolonoscopy for the colon and the terminal ileum. Bowel stenosis should be ruled out before VCE is performed in established CD due to the increased risk of capsule retention (about 5%). Routine use of patency capsule diminishes the risk of retention to almost negligible (see below).
VCE could be particularly useful in the following clinical scenarios in known CD (Table 3): (1) Monitoring of mucosal healing; (2) Detecting postsurgical recurrence; and (3) Discrepancy between clinical and laboratory data and endoscopic findings.
Table 3.
Key studies describing the role of videocapsule endoscopy in established Crohn’s disease
| Indication | Ref. | n | Inclusion criteria | Diagnostic criteria | Results |
| Mucosal healing | Efthimyou et al[28] | 40 | Patients with active CD (CDAI > 150) who responded to anti-inflammatory treatment, VCE was performed before and after treatment | Number of aphthous ulcers/large ulcers/length of involved segment | Only number of large ulcers correlated with response (8.3 ± 1.4 and 5/0.8, 95%CI: 0.8-5.9, P < 0.01) |
| Postoperative recurrence | Bourreille et al[31] | 31 | CD with ileocolonic anastomosis | Rutgeerts score ≥ 1 | VCE-21/31 (68%), IC-19/31 (61%) |
| Pons Beltrán et al[32] | 24 | CD with ileocolonic anastomosis | Rutgeerts score ≥ 2 | VCE-14/22 (55%), IC-6/24 (25%) | |
| Unexplained symptoms | Dubcenco et al[34] | 28 | Active CD patients | ≥ 3 ulcers | VCE-23 (82%), IC-14 50%, barium radiography-9 (32%) |
| Dussault et al[35] | 25 | Active CD patients with unexplained symptoms | Severity graded by number and appearance of ulcers and presence of stenosis | Active SB inflammation: 11/25 (44%) |
In 6 patients treated with immunomodulators, biologics or corticosteroids, a significant improvement was demonstrated in all 3 parameters. CD: Crohn’s disease; VCE: Vidoecapsule endoscopy; IC: Ileocolonoscopy; SB: Small bowel.
Mucosal healing: Mucosal healing, defined as absence of visible endoscopic inflammation, has emerged as a very important marker of long-term clinical efficacy associated with decreased risk of long-term complications in both ulcerative colitis (UC) and CD[24-27]. Conventional ileocolonoscopy is the current gold-standard modality for assessment of mucosal healing. A small prospective study had evaluated monitoring of mucosal healing with VCE performed before and after treatment for acute CD flare-up[28]. Forty patients with CD flares were included in the study and all have responded to treatment within 4-8 wk of treatment. Three parameters (number of large ulcers, number of aphthous ulcers and percentage of time with lesions visible) were examined. Of these only the first one improved significantly. In a subgroup of patients treated with corticosteroids combined with immunomodulators or biologics, a significant improvement in all three parameters was demonstrated. The most important limitations of this study were a significant heterogeneity in the instituted treatment, with majority of patients treated with mesalamine or corticosteroids, along with absence of a validated scoring system for mucosal inflammation. The data from our center demonstrated a significant reduction in the Lewis score in 4 patients with spondyloarthropathy and newly diagnosed SB CD after 6 mo of treatment with Adalimumab[14]. Importantly, no diagnostic score, including the commonly used Lewis score, has been validated for evaluation of mucosal healing in SB CD.
Postoperative CD recurrence: Recurrence of SB CD in the neo-terminal ileum following surgical resection can be demonstrated in 73%-93% of the patients within 1 year of ileocolonic resection[29,30]. SB lesions associated with postoperative recurrence are frequently quantified using the Rutgeerts score[29]. The accuracy of VCE in detection of postoperative recurrence was evaluated in 31 patients[31]. Recurrence occurred in 21 patients (68%) and was detected by ileocolonoscopy in 19 patients. Sensitivity of VCE using the Rutgeerts score was 62%-76% and specificity was 90%-100%. The severity of lesions as assessed by both methods correlated significantly (P < 0.05). In an additional study, 24 patients with CD, neo-terminal ileum recurrence defined as Rutgeerts score > 2 was demonstrated by ileocolonoscopy in 25% and capsule endoscopy in 62% (VCE was performed in 22/24 patients due to failure to excrete the patency capsule in 2 patients). Capsule endoscopy detected proximal SB lesions inaccessible by ileocolonoscopy in 13 patients[32]. VCE is an attractive monitoring modality for postoperative patients, providing a non-invasive and accurate visualization of the entire small bowel including the neo-terminal ileum.
Unexplained symptoms: Many symptoms of CD, such as diarrhea, abdominal pain, bloating, can be attributed to a multitude of etiologies other than active inflammation [underlying irritable bowel syndrome (IBS), bacterial overgrowth, bile salt diarrhea etc.]. Clear identification of inflammatory etiology is of crucial importance and may lead to significant changes in the treatment, such as initiation or escalation of anti-inflammatory treatment. Negative VCE results are also of clinical importance, as this would lead to diagnosis and initiation of treatment for a concomitant condition such as IBS, and prevent further unnecessary and expensive escalation of an anti-inflammatory regimen. Clinical indices and laboratory inflammatory markers may indicate ongoing inflammation, but lack sensitivity. In a study including 140 patients with CD, the Spearman’s rank correlation of simple endoscopic index with fecal calprotectin, CRP, blood leukocyte count and CDAI was 0.75, 0.53, 0.42 and 0.38, respectively[33]. Although ileocolonoscopy is a gold standard test for identification of active inflammation, it would potentially miss lesions located proximally to the ileocecal valve. Dubcenco et al[34] have prospectively evaluated 28 symptomatic Crohn’s patients with ileocolonoscopy, barium radiography and capsule endoscopy. Active disease was identified by VCE, ileocolonoscopy and barium radiography in 82%, 49% and 32% of patients, respectively. In a study by Dussault et al[35], in 25 out of the included symptomatic CD patients, VCE was indicated for a discrepancy between clinical symptoms and diagnostic findings. Abnormal SB findings were diagnosed in 44% of the patients, and in 45% of these patients the treatment was escalated following the performance of VCE.
VCE can also be used for monitoring of ileal recurrence in CD patients following bowel resection and ileocolonic anastomosis. In one study, VCE detected CD recurrence in 15 (62%) patients, whereas ileocolonoscopy detected inflammatory lesions in the neo-terminal ileum in only 6 (25%) patients[32]. VCE was also evaluated for a potential role in the assessment of mucosal healing after drug therapy in CD[28].
Therapeutic yield of VCE in established CD
VCE frequently produces clinically significant data that can lead to a change in a therapeutic management. In a retrospective series of 71 CD patients, medical treatment was changed in 38 (53%) of the patients within 3 mo of VCE performance[35]. In an additional series that included 86 patients with established CD, 61.6% had a change in medication in the 3 mo after the CE, with 39.5% initiating a new anti-inflammatory medication[36].
VCE in unclassified IBD and UC
Colonic inflammatory bowel disease cannot be classified as CD or UC using current colonoscopic and pathologic criteria in 10%-15% of the patients[37]. At least 30% of these patients with unclassified IBD (IBDU) will be reclassified as CD during the course of their illness[38], usually after identification of small bowel lesions. Correct classification of the patients is especially important when deciding on surgical intervention, as rates of chronic pouchitis, fistula formation and pouch failure after ileal pouch-anal anastomosis (IPAA) are significantly higher in patients with CD[39].
Several small studies have evaluated the utility of VCE for reclassification of IBDU patients. Mow et al[40] have described 22 patients with either isolated colitis or chronic symptoms following IPAA (n = 18) who were evaluated with VCE. All patients had prior unremarkable small bowel radiography. Multiple ulcerations (3 and above) considered diagnostic for CD were identified in 68 of 18 patients. Mehdizadeh et al[41] described 120 patients with a history of UC or IBDU who underwent VCE. Findings consistent with SB CD were demonstrated in 15.8% of the patients. Eighteen/19 patients with CD diagnosed by VCE have previously underwent SBFT, with positive findings in only 1 patient. Another series included 30 patients with IBDU, in whom CD (defined as 3 or more SB ulcers) was identified in 5. Interestingly, in 6/25 VCE-negative patients CD was diagnosed on a subsequent ileocolonoscopy with biopsies[42]. In a series of pediatric patients, 5/7 patients initially diagnosed as UC or IBDU were reclassified as having CD as a result of VCE findings[43] (Table 4).
Table 4.
Key studies describing the role of videocapsule endoscopy in unclassified Inflammatory bowel disease pouchitis and ulcerative colitis
| Ref. | Indication | n | Definition of CD | Results n (%) | |
| IBD-U | Mehdizadeh et al[41] | IBDU | 6 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 1 (17) |
| SB findings | |||||
| Maonoury et al[42] | IBDU | 30 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 5 (17), CD | |
| Cohen et al[43] | IBDU | 2 | NA | 1 (50), CD | |
| s/p IPAA | Mow et al[40] | Isolated colitis | 6 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 3 (50)-definite CD, 2 (20), possible CD |
| Mehdizadeh et al[41] | Persistent symptoms after IPAA | 21 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 7 (33%) | |
| SB findings | |||||
| Calabrese et al[47] | Chronic pouchitis after IPAA | 15 | NA | Gastric or SB lesions, 15 (100) | |
| Ulcerative colitis | Mow et al[40] | Isolated colitis | 12 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 3 (25)-definite CD, 3 (25), possible CD |
| Mehdizadeh et al[41] | Treatment-resistant UC | 22 | > 3 ulcerations-diagnostic of CD, 1-2 ulcerations-suggestive of CD | 2 (9), SB findings | |
| Cohen et al[43] | UC | 5 | NA | 4 (80), CD | |
| Higurashi et al[44] | UC | 23 | Lewis score | 13 (56.5), small bowel lesions | |
| 9 (39), Lewis score > 135 |
IBDU: Unclassified inflammatory bowel disease; IPAA: Ileoanal pouch anastomosis; CD: Crohn’s disease; UC: Ulcerative colitis; SB: Small bowel.
Higurashi et al[44] evaluated small bowel inflammation in patients with established UC. Of the 23 UC patients, 13 (57%) showed small-bowel lesions, and 8 (35%) had erosions, as opposed to 2/23 (7%) and 1/23 (4%) in the control group. In 9/23 patients with UC, the Lewis score of inflammation was consistent with mild to moderate small bowel inflammation (between 135 and 790). The clinical and pathological significance of these lesions is unclear (repeated biopsies were performed in only 2 patients, but these results are of great interest and emphasize the possible risk of misdiagnosis in many IBD patients.
VCE in evaluation of pouchitis in patients after IPAA
IPAA provides a continence-preserving surgical option in patients with UC unresponsive or unwilling to continue anti-inflammatory therapy, or those who have developed complications (such as colonic stenosis, colonic dysplasia etc.) that require total colectomy. The procedure is technically demanding and is associated with a significant incidence of postoperative complications, the most common being chronic and acute pouchitis and “de novo” CD[45]. Symptoms and endoscopic lesions consistent with chronic pouchitis are reported in 10%-59% in patients with UC, and even more frequently in patients with CD[46]. It is commonly argued that at least a subgroup of these patients actually represent a previously undiagnosed CD. In a series of 15 UC patients with chronic pouchitis, diffuse lesions involving the stomach or different segments of the small bowel were demonstrated in all patients[47]. Similar lesions were demonstrated in 27% of the control patients. Unfortunately, histological evaluation (showing non-specific inflammation) was available for only 2 patients with gastric involvement. The clinical significance of these lesions is unclear (Table 4).
The role of VCE in preoperative evaluation of UC/IBDU patients was examined in one retrospective series. The study evaluated the incidence of acute pouchitis, chronic pouchitis and de novo CD within 12 mo of the surgery in patients with and without pathological findings on a preoperative VCE. No significant difference was demonstrated for any of the outcomes[48]. However, an important limitation of this study was the definition of “positive” VCE as any ulceration or lesion, possibly leading to a high false positive rate. In addition, a significant selection bias stemming from the retrospective design of the study (patients with a high preoperative probability of CD were not likely to undergo IPAA) interferes with the interpretation of the results. In our opinion, preoperative evaluation of IPAA candidates with VCE merits further evaluation in prospective studies.
Anemia is another frequent complication of IPAA surgery, occurring in about 17% of UC patients[49]. Possible etiologies may include newly discovered CD, arteriovenous malformations, celiac disease and others. In a series of post-IPAA patients with chronic anemia, VCE detected the cause of anemia in 29.4%. Sixty percent of the patients were diagnosed as having a new-onset CD[50].
COLONIC CAPSULE ENDOSCOPY IN UC
A colonic capsule [PillCam colonic capsule (PCCE) Given Imaging, Yokneam, Israel] has been available for colorectal cancer screening for several years. This device includes 2 cameras which records 2 different sets of images. The colonic capsule was compared with colonoscopy with promising results, with the second-generation capsule reaching sensitivity of 88% for detection of polyps > 6 mm in comparison to colonoscopy[51,52].
PCCE was evaluated for diagnosis and monitoring of UC. In the study by Ye et al[53], 25 patients were evaluated for presence and severity (Mayo Score) of UC by PCCE and conventional colonoscopy. A significant correlation in the severity (k = 0.751, P < 0.001) and extent (k = 0.522, P < 0.001) of UC between the PCCE and conventional colonoscopy was demonstrated. Similar findings were reported by Hosoe et al[54]. However, PCCE is not suitable for monitoring of dysplasia and cancer surveillance in UC patients due to its lack of tissue sampling ability.
Contraindications and risks
The main complication of CE is capsule retention, defined as a failure to excrete the capsule for 2 wk or more, requiring directed medical, endoscopic or surgical intervention[55]. CE is contraindicated in patients with known bowel strictures or swallowing disorders, and history of bowel obstruction. Recent abdominal surgery is a relative contraindication[56]. In patients with obstructive symptoms or one of the aforementioned risk factors, cross-sectional imaging should be performed before VCE; however, absence of strictures on cross-sectional imaging does not preclude capsule retention[57]. The rate of capsule retention depends on the indication for performance of VCE[58]: 0% in healthy controls[59], 1.4% in obscure gastrointestinal bleeding[60-62], 1.48% in suspected CD[63-65], 5%-13% in known CD[40,66] and 21% in suspected small bowel obstruction[67]. Slow transit of the capsule, with delayed excretion of the capsule is very common, seen in up to 20% of the cases[56]. A retained capsule is usually asymptomatic[68], but may be associated with symptoms of partial or complete bowel obstruction. Only 6 cases of bowel perforation were reported[56,69]. Usually, the retained capsule can be extracted with surgery or enteroscopy. If the cause is an inflammatory stricture, corticosteroids have been useful in some cases. No consensus on the timing of intervention exists, and it is unclear how long one should wait before intervention in asymptomatic patients.
Patency capsule
The patency capsule has the same shape and dimensions as the real videocapsule. It is constructed of cellophane with wax plugs at either end and it contains lactose mixed with 10% barium to make it radiopaque. The wax plugs have holes that allow succus entericus to dissolve the lactose, resulting in capsule disintegration[70]. The dissolution of the patency capsule (Agile, Given Imaging) starts to occur after 30 h. The patency capsule can be detected by radiography or a portable radiofrequency scanner. When the patency capsule is successfully excreted or not detectable on radiography in the small bowel at 30 h post ingestion, it is usually safe to perform the diagnostic VCE. If the patency capsule location is uncertain, it is possible to localize it with the assistance of contrast or air enhanced fluorography or CT[71]. The rate of excretion of the patency capsule varies from 45%-88%[58,72-75], depending upon patient selection. In a series of 77 CD patients who underwent a patency capsule examination before proceeding to diagnostic VCE, the patency capsule was not excreted within 30 h in 7.8% of the patients[35]. The main complication of patency capsule is mild abdominal pain, occurring in about 20% of the patients. Clinically evident intestinal obstruction requiring surgical intervention was reported in very few cases[58]. This phenomenon may be explained by the lodging of the capsule in sites of obstruction not easily assessable by intestinal fluids necessary for the dissolution of the lactose in the patency capsule[76]. The rate of uneventful completion of the VCE examination after successful excretion of the patency capsule approximates 100%, even though excretion times may vary between patients[58]. In cases of unsuccessful patency capsule procedure, the small bowel should be investigated by alternative diagnostic modalities such as cross sectional imaging (MR-E).
CONCLUSION
VCE possesses several important diagnostic advantages for IBD patients, mainly excellent visualization of the entire small bowel mucosa and excellent tolerability. The main challenge for further implementation of VCE in monitoring of IBD patients is an establishment of a validated quantitative score for assessment of mucosal healing and postoperative recurrence, that would allow routine utilization of this modality in both clinical practice and clinical trials. This could be especially important in CD, where outcomes in clinical trials are frequently assessed using surrogate markers (clinical scores, inflammatory markers) and evaluation of the mucosal healing limited to the colon and terminal ileum, that frequently does not reflect the inflammatory burden of the small bowel.
Small bowel lesions are frequently diagnosed in patients initially diagnosed with UC or after IPAA. The true clinical significance of these lesions, and whether they actually represent undiagnosed cases of CD is an important question that merits further clinical and translational studies.
Another important pitfall limiting the use of VCE for CD monitoring is the clinician’s reluctance to perform VCE in these patients due to an exaggerated concern of retention. However, routine utilization of a patency capsule improves the safety of this procedure significantly, rendering the actual risk of retention extremely low. However, patency capsule frequently results in additional costs.
Further technological enhancements in the future may potentially lead to a further expansion of the indications for capsule endoscopy in IBD (Table 5). These improvements may include a development of an externally operated capsule, that has already been attempted[77,78]. An additional significant limitation of the capsule endoscopy is a lack of sampling ability, diminishing its usefulness for monitoring of neoplasms and colonic or small bowel dysplasia. In the future, additional technological features that are under development including tissue diagnosis capabilities, fluid aspiration, drug delivery and therapeutic (coagulation) capabilities may further increase the clinical utility of this modality[79].
Table 5.
Potential future technological developments in capsule endoscopy relevant to inflammatory bowel disease
VCE is a very important tool for diagnosis of CD, and also has a potentially significant role in the therapeutic monitoring of these patients. Capsule endoscopy may also provide important clinical information for patients with IBDU, UC and pouchitis, with an excellent tolerability and safety profile. Indications for VCE in IBD are likely to increase in the future with further technological and clinical developments.
Footnotes
Supported by Speaker bureau for Given Imaging to Seidman EG
P- Reviewers: Dignass AU, Geboes K, Triantafyllou K, Van Gossum A S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Terano A. Capsule endoscopy: past, present, and future. J Gastroenterol. 2008;43:93–99. doi: 10.1007/s00535-007-2153-6. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG, et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618–637. doi: 10.1055/s-0029-1214790. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Meir S. Review article: capsule endoscopy - are all small intestinal lesions Crohn’s disease? Aliment Pharmacol Ther. 2006;24 Suppl 3:19–21. doi: 10.1111/j.1365-2036.2006.03054.x. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 7.Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, Bjarnason I. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 9.Niv Y, Ilani S, Levi Z, Hershkowitz M, Niv E, Fireman Z, O’Donnel S, O’Morain C, Eliakim R, Scapa E, et al. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21–26. doi: 10.1055/s-0031-1291385. [DOI] [PubMed] [Google Scholar]
- 10.Atzeni F, Defendenti C, Ditto MC, Batticciotto A, Ventura D, Antivalle M, Ardizzone S, Sarzi-Puttini P. Rheumatic manifestations in inflammatory bowel disease. Autoimmun Rev. 2014;13:20–23. doi: 10.1016/j.autrev.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Taddio A, Simonini G, Lionetti P, Lepore L, Martelossi S, Ventura A, Cimaz R. Usefulness of wireless capsule endoscopy for detecting inflammatory bowel disease in children presenting with arthropathy. Eur J Pediatr. 2011;170:1343–1347. doi: 10.1007/s00431-011-1505-7. [DOI] [PubMed] [Google Scholar]
- 12.Stolwijk C, Pierik M, Landewé R, Masclee A, van Tubergen A. Prevalence of self-reported spondyloarthritis features in a cohort of patients with inflammatory bowel disease. Can J Gastroenterol. 2013;27:199–205. doi: 10.1155/2013/139702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo E, Latiano A, Palmieri O, Bossa F, Andriulli A, Annese V. Enteropathic spondyloarthropathy: a common genetic background with inflammatory bowel disease? World J Gastroenterol. 2009;15:2456–2462. doi: 10.3748/wjg.15.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopylov U, Adelson B, Starr M, Watts C, Seidman E. P171 Prevalence of Crohn’s disease in patients with spondyloarthropathies: interim analysis of the SPaCE study. J Crohns Colitis. 2013;7:S77. [Google Scholar]
- 15.de Melo SW, Di Palma JA. The role of capsule endoscopy in evaluating inflammatory bowel disease. Gastroenterol Clin North Am. 2012;41:315–323. doi: 10.1016/j.gtc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Marmo R, Rotondano G, Piscopo R, Bianco MA, Cipolletta L. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther. 2005;22:595–604. doi: 10.1111/j.1365-2036.2005.02625.x. [DOI] [PubMed] [Google Scholar]
- 17.Marmo R, Rotondano G, Piscopo R, Bianco MA, Siani A, Catalano O, Cipolletta L. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn’s disease: a prospective trial. Clin Gastroenterol Hepatol. 2005;3:772–776. doi: 10.1016/s1542-3565(05)00483-0. [DOI] [PubMed] [Google Scholar]
- 18.Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954–964. doi: 10.1111/j.1572-0241.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 19.Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–1248; quiz 1249. doi: 10.1038/ajg.2009.713. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–129. doi: 10.1016/j.cgh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Tillack C, Seiderer J, Brand S, Göke B, Reiser MF, Schaefer C, Diepolder H, Ochsenkühn T, Herrmann KA. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1219–1228. doi: 10.1002/ibd.20466. [DOI] [PubMed] [Google Scholar]
- 22.Crook DW, Knuesel PR, Froehlich JM, Eigenmann F, Unterweger M, Beer HJ, Kubik-Huch RA. Comparison of magnetic resonance enterography and video capsule endoscopy in evaluating small bowel disease. Eur J Gastroenterol Hepatol. 2009;21:54–65. doi: 10.1097/meg.0b013e32830ce7a7. [DOI] [PubMed] [Google Scholar]
- 23.Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, Proctor DD, Regueiro M, Rioux JD, Schumm PP, et al. Relationship between proximal Crohn’s disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106–112. doi: 10.1038/ajg.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanauer SB, Kirsner JB. Treat the patient or treat the disease? Dig Dis. 2012;30:400–403. doi: 10.1159/000338139. [DOI] [PubMed] [Google Scholar]
- 25.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698. doi: 10.1038/ajg.2011.103. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ. The future of inflammatory bowel disease therapy: where do we go from here? Dig Dis. 2012;30 Suppl 3:140–144. doi: 10.1159/000342742. [DOI] [PubMed] [Google Scholar]
- 27.Hommes D, Colombel JF, Emery P, Greco M, Sandborn WJ. Changing Crohn’s disease management: need for new goals and indices to prevent disability and improve quality of life. J Crohns Colitis. 2012;6 Suppl 2:S224–S234. doi: 10.1016/S1873-9946(12)60502-9. [DOI] [PubMed] [Google Scholar]
- 28.Efthymiou A, Viazis N, Mantzaris G, Papadimitriou N, Tzourmakliotis D, Raptis S, Karamanolis DG. Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis. 2008;14:1542–1547. doi: 10.1002/ibd.20509. [DOI] [PubMed] [Google Scholar]
- 29.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 30.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourreille A, Jarry M, D’Halluin PN, Ben-Soussan E, Maunoury V, Bulois P, Sacher-Huvelin S, Vahedy K, Lerebours E, Heresbach D, et al. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut. 2006;55:978–983. doi: 10.1136/gut.2005.081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533–540. doi: 10.1016/j.gie.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 33.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 34.Dubcenco E, Jeejeebhoy KN, Petroniene R, Tang SJ, Zalev AH, Gardiner GW, Baker JP. Capsule endoscopy findings in patients with established and suspected small-bowel Crohn’s disease: correlation with radiologic, endoscopic, and histologic findings. Gastrointest Endosc. 2005;62:538–544. doi: 10.1016/j.gie.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Dussault C, Gower-Rousseau C, Salleron J, Vernier-Massouille G, Branche J, Colombel JF, Maunoury V. Small bowel capsule endoscopy for management of Crohn’s disease: a retrospective tertiary care centre experience. Dig Liver Dis. 2013;45:558–561. doi: 10.1016/j.dld.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Long MD, Barnes E, Isaacs K, Morgan D, Herfarth HH. Impact of capsule endoscopy on management of inflammatory bowel disease: a single tertiary care center experience. Inflamm Bowel Dis. 2011;17:1855–1862. doi: 10.1002/ibd.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233–1244. doi: 10.1136/jcp.2003.015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliakim R. The impact of wireless capsule endoscopy on gastrointestinal diseases. South Med J. 2007;100:235–236. doi: 10.1097/01.smj.0000257405.87268.48. [DOI] [PubMed] [Google Scholar]
- 39.Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, Papadakis KA, Vasiliauskas EA. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31–40. doi: 10.1016/s1542-3565(03)00289-1. [DOI] [PubMed] [Google Scholar]
- 41.Mehdizadeh S, Chen G, Enayati PJ, Cheng DW, Han NJ, Shaye OA, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU) Endoscopy. 2008;40:30–35. doi: 10.1055/s-2007-995359. [DOI] [PubMed] [Google Scholar]
- 42.Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified) Inflamm Bowel Dis. 2007;13:152–155. doi: 10.1002/ibd.20060. [DOI] [PubMed] [Google Scholar]
- 43.Cohen SA, Gralnek IM, Ephrath H, Saripkin L, Meyers W, Sherrod O, Napier A, Gobin T. Capsule endoscopy may reclassify pediatric inflammatory bowel disease: a historical analysis. J Pediatr Gastroenterol Nutr. 2008;47:31–36. doi: 10.1097/MPG.0b013e318160df85. [DOI] [PubMed] [Google Scholar]
- 44.Higurashi T, Endo H, Yoneda M, Hosono K, Sakai E, Takahashi H, Inamori M, Uchiyama S, Kojima T, Kawana K, et al. Capsule-endoscopic findings of ulcerative colitis patients. Digestion. 2011;84:306–314. doi: 10.1159/000333086. [DOI] [PubMed] [Google Scholar]
- 45.Francone TD, Champagne B. Considerations and complications in patients undergoing ileal pouch anal anastomosis. Surg Clin North Am. 2013;93:107–143. doi: 10.1016/j.suc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Simchuk EJ, Thirlby RC. Risk factors and true incidence of pouchitis in patients after ileal pouch-anal anastomoses. World J Surg. 2000;24:851–856. doi: 10.1007/s002680010136. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese C, Fabbri A, Gionchetti P, Rizzello F, Morselli C, Liguori G, Poggioli G, Campieri M, Di Febo G. Controlled study using wireless capsule endoscopy for the evaluation of the small intestine in chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;25:1311–1316. doi: 10.1111/j.1365-2036.2007.03323.x. [DOI] [PubMed] [Google Scholar]
- 48.Murrell Z, Vasiliauskas E, Melmed G, Lo S, Targan S, Fleshner P. Preoperative wireless capsule endoscopy does not predict outcome after ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:293–300. doi: 10.1007/DCR.0b013e3181b71a2c. [DOI] [PubMed] [Google Scholar]
- 49.Oikonomou IK, Fazio VW, Remzi FH, Lopez R, Lashner BA, Shen B. Risk factors for anemia in patients with ileal pouch-anal anastomosis. Dis Colon Rectum. 2007;50:69–74. doi: 10.1007/s10350-006-0752-6. [DOI] [PubMed] [Google Scholar]
- 50.Shen B, Remzi FH, Santisi J, Lashner BA, Brzezinski A, Fazio VW. Application of wireless capsule endoscopy for the evaluation of iron deficiency anemia in patients with ileal pouches. J Clin Gastroenterol. 2008;42:897–902. doi: 10.1097/MCG.0b013e318074dd73. [DOI] [PubMed] [Google Scholar]
- 51.Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220–227. doi: 10.1055/s-0029-1243968. [DOI] [PubMed] [Google Scholar]
- 52.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 53.Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117–124. doi: 10.1111/1751-2980.12005. [DOI] [PubMed] [Google Scholar]
- 54.Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T, et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28:1174–1179. doi: 10.1111/jgh.12203. [DOI] [PubMed] [Google Scholar]
- 55.Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065–1067. doi: 10.1055/s-2005-870264. [DOI] [PubMed] [Google Scholar]
- 56.Eliakim R. Video capsule endoscopy of the small bowel. Curr Opin Gastroenterol. 2013;29:133–139. doi: 10.1097/MOG.0b013e32835bdc03. [DOI] [PubMed] [Google Scholar]
- 57.Sidhu R, Sanders DS, McAlindon ME, Kapur K. Capsule endoscopy for the evaluation of nonsteroidal anti-inflammatory drug-induced enteropathy: United Kingdom pilot data. Gastrointest Endosc. 2006;64:1035. doi: 10.1016/j.gie.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and Agile capsules. World J Gastroenterol. 2008;14:5269–5273. doi: 10.3748/wjg.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 60.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 61.Sears DM, Avots-Avotins A, Culp K, Gavin MW. Frequency and clinical outcome of capsule retention during capsule endoscopy for GI bleeding of obscure origin. Gastrointest Endosc. 2004;60:822–827. doi: 10.1016/s0016-5107(04)02019-x. [DOI] [PubMed] [Google Scholar]
- 62.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716; quiz 752, 754. doi: 10.1016/j.gie.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eliakim R, Fischer D, Suissa A, Yassin K, Katz D, Guttman N, Migdal M. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn’s disease. Eur J Gastroenterol Hepatol. 2003;15:363–367. doi: 10.1097/00042737-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Herrerías JM, Caunedo A, Rodríguez-Téllez M, Pellicer F, Herrerías JM. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy. 2003;35:564–568. doi: 10.1055/s-2003-40241. [DOI] [PubMed] [Google Scholar]
- 66.Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 67.Cheifetz AS, Lewis BS. Capsule endoscopy retention: is it a complication? J Clin Gastroenterol. 2006;40:688–691. doi: 10.1097/00004836-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Li F, Gurudu SR, De Petris G, Sharma VK, Shiff AD, Heigh RI, Fleischer DE, Post J, Erickson P, Leighton JA. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68:174–180. doi: 10.1016/j.gie.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 69.Palmer JS, Marenah K, El Madani F, Jain K, Gupta S. Small bowel perforation following capsule endoscopy: a case report. Ann R Coll Surg Engl. 2011;93:e69–e70. doi: 10.1308/147870811X590829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartmann D. Capsule endoscopy and Crohn’s disease. Dig Dis. 2011;29 Suppl 1:17–21. doi: 10.1159/000331124. [DOI] [PubMed] [Google Scholar]
- 71.Postgate AJ, Burling D, Gupta A, Fitzpatrick A, Fraser C. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig Dis Sci. 2008;53:2732–2738. doi: 10.1007/s10620-008-0210-5. [DOI] [PubMed] [Google Scholar]
- 72.Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial? Endoscopy. 2005;37:808–815. doi: 10.1055/s-2005-870220. [DOI] [PubMed] [Google Scholar]
- 73.Delvaux M, Ben Soussan E, Laurent V, Lerebours E, Gay G. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy. 2005;37:801–807. doi: 10.1055/s-2005-870241. [DOI] [PubMed] [Google Scholar]
- 74.Signorelli C, Rondonotti E, Villa F, Abbiati C, Beccari G, Avesani EC, Vecchi M, de Franchis R. Use of the Given Patency System for the screening of patients at high risk for capsule retention. Dig Liver Dis. 2006;38:326–330. doi: 10.1016/j.dld.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 75.Spada C, Shah SK, Riccioni ME, Spera G, Marchese M, Iacopini F, Familiari P, Costamagna G. Video capsule endoscopy in patients with known or suspected small bowel stricture previously tested with the dissolving patency capsule. J Clin Gastroenterol. 2007;41:576–582. doi: 10.1097/01.mcg.0000225633.14663.64. [DOI] [PubMed] [Google Scholar]
- 76.Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49–58. doi: 10.1055/s-2005-921176. [DOI] [PubMed] [Google Scholar]
- 77.Keller J, Fibbe C, Rosien U, Layer P. Recent advances in capsule endoscopy: development of maneuverable capsules. Expert Rev Gastroenterol Hepatol. 2012;6:561–566. doi: 10.1586/egh.12.26. [DOI] [PubMed] [Google Scholar]
- 78.Woo SH, Kim TW, Mohy-Ud-Din Z, Park IY, Cho JH. Small intestinal model for electrically propelled capsule endoscopy. Biomed Eng Online. 2011;10:108. doi: 10.1186/1475-925X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swain P. The future of wireless capsule endoscopy. World J Gastroenterol. 2008;14:4142–4145. doi: 10.3748/wjg.14.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI) Dig Dis Sci. 2008;53:1933–1937. doi: 10.1007/s10620-007-0084-y. [DOI] [PubMed] [Google Scholar]
- 81.Eliakim R, Suissa A, Yassin K, Katz D, Fischer D. Wireless capsule video endoscopy compared to barium follow-through and computerised tomography in patients with suspected Crohn’s disease--final report. Dig Liver Dis. 2004;36:519–522. doi: 10.1016/j.dld.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–134. doi: 10.1148/radiol.2381050296. [DOI] [PubMed] [Google Scholar]
- 83.Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369–373. doi: 10.1136/gut.2004.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Solem CA, Loftus EV, Fletcher JG, Baron TH, Gostout CJ, Petersen BT, Tremaine WJ, Egan LJ, Faubion WA, Schroeder KW, et al. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–266. doi: 10.1016/j.gie.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 85.Albert JG, Martiny F, Krummenerl A, Stock K, Lesske J, Göbel CM, Lotterer E, Nietsch HH, Behrmann C, Fleig WE. Diagnosis of small bowel Crohn’s disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721–1727. doi: 10.1136/gut.2005.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leighton JA, Gralnek IM, Cohen SA, Toth E, Cave DR, Wolf DC, Mullin GE, Ketover SR, Legnani PE, Seidman EG, et al. Capsule Endoscopy Is Superior to Small-bowel Follow-through and Equivalent to Ileocolonoscopy in Suspected Crohn’s Disease. Clin Gastroenterol Hepatol. 2013:Sep 27; Epub ahead of print. doi: 10.1016/j.cgh.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 87.Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol. 2013;19:3726–3746. doi: 10.3748/wjg.v19.i24.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]


