Abstract
AIM: To investigate the early upper gastrointestinal endoscopy (endoscopy) significantly reduces mortality resulting from upper gastrointestinal (GI) bleeding.
METHODS: Upper GI bleeding was defined as 1a, 1b, 2a, and 2b according to the Forrest classification. The hemoglobin (Hb), and C-reactive protein (CRP) were examined at around the day of endoscopy and 3 mo prior to endoscopy. The rate of change was calculated as follows: (the result of blood examination on the day of endoscopy - the results of blood examination 3 mo prior to endoscopy)/(results of blood examination 3 mo prior to endoscopy). Receiver operating characteristic curves were created to determine threshold values.
RESULTS: Seventy-nine men and 77 women were enrolled. There were 17 patients with upper GI bleeding: 12 with a gastric ulcer, 3 with a duodenal ulcer, 1 with an acute gastric mucosal lesion, and 1 with gastric cancer. The area under the curve (AUC), threshold, sensitivity, and specificity of Hb around the day of endoscopy were 0.902, 11.7 g/dL, 94.1%, and 77.1%, respectively, while those of CRP were 0.722, 0.5 mg/dL, 70.5%, and 73%, respectively. The AUC, threshold, sensitivity, and specificity of the rate of change of Hb were 0.851, -21.3%, 76.4%, and 82.6%, respectively, while those of CRP were 0.901, 100%, 100%, and 82.5%, respectively.
CONCLUSION: Predictors for upper GI bleeding were Hb < 11.7 g/dL, reduction rate in the Hb > 21.3% and an increase in the CRP > 100%, 3 mo before endoscopy.
Keywords: Receiver operating characteristic, Area under curve, Sensitivity, Specificity, Gastrointestinal bleeding
Core tip: Mortality rate of upper gastrointestinal (GI) bleeding is approximately 10%. Early upper gastrointestinal endoscopy significantly reduces mortality. We attempted to search for predictors of bleeding. Patient records were analyzed retrospectively. The rate of change was calculated as follows: (the result of blood examination on the day of endoscopy - the results of blood examination 3 mo prior to endoscopy)/(results of blood examination 3 mo prior to endoscopy). Receiver operating characteristic curves were created to determine threshold values. Predictors for upper GI bleeding were hemoglobin (Hb) < 11.7 g/dL, reduction rate in the Hb > 21.3% and an increase in the C-reactive protein > 100%, 3 mo before endoscopy.
INTRODUCTION
Upper gastrointestinal (GI) tract bleeding is diagnosed and treated with upper gastrointestinal endoscopy (endoscopy)[1]. Longstreth reported an annual incidence rate of 102.0 acute upper GI bleeding hospitalizations per 100000 population from a San Diego health maintenance organization; 61.6% of the patients were diagnosed with peptic ulcers[2]. Patients with bleeding are treated endoscopically using endoscopic clipping and bipolar electrocoagulation[3]. Endoscopic clipping significantly reduces recurrent bleeding as compared with epinephrine[4]. For patients who do not respond to these therapies, arteriographic embolization is performed[5,6]. Transcatheter embolization is a second line for patients failure of endoscopical hemostasis particularly in poor condition[7]. Despite these advances, mortality from the disorder has remained at approximately 10%[8]. The disease often occurs in patients using antiplatelet agents and anticoagulants.
Early endoscopy significantly reduces the mortality by peptic ulcer bleeding[9]. Patients at risk factors for mortality should be assessed for early endoscopy[10]. Clinical predictors of increased risk for mortality are age > 65 years, shock, poor overall health status, low initial hemoglobin (Hb) levels, melena, transfusion requirement, fresh red blood on rectal examination and nasogastric aspirate, sepsis, and elevated blood urea nitrogen (BUN) or creatinine (Cr) levels[11]. However, there are no data regarding the threshold levels of Hb, BUN, and Cr to assess patients for the risk of bleeding prior to endoscopy.
Mahlknecht et al[12] report age-related change of Hb. They excluded patients who had hematological history, oncological diseases, chronic infection or inflammation. They show that Hb decreases as aging, and that average Hb is, interestingly, below normal range of Hb. When patients with low Hb are encountered, Hb of previous days is always checked. Decreasae in Hb suggested that the patients had bleeding diseases, such as gastric ulcers. Therefore, we analyzed the change rate of Hb and other parameters.
Therefore, we attempted to evaluate the usefulness of Hb, BUN, and Cr levels as predictors of bleeding. The white blood cell (WBC) count and C-reactive protein (CRP) levels were also evaluated because they were useful indicators of inflammation including peptic ulcers[13].
MATERIALS AND METHODS
In our hospital, standard Olympus endoscopes (GIF Q260, GIF XQ260) (Olympus, Tokyo, Japan) were used. Patients with bleeding were subjected to endoscopic clipping (HX-610-135) (Olympus) or argon plasma coagulation (APC300) (ERBE, Tokyp, Japan).
Patient records were analyzed retrospectively from April 2012 to March 2013. During this period, 1101 patients underwent upper GI endoscopy (endoscopy). Indications of 1101 endoscopies were screening, anemia, examination of abdominal symptoms and other reasons. We chose patients who had laboratory data around the day of endoscopy and 3 mo before endoscopy to analyze reduction rate. Patient number, thus, reduced to 156 patients. Seventeen patients with bleeding matched the criteria and were analyzed. The other patients were excluded from the present study. No patients were thalassemia or sickle cell anemia. Bleeding was found in 15 patients with a peptic ulcer, 1 patient with an acute gastric mucosal lesion (AGML), and 1 patient with gastric cancer. Bleeding was classified according to the Forrest classification[14]. Patients were categorized into the bleeding group when they met the Forrest classification because our goal was the assessment of upper GI bleeding; therefore, subjects were not restricted to those with peptic ulcers. In brief, active bleeding lesions include spurting vessels (1a), oozing vessels (1b), visible vessels (2a), and clots (2b). Patients diagnosed as 1a, 1b, 2a, and 2b were classified into the bleeding group (15 patients), and all others made up the non-bleeding group (142 patients). Gastritis was diagnosed according to the Sydney system[15]. Blood examination results were analyzed between 2 d before endoscopy and the day of endoscopy (defined as around the day of endoscopy). Data were also collected 3 mo before endoscopy to analyze the rate of change in the results. Patients were enrolled only when their data were available, around the day of endoscopy and 3 mo prior to endoscopy. The parameters analyzed in the blood examination were WBC (× 104 cells/μL), Hb (mg/dL), CRP (mg/dL), Cr (mg/dL), and BUN (mg/dL) levels. Our study was subjected to our institutional ethics committee, and approved. It was not considered a clinical trial because it was performed as part of routine clinical practice. Patient anonymity was preserved.
The rate of change was calculated as follows: (the results of blood examination on the day of endoscopy-the results of blood examination 3 mo prior to endoscopy)/(results of blood examination 3 mo prior to endoscopy). Receiver operating characteristic (ROC) curves were created with JMP 8.0.2 (SAS Institute Japan, Tokyo, Japan). We investigated the Hb, CRP, Cr, and BUN levels, and WBC count around the day of endoscopy. The rate of change in the WBC count, and Hb, CRP, Cr, and BUN levels was also analyzed. The area under the curve (AUC) was used as a measure of diagnostic efficacy. The threshold value was determined as the highest sensitivity and specificity and calculated automatically by the software on the location where a line with a slope of 45° contacted the ROC curve. The Wilcoxon signed rank test was used for statistical analysis, and P < 0.05 was considered statistically significant. Adobe Illustrator CS4 (Adobe Systems, San Jose, CA) was used to make figures.
RESULTS
Seventy-nine patients were women (age, 68.2 ± 11.5 years, mean ± SD), and 77 were men (age, 70.5 ± 12.6 years). Table 1 presents the patient number, endoscopic diagnosis, and Forrest classification. Bleeding was noted in 17 patients: 12 patients with a gastric ulcer, 3 patients with a duodenal ulcer, 1 patient with an AGML, and 1 patient with gastric cancer. Spurting-type bleeding (1a) was found in 1 patient with a peptic ulcer and 1 patient with an AGML. Their bleeding was controlled using endoscopic clipping. The other patients with bleeding were treated with endoscopic clipping or argon plasma coagulation. None of the patients died in our study. Table 2 shows laboratory data of each disease. Table 3 shows the comparison of each parameter between the bleeding and non-bleeding groups. WBC, BUN and Cr levels, and BUN/Cr were higher in the bleeding group than in the non-bleeding group, but the difference was not significant. Hb (P < 0.0001) level was lower and CRP (P = 0.009) level was higher in the bleeding group than in the non-bleeding group, with a statistical significance difference. Hb and CRP were candidates for threshold values to diagnose bleeding before endoscopy.
Table 1.
Endoscopic diagnosis and patient number
| Diagnosis | Number of patients | Number of patients with bleeding |
Forrest classification |
|||
| 1a | 1b | 2a | 2b | |||
| Gastric ulcer | 38 | 12 | 1 | 1 | 5 | 5 |
| Duodenal ulcer | 9 | 3 | 0 | 1 | 2 | 0 |
| AGML | 1 | 1 | 1 | 0 | 0 | 0 |
| Gastric cancer | 5 | 1 | 0 | 0 | 1 | 0 |
| Esophageal varix | 1 | 0 | NA | NA | NA | NA |
| Gastritis | 50 | 0 | NA | NA | NA | NA |
| Esophageal hiatus hernia | 2 | 0 | NA | NA | NA | NA |
| Gastric polyp | 5 | 0 | NA | NA | NA | NA |
| Submucosal tumor | 1 | 0 | NA | NA | NA | NA |
| Normal | 45 | 0 | NA | NA | NA | NA |
| Total | 157 | 17 | 2 | 2 | 8 | 5 |
AGML: Acute gastric mucosal lesion; NA: Not applicable.
Table 2.
Laboratory data of each disease
| Diagnosis | WBC count (/μL) | Hb (g/dL) | CRP (mg/dL) | BUN (mg/dL) | Cr (mg/dL) | BUN/Cr |
| Gastric ulcer | 8080 ± 4104 | 10.4 ± 3.2 | 1.17 ± 1.43 | 23.3 ± 14.1 | 1.01 ± 0.44 | 31.7 ± 38.4 |
| Duodenal ulcer | 5520 ± 729 | 10.8 ± 2.7 | 0.28 ± 0.21 | 11.0 ± 5.5 | 0.80 ± 0.14 | 14.9 ± 10.2 |
| AGML | 6600 | 4.8 | 0.1 | 20.7 | 0.9 | 23 |
| Gastric cancer | 5860 ± 1132 | 11.1 ± 2.3 | 0.17 ± 0.11 | 16.1 ± 3.2 | 0.80 ± 0.16 | 20.5 ± 4.7 |
| Esophageal varix | 4700 | 10.1 | 0.13 | 21.7 | 0.8 | 27.1 |
| Gastritis | 6073 ± 2032 | 13.4 ±1.9 | 0.23 ± 0.22 | 14.5 ±5.4 | 0.81 ± 0.18 | 18.7 ± 6.4 |
| Esophageal hiatus hernia | 8350 ± 495 | 11.8 ± 1.1 | 1.45 ± 1.77 | 23.5 ± 4.9 | 1.30 ± 0.71 | 20.0 ± 7.07 |
| Gastric polyp | 6480 ± 1136 | 13.3 ± 0.5 | 0.95 ± 1.20 | 13.4 ± 4.6 | 0.78 ± 0.08 | 17.0 ± 4.6 |
| Submucosal tumor | 5000 | 12 | 1 | 20 | 0.6 | 33.3 |
| Normal | 5865 ± 1516 | 13.3 ± 1.6 | 0.64 ± 0.81 | 14.4 ± 2.1 | 0.77 ± 0.19 | 18.4 ± 4.3 |
WBC: White blood cell; Hb: Hemoglobin; CRP: C-reactive protein; BUN: Blood urea nitrogen; Cr: Creatinine; AGML: Acute gastric mucosal lesion.
Table 3.
Comparison between bleeding (+) and (-) around the day of endoscopy
| Laboratory data |
Bleeding (-) |
Bleeding (+) |
Wilcoxon (P value) | ||
| Mean | 95%CI | Mean | 95%CI | ||
| WBC count (/μL) | 6370 | 5946-6795 | 6700 | 5613-7786 | 0.341 |
| Hb (g/dL) | 12.8 | 12.5-13.3 | 8.4 | 6.9-9.8 | < 0.0001 |
| CRP (mg/dL) | 0.50 | 0.346-0.656 | 1.37 | 0.29-2.44 | 0.009 |
| BUN (mg/dL) | 16.4 | 14.6-18.1 | 22.5 | 15.4-29.7 | 0.096 |
| Cr (mg/dL) | 0.83 | 0.78-0.88 | 0.92 | 0.74-1.10 | 0.302 |
| BUN/Cr | 22.0 | 17.6-26.3 | 25.1 | 17.4-32.8 | 0.196 |
Bleeding (-), patients not diagnosed as 1a, 1b, 2a, or 2b by Forrest classification; bleeding (+), patients diagnosed as 1a, 1b, 2a, and 2b.Wilcoxon: Wilcoxon signed rank test; CI: Confidence interval; WBC: White blood cell; Hb: Hemoglobin; CRP: C-reactive protein; BUN: Blood urea nitrogen; Cr: Creatinine.
Figure 1 depicts the ROC curves of each parameter around the day of endoscopy. WBC (Figure 1A), CRP (Figure 1C), BUN (Figure 1D), Cr (Figure 1E), and BUN/Cr (Figure 1F) were closer to the reference line than Hb (Figure 1B). The AUC, threshold, sensitivity, and specificity are presented in Table 4. The AUC of Hb was > 0.9, whereas that of the other parameters was lower < 0.8. The threshold value of Hb was 11.7 g/dL. For this value, sensitivity and specificity were 94.1% and 77.1%, respectively. The AUC of CRP was 0.722, which was lower than expected. The threshold value of CRP was 0.5 mg/dL. Sensitivity and specificity of this value were 70.5% and 73%, respectively. Sensitivity and specificity did not seem useful for predicting bleeding before endoscopy.
Figure 1.
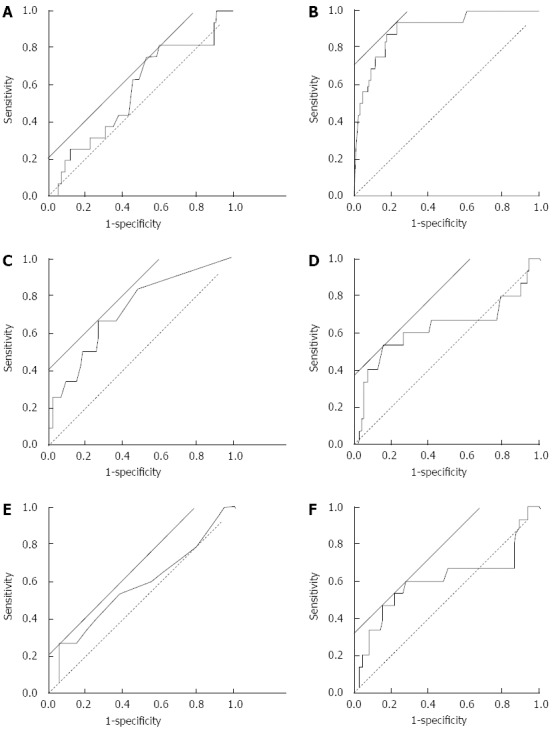
Receiver operating characteristic curves of the results of blood examination around the date of endoscopy. Receiver operating characteristic curves were created based on the results of blood examination around the day of endoscopy. The analyzed data were white blood cell count (A), hemoglobin (B), C-reactive protein (C), blood urea nitrogen (D), and creatinine (E), and blood urea nitrogen/creatinine (F). Solid straight line, a line with a slope of 45° to calculate threshold by the software (JMP 8.0.2); broken line, reference line.
Table 4.
Area under curve and threshold around the day of endoscopy
| Laboratory data | AUC | Threshold | Sensitivity | Specificity |
| WBC count | 0.573 | 5600 (/μL) | 70.5% | 46.4% |
| Hb | 0.902 | 11.7 (g/dL) | 94.1% | 77.1% |
| CRP | 0.722 | 0.5 (mg/dL) | 70.5% | 73.0% |
| BUN | 0.635 | 20.7 (mg/dL) | 52.9% | 85.0% |
| Cr | 0.580 | 1.20 (mg/dL) | 35.3% | 94.9% |
| BUN/Cr | 0.605 | 23.0 | 58.8% | 29.9% |
AUC: Area under curve; WBC: White blood cell; Hb: Hemoglobin; CRP: C-reactive protein; BUN: Blood urea nitrogen; Cr: Creatinine; BUN/Cr: The ratio of BUN divided by Cr.
Figure 2 depicts the ROC curve of the rate of change of each parameter between around the day of endoscopy and 3 mo prior to endoscopy. WBC (Figure 2A) and Cr (Figure 2E) were far from the reference line. BUN crossed the reference line (Figure 2D). Hb (Figure 2B) and CRP (Figure 2C) were close to the reference line. The AUC, threshold, sensitivity, and specificity are presented in Table 5. The AUC of Hb and CRP was 0.851 and 0.901, respectively. They were approximately 0.9. The other threshold values were < 0.6. The threshold of Hb was -21.3%. For this value, sensitivity and specificity were 76.4% and 82.6%, respectively. The threshold value of CRP was 100%. Sensitivity and specificity for this value were 100% and 82.5%, respectively.
Figure 2.
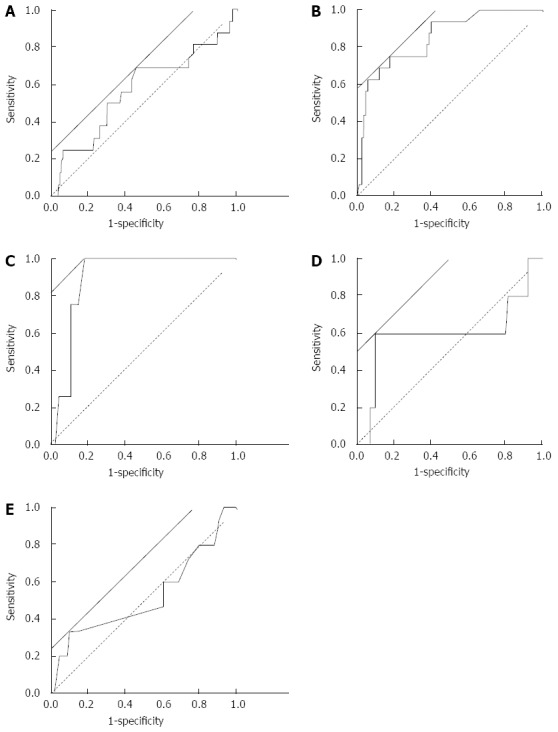
Receiver operating characteristic curves of the results of change rate of blood examination. Receiver operating characteristic curves were created based on the rate of change in the results of blood examination. The rate of change rate was calculated as a comparison between around the day of endoscopy and 3 mo prior to the date of endoscopy (see text). The analyzed data were white blood cell count (A), hemoglobin (B), C-reactive protein (C), blood urea nitrogen (D), and creatinine (E). Solid straight line, a line with a slope of 45° to calculate threshold by the software (JMP 8.0.2); broken line, reference line.
Table 5.
Area under curve and threshold of the rate of change rate
| Laboratory data | AUC | Threshold | Sensitivity | Specificity |
| WBC count | 0.577 | 6.0% | 64.7% | 55.3% |
| Hb | 0.851 | -21.3% | 76.4% | 82.6% |
| CRP | 0.901 | 100% | 100% | 82.5% |
| BUN | 0.592 | 50.3% | 58.8% | 89.6% |
| Cr | 0.527 | -14.3% | 35.3% | 90.4% |
AUC: Area under curve; WBC: White blood cell; Hb: Hemoglobin; CRP: C-reactive protein; BUN: Blood urea nitrogen; Cr: Creatinine.
DISCUSSION
A low Hb level is strongly associated with upper GI bleeding. Wong et al[16] followed up with 3386 patients with bleeding peptic ulcers after initial hemostasis. They found that an Hb level of < 10 g/dL is a predictive value for rebleeding (OR = 1.87; 95%CI: 1.18-2.96). Heldwein et al[17] reported that Forrest 1a and 2a classifications are associated with a poor prognosis, and a decreased Hb level and a requirement for blood transfusion are prospective values for emergency endoscopy. These reports focus on the prediction of rebleeding or emergency. In our study, the correlation between blood examination and bleeding diagnosed with endoscopy was analyzed. Our data clearly show that a low Hb level indicated bleeding from the upper GI tract. Similar to our study design, Giese et al[18] retrospectively analyzed the medical records of patients who underwent emergency endoscopy. The relative risks (P value) of the WBC count (> 12000/μL) and Hb (< 8.0 g/dL) level for bleeding were 1.18 (P = 0.6) and 1.38 (P = 0.3), respectively. A low Hb level did not correlate with bleeding. They excluded patients with symptoms of bleeding because their aim was to examine predictive values for bleeding. The reason of the discrepancy between the previous report and our results might be that we included patients with symptoms of bleeding such as low blood pressure and tachycardia. Srygley et al[19] reported that the likely ratio of Hb < 8.0 g/dL is 4.5-6.2 for severe bleeding. Our threshold value of Hb was 11.7 g/dL. The reason for this difference might be the inclusion of patients with mild bleeding. It might be proposed that Hb < 11.7 g/dL is the threshold for patients with mild and severe bleeding, and Hb < 8.0 g/dL is the threshold for those with severe bleeding.
It is hard to discriminate upper GI bleeding from lower GI bleeding with the parameters we presented. Endoscopy and colonoscopy should be performed for patients with low Hb[20]. Low Hb suggests patients with upper or lower GI bleeding, hematological disorders, such as thalassemia and sickle cell anemia. Those patients should be investigated following Goddard et al[21]. Endoscopy and colonoscopy were desirable for patients suspected with upper or lower GI bleeding. Parameters analyzed in our present study suggested bleeding from upper or lower GI. It was expected to be applied to lower GI bleeding.
Erosive esophagitis, gastritis, and duodenitis are associated with a low Hb level because of slow and long-term bleeding[22]. The rate of reduction in the Hb level might be another predictor of bleeding from the upper GI tract. Pundzius[23] analyzed 659 patients whose bleeding peptic ulcer was treated endoscopically. They determined that the rate of reduction in the Hb level is a predictive value for rebleeding. In our study, the rate of reduction in the Hb level during 3 mo strongly correlated with bleeding from the upper GI tract. The previous report and our data suggested that a higher rate of reduction in the Hb level predicted bleeding prior to endoscopy. Our results clearly showed that 21.3% was the threshold of the rate of reduction rate in the Hb level.
CRP is a plasma protein produced in the liver in response to tissue inflammation[24]. Boehme et al[25] reported that an elevated CRP level was a positive predictive value for peptic ulcers caused by active inflammation. In the present study, CRP around the day endoscopy was performed was significantly higher in the bleeding group than in the non-bleeding group, although the AUC was not high. The increase in the CRP level significantly correlated with bleeding. Our data indicated that a > 2-fold increase in the CRP level was indicative of bleeding. Serum CRP level correlates with serum interleukin (IL)-6 level, a key cytokine involved in the acute-phase response[26]. Tomita et al[27] compared the expression levels of IL-6 in the biopsy specimens of peptic ulcers with those from the antrum. They found that IL-6 is expressed more in peptic ulcers than the antrum. These reports indicate that peptic ulcer upregulates IL-6 and, consequently, increases the production of CRP. The problem with CRP is that its increase is nonspecific to peptic ulcers or upper GI bleeding. It is also used as a biomarker for predicting cardiovascular diseases[28]. The nonspecific nature of CRP might be the reason for the relatively low specificity of its rate of change, although its sensitivity was 100%. CRP, Hb, clinical symptoms, and patient conditions are cumulatively essential to predict bleeding from the upper GI tract prior to endoscopy.
A BUN/Cr ratio > 30 is a useful value to diagnose upper GI bleeding[19]. We hypothesized that BUN levels and BUN/Cr were higher in bleeding patients than in non-bleeding patients. However, according to our results, BUN and BUN/Cr did not correlate with bleeding. The reason for the discrepancy between Srygley et al[19] and our results is unclear. Chalasani et al[29] compared the BUN/Cr ratio between upper GI bleeding patients and lower GI bleeding patients. They concluded that the ratio is not useful to differentiate between the 2 types of bleeding.
In conclusion, an Hb level < 11.7 g/dL indicated bleeding from the upper GI tract. Other predictors for bleeding were a > 21.3% rate of reduction in the Hb level and an increase in the CRP level by > 100%, 3-6 mo before endoscopy.
COMMENTS
Background
Upper gastrointestinal (GI) bleeding is fatal when patients are not diagnosed correctly and treated adequately. Early upper GI endoscopy (endoscopy) significantly reduces mortality. Clues for decision making of endoscopy is awaited.
Research frontiers
Clinical predictors of increased risk for mortality are age > 65 years, shock, poor overall health status, low initial hemoglobin (Hb) levels, melena, transfusion requirement, fresh red blood on rectal examination and nasogastric aspirate, sepsis, and elevated blood urea nitrogen or creatinine levels.
Innovations and breakthroughs
Threshold values of hemoglobin and C-reactive protein (CRP) to diagnose bleeding peptic ulcer were investigated with receiver operating characteristic (ROC) curves. An Hb level < 11.7 g/dL indicated bleeding from the upper GI tract. Other predictors for bleeding were a > 21.3% rate of reduction in the Hb level and an increase in the CRP level by > 100%, 3-6 mo before endoscopy.
Applications
Endoscopy is strongly recommended for patients with Hb and CRP above threshold values.
Terminology
Receiver operating characteristic: ROC is a graph that plots the fraction of true positives out of the positives true positive rate) vs the fraction of false positives out of the negatives false positive rate), at various thresholds.
Peer review
This study attempted to establish biomarkers for upper gastrointestinal bleeding by measuring Hb and CRP. The study design is rational. It is hard to discriminate upper and lower gastrointestinal bleeding with lower Hb and higher CRP because blood loss from any part of the body will lead to low Hb and CRP is a non-specific indicator of inflammatory response. Endoscopy is strongly recommended for patients with lower Hb and higher CRP. If upper GI does not show any evidence of bleeding, the patients should be subjected to colonoscopy. The study is of particular interest to the practical medicine.
Footnotes
P- Reviewers: Kuo SM, Reshetnyak VI, Rosenthal P S- Editor: Cui XM L- Editor: A E- Editor: Wang CH
References
- 1.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–360; quiz 361. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206–210. [PubMed] [Google Scholar]
- 3.Kim SY, Hyun JJ, Jung SW, Lee SW. Management of non-variceal upper gastrointestinal bleeding. Clin Endosc. 2012;45:220–223. doi: 10.5946/ce.2012.45.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljubicic N, Budimir I, Biscanin A, Nikolic M, Supanc V, Hrabar D, Pavic T. Endoclips vs large or small-volume epinephrine in peptic ulcer recurrent bleeding. World J Gastroenterol. 2012;18:2219–2224. doi: 10.3748/wjg.v18.i18.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician. 2012;85:469–476. [PubMed] [Google Scholar]
- 6.Katano T, Mizoshita T, Senoo K, Sobue S, Takada H, Sakamoto T, Mochiduki H, Ozeki T, Kato A, Matsunami K, et al. The efficacy of transcatheter arterial embolization as the first-choice treatment after failure of endoscopic hemostasis and endoscopic treatment resistance factors. Dig Endosc. 2012;24:364–369. doi: 10.1111/j.1443-1661.2012.01285.x. [DOI] [PubMed] [Google Scholar]
- 7.Loffroy R, Guiu B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J Gastroenterol. 2009;15:5889–5897. doi: 10.3748/wjg.15.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang YS, Chan FK. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013;381:2033–2043. doi: 10.1016/S0140-6736(13)60596-6. [DOI] [PubMed] [Google Scholar]
- 9.Liu N, Liu L, Zhang H, Gyawali PC, Zhang D, Yao L, Yang Y, Wu K, Ding J, Fan D. Effect of intravenous proton pump inhibitor regimens and timing of endoscopy on clinical outcomes of peptic ulcer bleeding. J Gastroenterol Hepatol. 2012;27:1473–1479. doi: 10.1111/j.1440-1746.2012.07191.x. [DOI] [PubMed] [Google Scholar]
- 10.Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 11.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 12.Mahlknecht U, Kaiser S. Age-related changes in peripheral blood counts in humans. Exp Ther Med. 2010;1:1019–1025. doi: 10.3892/etm.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomizawa M, Shinozaki F, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Ultrasonography for leukocytosis or elevated C-reactive protein. Hepatogastroenterology. 2011;58:1156–1158. doi: 10.5754/hge10606. [DOI] [PubMed] [Google Scholar]
- 14.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394–397. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 15.Tytgat GN. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991;6:223–234. doi: 10.1111/j.1440-1746.1991.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 16.Wong SK, Yu LM, Lau JY, Lam YH, Chan AC, Ng EK, Sung JJ, Chung SC. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002;50:322–325. doi: 10.1136/gut.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heldwein W, Schreiner J, Pedrazzoli J, Lehnert P. Is the Forrest classification a useful tool for planning endoscopic therapy of bleeding peptic ulcers? Endoscopy. 1989;21:258–262. doi: 10.1055/s-2007-1010729. [DOI] [PubMed] [Google Scholar]
- 18.Giese A, Grunwald C, Zieren J, Buchner NJ, Henning BF. Can pre-endoscopic assessment predict active upper gastrointestinal bleeding? A retrospective study in patients with symptoms of upper gastrointestinal bleeding outside regular working hours. Hepatogastroenterology. 2012;59:2508–2511. doi: 10.5754/hge12123. [DOI] [PubMed] [Google Scholar]
- 19.Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307:1072–1079. doi: 10.1001/jama.2012.253. [DOI] [PubMed] [Google Scholar]
- 20.Bull-Henry K, Al-Kawas FH. Evaluation of occult gastrointestinal bleeding. Am Fam Physician. 2013;87:430–436. [PubMed] [Google Scholar]
- 21.Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 22.Droogendijk J, Beukers R, Berendes PB, Tax MG, Sonneveld P, Levin MD. Screening for gastrointestinal malignancy in patients with iron deficiency anemia by general practitioners: an observational study. Scand J Gastroenterol. 2011;46:1105–1110. doi: 10.3109/00365521.2011.594082. [DOI] [PubMed] [Google Scholar]
- 23.Pundzius J. Clinical and endoscopic signs for the prediction of recurrent bleeding from gastroduodenal ulcers. Eur J Surg. 1994;160:689–692. [PubMed] [Google Scholar]
- 24.Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56:131–142. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 25.Boehme MW, Autschbach F, Ell C, Raeth U. Prevalence of silent gastric ulcer, erosions or severe acute gastritis in patients with type 2 diabetes mellitus--a cross-sectional study. Hepatogastroenterology. 2007;54:643–648. [PubMed] [Google Scholar]
- 26.Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P’eng FK, Chi CW. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996;91:1417–1422. [PubMed] [Google Scholar]
- 27.Tomita M, Ando T, Minami M, Watanabe O, Ishiguro K, Hasegawa M, Miyake N, Kondo S, Kato T, Miyahara R, et al. Potential role for matrix metalloproteinase-3 in gastric ulcer healing. Digestion. 2009;79:23–29. doi: 10.1159/000203637. [DOI] [PubMed] [Google Scholar]
- 28.van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G, Prins MW, Roest M. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8:e62080. doi: 10.1371/journal.pone.0062080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalasani N, Clark WS, Wilcox CM. Blood urea nitrogen to creatinine concentration in gastrointestinal bleeding: a reappraisal. Am J Gastroenterol. 1997;92:1796–1799. [PubMed] [Google Scholar]


