Abstract
AIM: To assess the value of contrast-enhanced ultrasound (CEUS) in diagnosing splenic artery complications (SACs) after acute pancreatitis (AP).
METHODS: One hundred and eighteen patients with AP were enrolled in the study. All patients were examined by CEUS and contrast-enhanced computed tomography (CECT). CECT was accepted as a gold standard for the diagnosis of SACs in AP. The diagnostic accuracy of splenic CEUS and pancreatic CEUS was compared with that of CECT. Splenic infarction was the diagnostic criterion for splenic artery embolism and local dysperfusion of the splenic parenchyma was the diagnostic criterion for splenic arterial stenosis. The incidence of splenic sub-capsular hemorrhage, splenic artery aneurysms, and splenic rupture was all lower than that of SACs.
RESULTS: Nine patients were diagnosed as having SACs after AP by CECT among the 118 patients. The patients with SACs were diagnosed with severe acute pancreatitis (SAP). Among them, 6 lesions were diagnosed as splenic artery embolism, 5 as splenic artery aneurysms, and 1 as splenic arterial stenosis. No lesion was diagnosed by pancreatic CEUS and 5 lesions were diagnosed by splenic CEUS. By splenic CEUS, 4 cases were diagnosed as splenic artery embolism and 1 as splenic arterial stenosis. The accuracy of splenic CEUS in diagnosis of SACs in SAP was 41.7% (5/12), which was higher than that of pancreatic CEUS (0%).
CONCLUSION: Splenic CEUS is a supplementary method for pancreatic CEUS in AP patients, which can decrease missed diagnosis of SACs.
Keywords: Acute pancreatitis, Severe acute pancreatitis, Contrast enhanced ultrasound, Splenic artery complications, Splenic contrast-enhanced computed tomography
Core tip: We prospectively investigated splenic contrast-enhanced ultrasound (CEUS) in diagnosis of splenic artery complications in acute pancreatitis (AP). The diagnostic yield of splenic CEUS for detecting splenic artery complications (SACs) in AP was higher than that of pancreatic CEUS. Splenic CEUS is a supplementary method for pancreatic CEUS when an AP patient needs pancreatic CEUS examination, which can decrease missed diagnosis of SACs.
INTRODUCTION
Contrast-enhanced ultrasound (CEUS) has become an important imaging method to evaluate the degree of severity and predict the prognosis of diseases[1]. Acute pancreatitis (AP) can be examined by CEUS to identify the necrosis range of the pancreas, which is related to treatment and prognosis. Splenic artery complications (SACs) have a low morbidity in AP. Although SACs are rare complications in AP and most patients present few clinical symptoms, it may lead to a severe outcome. Rupture of splenic artery aneurysms is frequent and sometimes occurs as the first symptom, and sometimes it is fatal[2]. So, it is of great clinical significance to determine the diagnostic accuracy of CEUS for SACs in AP. However, the sensitivity of gray scale ultrasound and color Doppler ultrasound is low, and few studies have reported on CEUS[3]. In this article, we discussed the diagnostic value and accuracy of splenic CEUS for SACs in AP.
MATERIALS AND METHODS
Patient data
This study was conducted with the approval of the Ethics Committee of West China Hospital and informed consent was obtained from all the patients. We collected 129 in-patients with AP treated at our hospital from March 2010 to March 2013. Eleven patients were excluded from the study either because the gray scale sonography of the pancreas hardly displayed meteorism or because patients had a history of splenic disease. Of the remaining 118 patients included in the study, there were 69 males and 49 females, with a mean age of 41.27 ± 12.15 years (range, 15-79). All patients were examined by pancreatic CEUS to diagnose the SACs in AP. Splenic CEUS was also used to diagnose the splenic lesions. All contrast-enhanced computed tomography (CECT) and CEUS examinations were performed at an interval of 72 h. The study protocol was kept blind for doctors performing CEUS and CECT.
Sonographic examination
A LOGIQ E9 (GE Healthcare, Milwaukee, WI, United States) ultrasound system with a C1-5 MHz probe or a PHILIPS IU22 (Philips Medical Systems, Bothell, WA, United States) ultrasound system with a C5-2 MHz transducer was selected. Both the ultrasound systems were equipped with harmonic contrast pulse sequencing apparatus. The contrast agent used was SonoVue (BraccoSpa, Milan, Italy) and the suspension contained stabilized sulfur hexafluoride microbubbles.
Two sonologists, who had over ten years of experience in abdominal conventional ultrasound and over three years of experience in CEUS evaluation for pancreatic diseases, performed the examinations. All patients were asked to fast before ultrasound examination. Firstly, gray scale sonography and color Doppler flow imaging were performed to observe the size and shape of the pancreas and spleen, peri-pancreatic fluid collection, echogenicity of the parenchyma, and the inner diameters of the splenic artery. Then, CEUS was started at a low mechanical index (GE MI: 0.12; PHILIPS MI: 0.06). SonoVue suspension of 1.5-2.0 mL was administered as a bolus injection through the antecubital vein, followed by a flush with 5 mL saline solution. Real-time contrast-enhanced imaging was recorded on the hard disk when the suspension (SonoVue) was injected, and the times were calculated simultaneously. Based on the entire arterial pancreatic or splenic system, the contrast phase was identified as the arterial phase (0-30 s after contrast agent injection) and the venous phase (starting at 31 s after contrast agent injection). When a sonologist finished pancreatic CEUS, the other sonologist started splenic CEUS successively. And the results of SACs by pancreatic CEUS and by splenic CEUS were recorded.
The splenic CEUS images for SACs were correlated with the pathophysiologic results (Table 1). The results of splenic lesions were transformed into corresponding SACs and recorded immediately.
Table 1.
Splenic lesions and splenic artery complications
| Splenic lesions | Splenic artery complications |
| Splenic infarction/subcapsular hemorrhage | Splenic artery embolism |
| Local dysperfusion of splenic parenchyma | Splenic artery stenosis |
| Splenic artery aneurysms | Splenic artery aneurysms |
| Splenic rupture | Splenic artery embolism |
Computer tomography
A 64-slice spiral computer tomography (CT) (Philips Brilliance; Philips Medical Systems, Cleveland, OH, United States) system or 16-slice spiral CT system (Somatom Sensation 16; Siemens, Erlangen, Germany) was used in this study. The contrast agent used was iopamidol (Iopamiro; Bracco Imaging) or iopromide (Ultravist; Bayer Schering, Germany) at a concentration of 37 gI/100 mL.
The range of CECT scanning was from the chest to the pelvic floor. Iopamidol or iopromide was injected with a total amount of 90-120 mL to each patient at a rate of 3 mL/s with a power injector through the antecubital vein.
RESULTS
The 118 patients were diagnosed with AP by CECT; among them only 9 patients (including 12 lesions) were diagnosed as having SACs. Among the 118 patients, there were 38 patients with mild AP and 80 with severe AP (SAP). All SACs patients were from the SAP group. Six cases were diagnosed as splenic artery embolism; 5 cases as splenic artery aneurysms and 1 case as splenic arterial stenosis by CECT. Three patients had splenic artery embolism with splenic artery aneurysms found by CECT. In the splenic CEUS group, SACs were found in 5 cases, including 4 cases of splenic artery embolism (Figure 1) and 1 case of splenic arterial stenosis (Figure 2). And in the pancreatic CEUS group, all lesions of SACs were misdiagnosed (Table 2). The diagnostic accuracy of splenic CEUS for SACs in AP was 41.7% (5/12), which was obviously higher than that of pancreatic CEUS (0%, 0/12).
Figure 1.
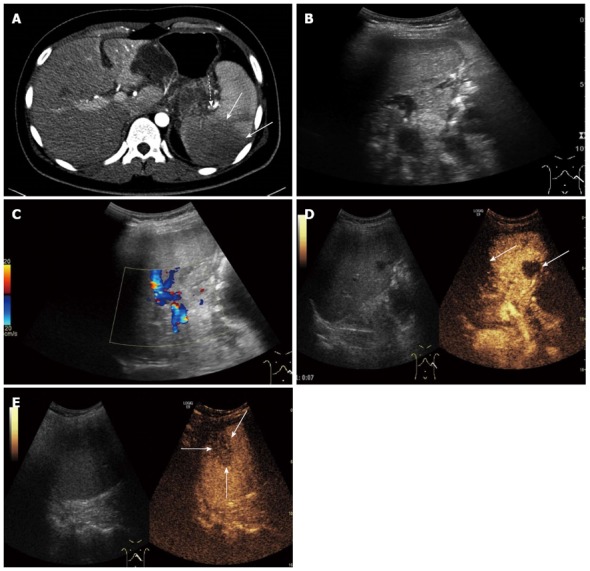
A 22-year-old man with splenic artery embolism. A: Enhanced-contrast computed tomography showing splenic artery embolism (dotted arrow), and the lesion of splenic infarction (solid arrow); B, C: Gray scale ultrasonography and color Doppler ultrasonography of the spleen showed no obvious lesion of splenic infarction; D: Some regions with no enhancement appeared in the splenic arterial phase (solid arrow) on contrast enhanced ultrasound (CEUS); E: A wedge-shaped splenic infarction with no enhancement was displayed in the splenic venous phase (solid arrow) on CEUS.
Figure 2.
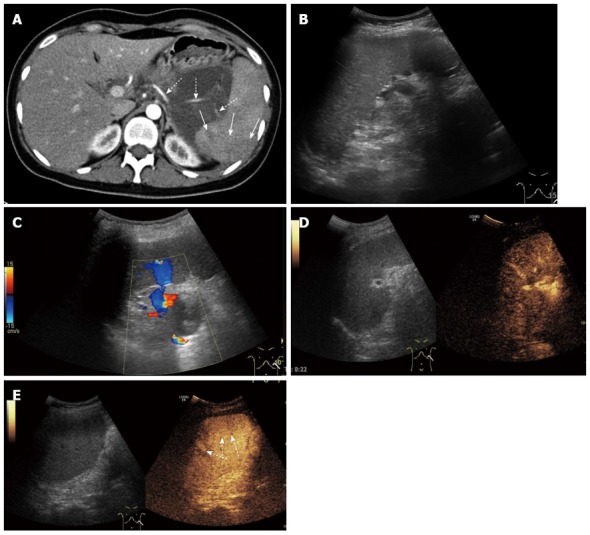
A 26-year-old woman with splenic arterial stenosis. A: Enhanced-contrast computed tomography showing splenic arterial stenosis (dotted arrow) and a focal splenic parenchyma region showed low enhancement (solid arrow); B, C: Gray scale ultrasonography and color Doppler ultrasonography showed no obvious lesions in the spleen; D: No obvious lesion was displayed in the splenic arterial phase on contrast enhanced ultrasound (CEUS); E: Some regions of focal dysperfusion of the splenic parenchyma showed low enhancement in the splenic venous phase (dotted arrow).
Table 2.
Splenic artery complications examined by three methods in acute pancreatitis
| Splenic artery embolism | Splenic artery aneurysms | Splenic artery stenosis | |
| CECT | 6 | 5 | 1 |
| Pancreatic CEUS | 0 | 0 | 0 |
| Splenic CEUS | 4 | 0 | 1 |
CECT: Contrast-enhanced computed tomography; CEUS: Contrast enhanced ultrasound.
By splenic CEUS, 4 cases were diagnosed as splenic infarction, including 1 case complicated with sub-capsular hemorrhage, and 1 case of focal dysperfusion of the splenic parenchyma (Table 3). The CEUS imaging of splenic infarction showed that in some regions, no enhancement appeared after contrast agent injection in the splenic artery phase and venous phase, and hence infarcted splenic parenchyma was displayed as a hypoechoic area. The shape of splenic infarction varied from wedge-shaped (3 cases) to round or irregular (1 case).
Table 3.
Splenic artery complications by splenic contrast enhanced ultrasound in acute pancreatitis
| Imaging of splenic CEUS | No. |
| Splenic infarction | 4 |
| Subcapsular hemorrhage | 1 |
| Splenic parenchymal dysperfusion | 1 |
| Splenic rupture | 0 |
| Splenic artery aneurysms | 0 |
CEUS: Contrast enhanced ultrasound.
Sub-capsular hemorrhage is a complication of splenic artery embolism. CEUS imaging of sub-capsular hemorrhage showed an anechoic region under the splenic sub-capsule, which could differentiate better than pancreatic ultrasound, and there appeared an anechoic region and no enhancement after contrast agent injection in the splenic arterial phase and venous phase. There was one case diagnosed by CEUS as splenic infarction in the splenic CEUS group.
The imaging of focal dysperfusion of the splenic parenchyma revealed a region where the degree of enhancement inside the lesion area was lower than that of normal surrounding splenic parenchyma in the arterial and venous phases (especially the venous phase). The region of focal dysperfusion of the splenic parenchyma was displayed as a slightly hypoechoic area on CEUS, which did not appear on pancreatic ultrasound. Hence, a case of SAC was diagnosed by splenic CEUS, and there was no evidence of splenic rupture during his stay in hospital.
Five cases of splenic artery aneurysms were all miss-diagnosed by pancreatic CEUS and splenic CEUS in AP. Because the whole splenic artery is difficult to display by ultrasound, especially the region which is close to the hiluslienis, gray scale ultrasound or CEUS may be necessary to identify the size or the location of splenic artery aneurysms.
DISCUSSION
AP is a common abdomen emergency and SACs in AP are rarely seen with a low incidence[4]. Due to the low sensitivity of ultrasound in diagnosing SACs, the missed diagnosis of the complications is often ignored[5], even by CEUS[6]. CECT was regarded as the gold standard to evaluate AP, especially the degree of severity[7,8]. CEUS changes the conventional viewpoint that ultrasound can only examine AP roughly[1,9], such as the pancreas size, shape, and peri-pancreatic fluid collection[10]. With the development of contrast agents, corresponding contrast-enhanced software and ultrasound equipment, CEUS is being utilized in more and more diseases, including pancreatic diseases, especially examination of SAP because it can display pancreatic parenchymal necrosis and the degree of necrosis, and the extra-pancreatic acute fluid collection. Previous studies concluded that ultrasound severity index (USSI) has a strong correlation with CT severity index (CTSI) and has been regarded as a substitute for CECT[6,9]. Only a few cases have been reported worldwide about CEUS for SACs compared with CECT for splenic venous complications[11-13]. The reason may be that the splenic artery is located between the tail of the pancreas and the hilum of the spleen with the body of the stomach in front. Gray scale sonography is influenced greatly by meteorism in the gastrointestinal tract, and diagnosis is sometimes difficult[14]. Because the quality of CEUS depends on the gray scale sonography, its quality is consequently affected, and the diagnostic accuracy for SACs is often ignored compared with CT or MRI[15].
Although the whole splenic artery is difficult to be displayed directly by ultrasound, we convert the observational objective to evaluate the lesions of the splenic artery indirectly. The diagnostic criteria for SACs were defined by observing the pattern of images obtained from the splenic CEUS. Blood supply to the spleen is solely from the splenic artery. Any lesions or changes in the splenic artery may result in splenic parenchymal infarction. In AP, splenic parenchymal infarction occurs due to SACs. SACs are infrequent complications with pancreatitis, which mainly occur in chronic pancreatitis and SAP[16]. Mortelé et al[17] found that the incidence of splenic infarction in AP was about 7%, and there was a strong relationship between this complication and the severity of pancreatitis. In our study, 6 cases were diagnosed as splenic infarction by CECT. The incidence of splenic infarction in AP was 5.1% (6/118) and all cases with the complications were SAP. The findings in our study are consistent with the results reported by Mortelé et al.
SACs include splenic artery embolism, splenic artery aneurysms and splenic arterial stenosis[18]. SACs can lead to splenic infarction, sub-capsular hemorrhage, splenic rupture, or splenic aneurysm rupture. Color Doppler ultrasound is less sensitive to display acute infarction because there is no difference between the echo area of infarcted and normal parenchyma[19]. Acute splenic arterial stenosis is the main presentation of SAP. With the development of CEUS, splenic trauma or aortic aneurysm rupture can be diagnosed in clinical practice[20-22]. The microcirculation of the splenic parenchyma can be displayed by CEUS and it is possible to observe the ischemic alteration of the splenic parenchyma. These characteristics guarantee the diagnosis of acute infarction as early as possible and SAPs indirectly. The sensitivity of splenic CEUS for detecting SACs has increased obviously, and the accuracy rate was 41.7% (5/12, including 4 cases of splenic artery embolism and 1 case of splenic arterial stenosis) in diagnosing SACs compared with CECT.
There were no SACs diagnosed by pancreatic CEUS, indicating that pancreatic CEUS is a low sensitive method to diagnose SACs in AP. This finding also demonstrates that splenic CEUS is an indispensable technique in diagnosis of SACs in AP. It is of great clinical significance to optimize methods of diagnosis for AP complications, such as CEUS. Splenic CEUS is a felicitous supplement to overcome the disadvantage of pancreatic CEUS for SAPs in AP.
However, this study of diagnosis for splenic artery aneurysms is not satisfactory. In this study, all cases of splenic artery aneurysms were miss- diagnosed. We finally analyzed the reason for missed diagnosis of splenic artery aneurysms. The longest diameter of splenic artery aneurysms was less than 2 cm and the location was far from the hilum of the spleen so that sonologists could not observe the lesions, even by CEUS. And there were 2 cases of splenic infarction which were miss-diagnosed by splenic CEUS. We retrospectively analyzed the reasons why splenic CEUS missed the diagnosis. The reason was that the splenic infarction in 2 cases was located close to the diaphragm and at the sub-capsular area of the spleen, where ultrasound imaging would be obstructed by the ribs. Sonologists did not notice the missed area, which was just located in the shadow of the imaging. By controlling the quality of ultrasound imaging and avoiding the influence of the ribs, the incidence of missed diagnosis would be decreased.
The ultrasound contrast agent (suspension made of 5 mL normal saline mixed with SonoVue dry powder) was administered by intravenous injection at 1.5-2.0 mL for each pancreatic CEUS examination. Pancreatic CEUS examination generally did not run out of the suspension. Usually the remaining suspension of about 1.0-2.0 mL was sufficient for splenic CEUS examination. Therefore, it is economic to perform the splenic CEUS after pancreatic CEUS examination. Splenic CEUS examination could diagnose not only splenic artery lesions but also any other pathological changes, such as pancreatic pseudocysts in the splenic parenchyma[23,24].
Although CECT is the gold standard in diagnosing AP, ultrasound has an important position in pancreatitis examination[25]. When the patient’s condition is not fit for CECT, such as iodine allergy, unavailability for bedside examination, and cases of renal insufficiency, CEUS is an effective substitute for CECT examination[26,27].
In conclusion, splenic CEUS diagnosis of SACs in AP was better than pancreatic CEUS because it had a higher diagnostic accuracy than the latter. In splenic CEUS examination, the contrast agent can be utilized more effectively and economically. Splenic CEUS can obviously increase the diagnostic accuracy for SACs in AP, which is simple, convenient, efficient, safe, and partly solves the disadvantages of other modalities in diagnosis of SACs in AP. Splenic CEUS is a supplementary method for pancreatic CEUS when AP patients need pancreatic CEUS examination, which can decrease the missed diagnosis of SACs.
ACKNOWLEDGMENTS
The authors are indebted to Professor Qin Xia, Zhong-Wen Huang, and Wen-Fu Tang (Department of Integrated Chinese and Western Medicine of West China Hospital) for providing the cases in this study. We also thank Lin Lan and Xiao-Ying Feng for assistance with the injection of contrast agents.
COMMENTS
Background
Splenic artery complications (SACs) have a low morbidity in acute pancreatitis (AP). Although SACs are rare complications in AP and most patients present few clinical symptoms, they may lead to a severe outcome. Rupture of splenic artery aneurysms is frequent and sometimes occurs as the first symptom, and sometimes it is fatal. However, the sensitivity of ultrasound is low, even for pancreatic contrast-enhanced ultrasound (CEUS). The authors introduced an indirect method used for splenic CEUS to show the SACs in AP.
Research frontiers
CEUS has been used to reflect the necrosis range of the pancreas in AP. The enhancement patterns of AP have been described previously. However, the diagnostic value of splenic CEUS for SACs in AP has not been investigated or reported in the English-language literature.
Innovations and breakthroughs
The diagnostic value of splenic CEUS for SACs in AP was discussed in this study. When the splenic parenchyma shows a lower enhanced area than the surrounding parenchyma, or an unenhanced area of the infarct lesion or an unenhanced area of the fluid collection under the splenic subcapsule, a diagnosis of SACs in AP should be suspected.
Applications
Splenic CEUS is a convenient and useful method for the detection and discrimination of SACs in AP. SACs could be better managed if ultrasound technicians and physicians are familiar with their features on CEUS.
Terminology
Contrast enhanced ultrasonography (CEUS) is the application of an ultrasound contrast agent to traditional color Doppler sonography. Microbubble contrast agents produce a unique sonogram with increased contrast due to the high echogenicity difference. CEUS can be used to image blood perfusion in organs and tissues.
Peer review
This is a well written and interesting paper.
Footnotes
P- Reviewer: Poma EM S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Ripollés T, Martínez MJ, López E, Castelló I, Delgado F. Contrast-enhanced ultrasound in the staging of acute pancreatitis. Eur Radiol. 2010;20:2518–2523. doi: 10.1007/s00330-010-1824-5. [DOI] [PubMed] [Google Scholar]
- 2.Carr SC, Mahvi DM, Hoch JR, Archer CW, Turnipseed WD. Visceral artery aneurysm rupture. J Vasc Surg. 2001;33:806–811. doi: 10.1067/mva.2001.112320. [DOI] [PubMed] [Google Scholar]
- 3.Rickes S, Rauh P, Uhle C, Ensberg D, Mönkemüller K, Malfertheiner P. Contrast-enhanced sonography in pancreatic diseases. Eur J Radiol. 2007;64:183–188. doi: 10.1016/j.ejrad.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Horváth K, Györi-Molnár L, Bíró E, György E. [Ultrasonic diagnosis of splenic complications of chronic pancreatitis] Orv Hetil. 1986;127:2311–2312. [PubMed] [Google Scholar]
- 5.Fishman EK, Soyer P, Bliss DF, Bluemke DA, Devine N. Splenic involvement in pancreatitis: spectrum of CT findings. AJR Am J Roentgenol. 1995;164:631–635. doi: 10.2214/ajr.164.3.7863884. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q, Zhong Y, Wen XR, Huang ZW, Fan YT, Xia Q, Luo Y. Can contrast-enhanced ultrasound evaluate the severity of acute pancreatitis? Dig Dis Sci. 2011;56:1578–1584. doi: 10.1007/s10620-010-1460-6. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–613. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 9.Rickes S, Uhle C, Kahl S, Kolfenbach S, Monkemuller K, Effenberger O, Malfertheiner P. Echo enhanced ultrasound: a new valid initial imaging approach for severe acute pancreatitis. Gut. 2006;55:74–78. doi: 10.1136/gut.2005.070276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound. 2003;16:141–159. doi: 10.1016/s0929-8266(02)00068-x. [DOI] [PubMed] [Google Scholar]
- 11.Malka D, Hammel P, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J, Bernades P. Splenic complications in chronic pancreatitis: prevalence and risk factors in a medical-surgical series of 500 patients. Br J Surg. 1998;85:1645–1649. doi: 10.1046/j.1365-2168.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller LA, Mirvis SE, Shanmuganathan K, Ohson AS. CT diagnosis of splenic infarction in blunt trauma: imaging features, clinical significance and complications. Clin Radiol. 2004;59:342–348. doi: 10.1016/j.crad.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Liu GJ, Chen YX, Dong HP, Wang LX. Sinistral portal hypertension: clinical features and surgical treatment of chronic splenic vein occlusion. Med Princ Pract. 2012;21:20–23. doi: 10.1159/000329888. [DOI] [PubMed] [Google Scholar]
- 14.Rickes S, Mönkemüller K, Malfertheiner P. Acute severe pancreatitis: contrast-enhanced sonography. Abdom Imaging. 2007;32:362–364. doi: 10.1007/s00261-007-9250-0. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich CF. Comments and illustrations regarding the guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS)--update 2008. Ultraschall Med. 2008;29 Suppl 4:S188–S202. doi: 10.1055/s-2008-1027799. [DOI] [PubMed] [Google Scholar]
- 16.Patil PV, Khalil A, Thaha MA. Splenic parenchymal complications in pancreatitis. JOP. 2011;12:287–291. [PubMed] [Google Scholar]
- 17.Mortelé KJ, Mergo PJ, Taylor HM, Ernst MD, Ros PR. Splenic and perisplenic involvement in acute pancreatitis: determination of prevalence and morphologic helical CT features. J Comput Assist Tomogr. 2001;25:50–54. doi: 10.1097/00004728-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Miller FH, Keppke AL, Dalal K, Ly JN, Kamler VA, Sica GT. MRI of pancreatitis and its complications: part 1, acute pancreatitis. AJR Am J Roentgenol. 2004;183:1637–1644. doi: 10.2214/ajr.183.6.01831637. [DOI] [PubMed] [Google Scholar]
- 19.Ray S, Mridha AR, Ahammed M. Diffuse splenic infarction in a case of severe acute pancreatitis. Am J Surg. 2011;201:e23–e25. doi: 10.1016/j.amjsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, Zhang H, Lv F, Li W, Luo Y, Wang Y, Li J. Percutaneous injection therapy for blunt splenic trauma guided by contrast-enhanced ultrasonography. J Ultrasound Med. 2008;27:925–932; quiz 933. doi: 10.7863/jum.2008.27.6.925. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Lv F, Luo Y, An L, Li J, Xie X, Tian J, Zhao W, Tang J. Comparison of transcutaneous contrast-enhanced ultrasound-guided injected hemostatic agents with traditional surgery treatment for liver, spleen and kidney trauma: a retrospective study. Hepatogastroenterology. 2012;59:2021–2026. doi: 10.5754/hge12255. [DOI] [PubMed] [Google Scholar]
- 22.Catalano O, Lobianco R, Cusati B, Siani A. Contrast-enhanced sonography for diagnosis of ruptured abdominal aortic aneurysm. AJR Am J Roentgenol. 2005;184:423–427. doi: 10.2214/ajr.184.2.01840423. [DOI] [PubMed] [Google Scholar]
- 23.Heider R, Behrns KE. Pancreatic pseudocysts complicated by splenic parenchymal involvement: results of operative and percutaneous management. Pancreas. 2001;23:20–25. doi: 10.1097/00006676-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Siablis D, Papathanassiou ZG, Karnabatidis D, Christeas N, Katsanos K, Vagianos C. Splenic arteriovenous fistula and sudden onset of portal hypertension as complications of a ruptured splenic artery aneurysm: Successful treatment with transcatheter arterial embolization. A case study and review of the literature. World J Gastroenterol. 2006;12:4264–4266. doi: 10.3748/wjg.v12.i26.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, Yuan CX, Peng YL, Wei PL, Zhang ZD, Jiang JM, Dai L, Hu YK. Can ultrasound predict the severity of acute pancreatitis early by observing acute fluid collection? World J Gastroenterol. 2001;7:293–295. doi: 10.3748/wjg.v7.i2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723. doi: 10.1053/j.gastro.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi R, Brizi MG, Canadè A, Vecchioli A, Marano P. Imaging of acute pancreatitis. Rays. 2001;26:135–142. [PubMed] [Google Scholar]


