Abstract
Colorectal cancer (CRC) is the second leading cause of cancer related deaths in the United States. There are significant differences in CRC incidence and mortality by race with the highest burden occurring among blacks. The underlying factors contributing to CRC disparities are multiple and complex. Studies have suggested that a higher prevalence of putative risk factors for CRC, limited access to healthcare services, lower utilization of healthcare resources and increased biological susceptibilities contribute to this disparity by race. This article reviews the factors associated with the disproportionally higher burden of CRC among blacks; addresses the controversies regarding the age to begin CRC screening and the screening modality to use for blacks; and proffers solutions to eliminate CRC disparity by race.
Keywords: Colorectal cancer disparities, Adenomatous polyps, Colon cancer, Colonoscopy, Screening
Core tip: This article reviews the underlying factors for the disproportionally higher burden of colorectal cancer (CRC) among blacks, addresses controversies regarding race-based screening recommendations and concludes by suggesting that a comprehensive approach that increases access and utilization of CRC screening, timely follow-up of abnormal results and treatment of CRC will be needed to reduce or eliminate CRC disparity.
DISPARITIES IN COLORECTAL CANCER INCIDENCE AND MORTALITY BY RACE
Widening racial disparities in cancer burden continues to be recognized[1-5]. Since 1960, colorectal cancer (CRC) mortality has declined by 39% among whites, but increased by 28% among blacks[5]. The incidence and mortality from CRC is higher among blacks when compared with other race-ethnicities[2]. An estimated 142820 new cases of CRC and 50830 CRC related deaths were expected in 2013. The incidence of CRC among black males is 65.1 per 100000 population but 52.8 per 100000 among white males. The incidence of CRC among black females is 48 per 100000 as compared to 39.2 per 100000 among white females. Similar pattern has been observed with mortality rates from the disease. The CRC mortality rate among black males is 29.8 per 100000 as compared to 19.5 per 100000 among white males. The mortality rate from CRC is 19.8 per 100000 among black females but 13.6 per 100000 among white females.
POSTULATED FACTORS UNDERLYING RACIAL DISPARITY IN CRC BURDEN
Differences in the prevalence of putative diet and lifestyle risk factors
Studies have suggested that alcohol ingestion, cigarette smoking, obesity, and meat consumption increases the risk of CRC whereas physical activity decreases the risk[6,7]. The prevalence of obesity and cigarette smoking is higher and physical activity is lower among blacks when compared to whites[8,9]. In South Africa, blacks have lower incidence of CRC (< 5 per 100000 ) as compared to South African whites (40 per 100000) who consumed a typical “western’’ diet[10]. When compared to South African blacks, American blacks exhibit a higher CRC risk and mucosal proliferation rates which is associated with higher dietary intakes of animal products and higher colonic populations of potentially toxic hydrogen and secondary bile-salt-producing bacteria[10]. Higher consumption of animal fat including beef, pork and lamb has been associated with an increased risk for CRC[11,12]. The consumption of red meat and pork is higher among blacks than other race-ethnicities in the United States[12].
Some studies have suggested an inverse association between micronutrients (Vitamin E, β carotene, vitamin C, Calcium) intake and CRC risk[13,14]. However, there are differences in the intake of micronutrients between blacks and whites with higher intake among whites primarily due to higher dietary supplements use. A possible basis of this association is that high concentrations of hydrogen (a byproduct of bacterial metabolism) in the colon can interfere with cellular metabolism[15]. Hydrogen concentration has been reported to be higher among blacks than among whites[10] and hydrogen is toxic to colonic epithelium. Although the exact mechanisms of action of micronutrients are not well established, they are postulated to have anti-oxidant effects, exert some effect on cell growth regulation and enhance immune response.
Although these dietary and lifestyle factors have been associated with CRC risk and may play a role in the observed disparity among blacks versus whites, the actual differential contributions of these factors to the overall CRC burden is not well established. For instance, while it has been suggested that high fat and low fiber diet are associated with increased susceptibility to CRC, reversal or adoption of the “healthy” lifestyle has not been proven to reduce CRC susceptibility in randomized trials. For instance, the adoption of low fat, high fiber, high fruits and vegetable diet in the Polyp Prevention Trial and fiber supplementation in the Wheat Bran Fiber Trial did not reduce the recurrence of adenoma[16,17]. Similarly, weight loss within 4 years did not reduce the risk of adenoma recurrence[18].
Furthermore, using the Behavioral Risk Factor Surveillance System, the prevalence of these putative risk factors such as obesity, cigarette smoking, alcohol consumption, lack of physical activities, low socioeconomic status and low health literacy are comparable among blacks and Hispanic population, yet Hispanic Americans have lower burden of CRC when compared to blacks and whites in the United States[2,9]. The incidence of CRC among Hispanic males and Hispanic females are 46.9 and 33.3 per 100000 population and the corresponding mortality rates are 15.3 and 10.2 per 100000 population.
Differences in tumor biology
Blacks tend to be diagnosed with CRC at younger ages, are more likely to present with proximal tumors and be diagnosed with more advanced diseases than whites[19-26]. This suggests that stage at presentation may account for some of the observed racial differences in survival after CRC diagnosis. Tumor grade is an independent prognostic predictor of CRC even after adjusting the effects of stage of disease at the time of diagnosis[3,27-31]. Studies have suggested that blacks suffer worse outcomes when they present with similar tumor characteristics as whites. Alexander et al[31] reported that blacks with high grade tumors were three times (HR = 3.05, 95%CI: 1.32-7.05) more likely to die of CRC within 5 years after surgery when compared with whites with high grade tumors. Blacks with lymph node-negative CRC have more than 40% excess mortality when compared to whites with the same disease[5,26,27]. It is noteworthy that approximately 30% of patients with lymph node negative CRC (stages I and II) develop recurrent disease, probably reflecting the presence of occult tumor in those lymph nodes[32-34]. Hyslop et al[35] suggested that blacks exhibited 4-fold greater occult metastases in individual lymph nodes compared with whites. Thus, occult tumor burden may be playing an important role in racial disparities in CRC outcomes between blacks and whites. However, in a systematic review of published studies, Bach et al[36] reported that there were no appreciable differences in cancer-specific survival between blacks and whites when treatment was comparable for similar stage cancers. The authors concluded that differences in cancer biology between racial groups are unlikely to be responsible for a substantial portion of the observed discrepancy in stage-specific CRC survival if blacks and whites receive similar treatments.
Differences in healthcare access, utilization and treatment
Blacks are more likely to be uninsured, have a fatalistic attitude towards medical illness, experience stigma, exhibit fear and denial related to a cancer diagnosis, have an aversion to health care treatments such as surgery, mistrust the healthcare system, and have misperceptions about cancer that ultimately interfere with treatment[9,37,38]. Although the efficacy of treatment for CRC appeared to be similar between blacks and whites in equal access systems and among participants in adjuvant chemotherapy trials[21,23] there are differences in the treatment received after CRC diagnosis among blacks and whites in general[25,26,39-45].
Blacks received surgery less often than whites for CRC[26,42-45]. Demissie et al[44] reported that the odds of non-receipt of surgical treatment was higher among blacks as compared with whites for stage I (OR = 2.08, 95%CI: 1.41-3.03 among males; OR = 2.38; 95%CI: 1.69-3.45 among females) and stage IV colon cancer (OR = 1.25, 95%CI: 1.01-1.56 among males; OR = 1.41, 95%CI: 1.14-1.72 among females). Similar results were reported by Le et al[26]. The authors reported that a higher percentage of blacks did not undergo surgical resection for stage I disease (5.4% vs 3.7%, P < 0.001) and stage IV disease (30.3% vs 23.3%, P < 0.001) when compared with whites. In general, the reasons for not receiving surgery among blacks included that it was less often recommended, refused by black patients, and because of higher prevalence of comorbid conditions among blacks[26].
While disparity in surgical treatment of CRC between blacks and whites is evident from above, studies have shown mixed results regarding chemotherapy and radiation therapy use for CRC management by race. Some studies suggest that blacks were less likely to undergo chemotherapy and radiation therapy even when they had undergone consultations with oncologists[25,40,46]. The reason for this is unclear but it is unknown if blacks are more fearful of the effects of chemotherapy even though blacks were less likely to develop chemotherapy related toxicity in a clinical trial[47]. However, it has been suggested that a higher proportion of black patients with CRC lived in census tracts with low high school graduation rates and these areas had lower chemotherapy use[48]. Blacks also tend to have less supplemental coverage when compared to whites which could affect co-payment for outpatient chemotherapy, and this can affect the initiation of chemotherapy among blacks[41]. Conversely, other studies did not find an association between race and chemotherapy use[26,49]. It is noteworthy that studies have reported that disparity in survival after CRC diagnosis are attenuated or eliminated with the receipt of similar treatments by blacks and whites[21,23,47,50,51].
Disparities in colorectal cancer screening
There has been growing evidence that CRC screening reduces mortality[52-54]. There are multiple acceptable options for CRC screening including fecal occult blood and fecal DNA testing, double contrast barium enema, CT colonography, flexible sigmoidoscopy, and colonoscopy[55]. Identified significant predictors of CRC screening include older age, having a regular doctor and participating in general medical examinations[56]. Screening rates are low among racial and ethnic minorities and persons from socioeconomically disadvantaged population[57-59]. Doubeni et al[60] reported that the screening rate for white Medicare beneficiaries improved from 49% in 2000 to 56.6% in 2005. During the same study period, the screening rates among blacks improved from 41% to 52% suggesting that screening rates for CRC are improving, but disparity in the screening rates still persists. Tehranifar et al[61] also reported that cancer survival disparities between blacks and whites widened as cancers become more amenable to medical interventions. This suggests that blacks lag behind when medical technologies that improve cancer healthcare are introduced for reasons that are not well understood.
Disparities in screening have been cited as one of the reasons for high incidence and mortality from CRC among blacks as compared with whites. Lansdorp-Vogelaar et al[62] reported that differences in CRC screening accounted for 42% of disparity in CRC incidence and 19% of disparity in CRC mortality between blacks and whites.
There are many barriers to CRC screening uptake among blacks. These are due to a complex combination of socioeconomic disadvantages from lower education and income, place of residence, inadequate insurance and mistrust of the medical system[63-65]. People in lower socioeconomic neighborhoods are less likely to undergo a colonoscopy, even among insured subjects receiving care in integrated healthcare systems[64]. Physician recommendation is important for completing CRC screening[66,67]. However, increasing availability of primary care physicians and colonoscopy providers did not narrow the racial gaps in CRC screening disparities. Rather, colonoscopy rates increased among whites but decreased among minorities[59]. Therefore, there is an urgent need to increase the participation of minorities as care providers in biomedical fields and improve cultural competencies of all care providers[68,69].
CONTROVERSIES IN COLORECTAL CANCER SCREENING BY RACE
Although it is well known that blacks suffer the highest burden from CRC and increasing CRC screening uptake among blacks is at the core of the solution, there is no consensus regarding the screening strategy to adopt in eliminating this disparity. In particular, it is unclear whether there should be different CRC screening recommendations based on race-ethnicity.
Should we begin CRC screening earlier among blacks?
Studies have suggested that when compared to whites, blacks are more likely to be diagnosed with CRC at younger ages, present with proximal tumors and be diagnosed with advanced diseases[19-26]. The widely adopted recommendation is to begin CRC screening from 50 years of age for average risk individuals in the United States[55,70]. Some experts have expressed the opinion that race-based recommendation for CRC screening will create more confusion for patients and their healthcare providers amidst a crowded field of recommendations based on age, family history of colorectal cancer and polyps, and colonic diseases such as inflammatory bowel disease. They argue for increasing efforts to boost participation in CRC screening by blacks and that it is not cost effective to lower the age of screening[71]. In contrast to this opinion, the American College of Physicians recommended that CRC screening should start at 40 years of age[72] while the American College of Gastroenterology (ACG)[73] and American Society for Gastrointestinal Endoscopy recommended CRC screening beginning at age 45 years[74]. In our opinion, blacks should begin screening at age 45 years as part of a comprehensive intervention to improve screening uptake, ensure adequate follow-up and resolution of abnormal screening results, and enhance access and utilization of good quality treatment of patients diagnosed with CRC in a timely fashion. This is because of the well documented earlier presentation with CRC among blacks and the proximal location of their tumors which raises the possibility of genetic predisposition such as Lynch syndrome. However, genetic predisposition to CRC is raised based on family history of CRC. It is well known that blacks tend not to know their family medical history due to lower health literacy and lack of knowledge of their family ancestry. There is a lack of information among African Americans with regards to the prevalence of inherited conditions that predispose to CRC. Murff et al[75] reported that blacks who have first-degree relatives (FDRs) with CRC are less likely to undergo colonoscopy screening compared with whites (27.3% vs 43.1%) who have affected relatives. Therefore, screening blacks earlier will serve as a “reset” that has the potential of early identification of those at increased risk of CRC in the next generation. Future studies should focus on quantifying genetic susceptibility to CRC among blacks.
What CRC screening modality should be recommended for blacks?
The predisposition to proximal CRC among blacks is a potent argument to support the recommendation of the ACG that colonoscopy should be the preferred screening modality. Furthermore, colonoscopy is the diagnostic procedure after an abnormal screening from the other modalities and polyps can be removed during the same procedure. However, it is the most invasive, most expensive and presents the most difficult logistic challenge in terms of bowel preparation, co-pays, missed work days and the need for an escort due to moderate sedation. Studies have suggested a higher completion rate of screening when the underserved are offered fecal occult blood tests[76,77]. It appears that there are wide geographic variations in availability of colonoscopy to the population and in the acceptability of fecal based tests to the underserved. We observed that blacks were less likely to undergo diagnostic colonoscopy following an abnormal flexible sigmoidoscopy screening in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial[78]. This raises a concern that follow-up of abnormal screening may be a challenge for blacks and a screening modality that can permit intervention may be better. Notwithstanding, we are of the opinion that healthcare providers should discuss CRC screening options with their patients, but should make a specific recommendation for a screening modality based on the patient’s preferences, comorbidities, social situation, and locally available resources.
THE WAY FORWARD
It will require a comprehensive patient, provider, and policy-maker partnership to reduce CRC disparity. As studies have shown that blacks who receive medical care and cancer treatment similar to those of whites experience similar outcomes, enabling blacks to achieve equal access to care as whites could substantially reduce the racial disparities in CRC burden[36]. It is imperative to improve health insurance coverage and health education of the population. The implementation of the Affordable Care Act has a potential to ensure coverage for millions of previously uninsured persons and provide the necessary access to CRC screening. However, White et al[79] reported that despite the expansion of Medicare coverage for CRC screening tests, racial/ethnic differences in CRC screening persisted over time among this insured population. Hence, health care access by provision of health insurance is necessary but not sufficient to improve CRC screening and reduce CRC disparity by race.
In a meta analysis by Naylor et al[80] provider-directed multi-modal interventions which comprised of education sessions and reminders and pure educational interventions were found to be effective in raising CRC screening rates in minorities by 10%-15%. A median improvement of 16% in endoscopic CRC screening completion was noted with the patient navigator model. Tailored patient education combined with patient navigation services, and physician training in communicating with patients of low health literacy, can modestly improve adherence to CRC screening.
The New York City Department of Health and Mental Hygiene has started a program called The Citywide Colon Cancer Control Coalition (C5) to help New York City attain CRC control goals through advocacy, resource development, and policy initiatives. They launched public campaigns and implemented patient navigation systems in many of the New York City area hospitals in an effort to increase colonoscopy screening rates and reduce racial/ethnic disparities. In 2003, low rates of screening as well as screening disparities were noted with only 36% of blacks, 38% Latinos and 48% of whites having had a colonoscopy. After this concerted citywide effort, screening colonoscopy rates had increased to approximately 62 percent by 2009 with virtual elimination of screening disparities among these ethnic groups[81].
In Delaware, a “Village approach” was implemented which involved a 3-step process to reduce CRC disparities by increasing CRC screening rates, providing quality treatment and resolution of abnormalities and by using an extensive patient navigation services. The program led to increased CRC screening rates among all race-ethnicities, reduction in CRC incidence, reduction in CRC mortality and narrowing of the CRC survival differences[82].
Figure 1 shows the leading causes of CRC disparities and proffer solutions to reduce the observed disparities.
Figure 1.
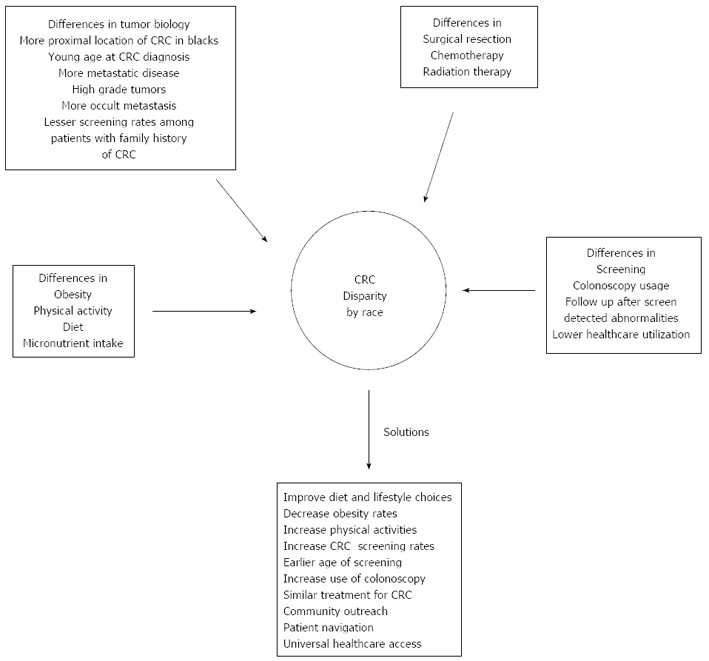
Leading causes of colorectal cancer disparities and potential solutions to reduce the observed disparities. CRC: Colorectal cancer.
CONCLUSION
There are several important factors contributing to differential CRC mortality rates among blacks and whites. A comprehensive approach that increases access and utilization of CRC screening, timely follow-up of abnormal results and treatment of CRC will be needed to reduce or eliminate disparity.
Footnotes
P- Reviewers: Bloomston M, NaitoY S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Henschke UK, Leffall LD, Mason CH, Reinhold AW, Schneider RL, White JE. Alarming increase of the cancer mortality in the U.S. black population (1950-1967) Cancer. 1973;31:763–768. doi: 10.1002/1097-0142(197304)31:4<763::aid-cncr2820310401>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Mayberry RM, Coates RJ, Hill HA, Click LA, Chen VW, Austin DF, Redmond CK, Fenoglio-Preiser CM, Hunter CP, Haynes MA. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 4.Schroy PC, Glick JT, Robinson PA, Lydotes MA, Evans SR, Emmons KM. Has the surge in media attention increased public awareness about colorectal cancer and screening? J Community Health. 2008;33:1–9. doi: 10.1007/s10900-007-9065-5. [DOI] [PubMed] [Google Scholar]
- 5.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010;100:1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol. 1999;150:390–398. doi: 10.1093/oxfordjournals.aje.a010018. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Cancer Trends Progress Report 2011-2012. Last accessed on October 1, 2013. Available from: http//progressreport.cancer.gov/doc.asp?
- 9.Bolen JC, Rhodes L, Powell-Griner EE, Bland SD, Holtzman D. State-specific prevalence of selected health behaviors, by race and ethnicity--Behavioral Risk Factor Surveillance System, 1997. MMWR CDC Surveill Summ. 2000;49:1–60. [PubMed] [Google Scholar]
- 10.O’Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137:175S–182S. doi: 10.1093/jn/137.1.175S. [DOI] [PubMed] [Google Scholar]
- 11.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 12.USDA Economic Research Service. 1994–1996 and 1998 Continuing Survey of Food Intakes by Individuals. 2000. Available from: http//www.ers.usda.gov/publications/ldp/Oct05/ldpm13502/ldpm13502.pdf.
- 13.Patterson RE, White E, Kristal AR, Neuhouser ML, Potter JD. Vitamin supplements and cancer risk: the epidemiologic evidence. Cancer Causes Control. 1997;8:786–802. doi: 10.1023/a:1018443724293. [DOI] [PubMed] [Google Scholar]
- 14.Satia-Abouta J, Galanko JA, Martin CF, Potter JD, Ammerman A, Sandler RS. Associations of micronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12:747–754. [PubMed] [Google Scholar]
- 15.Gibson GR, Macfarlane GT, Cummings JH. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993;34:437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, Shike M, Weissfeld J, Burt R, Cooper MR, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 17.Alberts DS, Martínez ME, Roe DJ, Guillén-Rodríguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 18.Laiyemo AO, Doubeni C, Badurdeen DS, Murphy G, Marcus PM, Schoen RE, Lanza E, Smoot DT, Cross AJ. Obesity, weight change, and risk of adenoma recurrence: a prospective trial. Endoscopy. 2012;44:813–818. doi: 10.1055/s-0032-1309837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessup JM, McGinnis LS, Steele GD, Menck HR, Winchester DP. The National Cancer Data Base. Report on colon cancer. Cancer. 1996;78:918–926. doi: 10.1002/(SICI)1097-0142(19960815)78:4<918::AID-CNCR32>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99:733–748. [PMC free article] [PubMed] [Google Scholar]
- 21.Dignam JJ, Colangelo L, Tian W, Jones J, Smith R, Wickerham DL, Wolmark N. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst. 1999;91:1933–1940. doi: 10.1093/jnci/91.22.1933. [DOI] [PubMed] [Google Scholar]
- 22.Doubeni CA, Field TS, Buist DS, Korner EJ, Bigelow C, Lamerato L, Herrinton L, Quinn VP, Hart G, Hornbrook MC, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109:612–620. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 23.Dominitz JA, Samsa GP, Landsman P, Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82:2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, Jamison P. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006;107:1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 25.Govindarajan R, Shah RV, Erkman LG, Hutchins LF. Racial differences in the outcome of patients with colorectal carcinoma. Cancer. 2003;97:493–498. doi: 10.1002/cncr.11067. [DOI] [PubMed] [Google Scholar]
- 26.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 27.Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54:359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 28.Alexander D, Jhala N, Chatla C, Steinhauer J, Funkhouser E, Coffey CS, Grizzle WE, Manne U. High-grade tumor differentiation is an indicator of poor prognosis in African Americans with colonic adenocarcinomas. Cancer. 2005;103:2163–2170. doi: 10.1002/cncr.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris GJ, Senagore AJ, Lavery IC, Church JM, Fazio VW. Factors affecting survival after palliative resection of colorectal carcinoma. Colorectal Dis. 2002;4:31–35. doi: 10.1046/j.1463-1318.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 30.Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, Slattery ML. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 31.Alexander D, Chatla C, Funkhouser E, Meleth S, Grizzle WE, Manne U. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101:66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edition. New York: Springer; 2010. p. 649. [Google Scholar]
- 33.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 35.Hyslop T, Weinberg DS, Schulz S, Barkun A, Waldman SA. Occult tumor burden contributes to racial disparities in stage-specific colorectal cancer outcomes. Cancer. 2012;118:2532–2540. doi: 10.1002/cncr.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 37.Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gockerman JP, Gautier M. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 38.Gregg J, Curry RH. Explanatory models for cancer among African-American women at two Atlanta neighborhood health centers: the implications for a cancer screening program. Soc Sci Med. 1994;39:519–526. doi: 10.1016/0277-9536(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 39.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 40.Morris AM, Billingsley KG, Hayanga AJ, Matthews B, Baldwin LM, Birkmeyer JD. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100:738–744. doi: 10.1093/jnci/djn396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Super N. Medigap: prevalence, premiums, and opportunities for reform. NHPF Issue Brief. 2002;(782):1–23. [PubMed] [Google Scholar]
- 42.Cooper GS, Yuan Z, Landefeld CS, Rimm AA. Surgery for colorectal cancer: Race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health. 1996;86:582–586. doi: 10.2105/ajph.86.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball JK, Elixhauser A. Treatment differences between blacks and whites with colorectal cancer. Med Care. 1996;34:970–984. doi: 10.1097/00005650-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Demissie K, Oluwole OO, Balasubramanian BA, Osinubi OO, August D, Rhoads GG. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between Whites and Blacks. Ann Epidemiol. 2004;14:215–221. doi: 10.1016/j.annepidem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637–645. doi: 10.1245/ASO.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin LM, Dobie SA, Billingsley K, Cai Y, Wright GE, Dominitz JA, Barlow W, Warren JL, Taplin SH. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, Fuchs CS. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94:1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, O’Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83:583–589. doi: 10.1136/pgmj.2007.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yothers G, Sargent DJ, Wolmark N, Goldberg RM, O’Connell MJ, Benedetti JK, Saltz LB, Dignam JJ, Blackstock AW; ACCENT Collaborative Group. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103:1498–506. doi: 10.1093/jnci/djr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabeneck L, Souchek J, El-Serag HB. Survival of colorectal cancer patients hospitalized in the Veterans Affairs Health Care System. Am J Gastroenterol. 2003;98:1186–1192. doi: 10.1111/j.1572-0241.2003.07448.x. [DOI] [PubMed] [Google Scholar]
- 52.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 53.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys SS, Crawford ED, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 55.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Fisher DA, Dougherty K, Martin C, Galanko J, Provenzale D, Sandler RS. Race and colorectal cancer screening: a population-based study in North Carolina. N C Med J. 2004;65:12–15. [PubMed] [Google Scholar]
- 57.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 58.Wilkins T, Gillies RA, Harbuck S, Garren J, Looney SW, Schade RR. Racial disparities and barriers to colorectal cancer screening in rural areas. J Am Board Fam Med. 2012;25:308–317. doi: 10.3122/jabfm.2012.03.100307. [DOI] [PubMed] [Google Scholar]
- 59.Benarroch-Gampel J, Sheffield KM, Lin YL, Kuo YF, Goodwin JS, Riall TS. Colonoscopist and primary care physician supply and disparities in colorectal cancer screening. Health Serv Res. 2012;47:1137–1157. doi: 10.1111/j.1475-6773.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doubeni CA, Laiyemo AO, Klabunde CN, Young AC, Field TS, Fletcher RH. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010;38:184–191. doi: 10.1016/j.amepre.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tehranifar P, Neugut AI, Phelan JC, Link BG, Liao Y, Desai M, Terry MB. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18:2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, van Ballegooijen M, Zauber AG, Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev. 2012;21:728–736. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18:2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doubeni CA, Jambaulikar GD, Fouayzi H, Robinson SB, Gunter MJ, Field TS, Roblin DW, Fletcher RH. Neighborhood socioeconomic status and use of colonoscopy in an insured population--a retrospective cohort study. PLoS One. 2012;7:e36392. doi: 10.1371/journal.pone.0036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fyffe DC, Hudson SV, Fagan JK, Brown DR. Knowledge and barriers related to prostate and colorectal cancer prevention in underserved black men. J Natl Med Assoc. 2008;100:1161–1167. doi: 10.1016/s0027-9684(15)31478-4. [DOI] [PubMed] [Google Scholar]
- 66.Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Med Care. 2008;46:738–745. doi: 10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- 67.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 68.Merchant JL, Omary MB. Underrepresentation of underrepresented minorities in academic medicine: the need to enhance the pipeline and the pipe. Gastroenterology. 2010;138:19–26.e1-19-26.e3. doi: 10.1053/j.gastro.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Abreu MT, Okolo PI. Re: underrepresentation of underrepresented minorities in academic medicine. Gastroenterology. 2010;139:359; author reply 359. doi: 10.1053/j.gastro.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 70.United States Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 71.Gupta S, Shah J, Balasubramanian BA. Strategies for reducing colorectal cancer among blacks. Arch Intern Med. 2012;172:182–184. doi: 10.1001/archinternmed.2011.594. [DOI] [PubMed] [Google Scholar]
- 72.Qaseem A, Denberg TD, Hopkins RH, Humphrey LL, Levine J, Sweet DE, Shekelle P. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 73.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 74.Cash BD, Banerjee S, Anderson MA, Ben-Menachem T, Decker GA, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Jue TL, et al. Ethnic issues in endoscopy. Gastrointest Endosc. 2010;71:1108–1112. doi: 10.1016/j.gie.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Murff HJ, Peterson NB, Fowke JH, Hargreaves M, Signorello LB, Dittus RS, Zheng W, Blot WJ. Colonoscopy screening in African Americans and Whites with affected first-degree relatives. Arch Intern Med. 2008;168:625–631. doi: 10.1001/archinte.168.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Muñoz R, Lau C, Somsouk M, El-Nachef N, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, Valdez L, Tong L, Ahn C, Kashner M, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173:1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE, Crawford ED, Kvale P, Fouad M, Hickey T, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102:538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White A, Vernon SW, Franzini L, Du XL. Racial and ethnic disparities in colorectal cancer screening persisted despite expansion of Medicare’s screening reimbursement. Cancer Epidemiol Biomarkers Prev. 2011;20:811–817. doi: 10.1158/1055-9965.EPI-09-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27:1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richards CA, Kerker BD, Thorpe L, Olson C, Krauskopf MS, Silver LS, Weber TK, Winawer SJ. Increased screening colonoscopy rates and reduced racial disparities in the New York Citywide campaign: an urban model. Am J Gastroenterol. 2011;106:1880–1886. doi: 10.1038/ajg.2011.191. [DOI] [PubMed] [Google Scholar]
- 82.Grubbs SS, Polite BN, Carney J, Bowser W, Rogers J, Katurakes N, Hess P, Paskett ED. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013;31:1928–1930. doi: 10.1200/JCO.2012.47.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]


