Abstract
Management of rectal cancer has markedly evolved over the last two decades. New technologies of staging have allowed a more precise definition of tumor extension. Refinements in surgical concepts and techniques have resulted in higher rates of sphincter preservation and better functional outcome for patients with this malignancy. Although, preoperative chemoradiotherapy followed by total mesorectal excision has become the standard of care for locally advanced tumors, many controversial matters in management of rectal cancer still need to be defined. These include the feasibility of a non-surgical approach after a favorable response to neoadjuvant therapy, the ideal margins of surgical resection for sphincter preservation and the adequacy of minimally invasive techniques of tumor resection. In this article, after an extensive search in PubMed and Embase databases, we critically review the current strategies and the most debatable matters in treatment of rectal cancer.
Keywords: Rectal cancer, Colorectal cancer, Staging, Sphincter preservation, Neoadjuvant chemo-radiotherapy, Surgery
Core tip: Rectal cancer management is currently a multidisciplinary effort, which incorporates new concepts and technologies, resulting in significant improvement in patients’ oncological and functional outcomes. Despite the evolution reported in the last decades, there are still many unanswered questions about treatment of rectal cancer. In this article, we critically analyzed the main controversial matters in current rectal cancer management.
INTRODUCTION
In the last two decades there have been significant changes in evaluation and management of rectal cancer. Incorporation of new technologies of staging and new therapeutic concepts has improved oncologic and functional results in patients with this malignancy. Treatment of the disease has become a multidisciplinary effort, which depends upon the integration between oncologists and colorectal surgeons. The goal of this article is to analyze the main controversial matters in current rectal cancer management.
LITERATURE SEARCH
We performed a literature search using PubMed and Embase databases up to September 2013. The following MeSH search terms were used: rectal cancer, tumor staging, total mesorectal excision, radiotherapy, chemoradiotherapy, neoadjuvant chemo-radiotherapy, ‘‘watch and wait’’, surgery and sphincter preservation. These terms were applied in various combinations to maximize the search. Only articles written in English were included. Additional searches of the embedded references from primary articles were performed to further improve the review.
ANATOMIC DEFINITIONS
Some anatomic concepts are essential for planning rectal cancer therapy (Figure 1). The rectum is the segment of the large bowel located between the sigmoid colon and the anal canal. Its upper limit is generally located at the level of the sacral promontory, roughly corresponding to a point where the taeniae coli spread out and can no longer be distinguished. For practical purposes, it is accepted that malignant tumors located within 15 cm from the anal verge (using a rigid proctoscope) should be diagnosed as rectal cancers. Below the peritoneal reflection, the rectum has no serosal layer and is surrounded by a circumferential fatty sheath known as mesorectum. It contains the perirectal lymph nodes, which usually represent the first sites to which rectal tumors disseminate[1,2].
Figure 1.
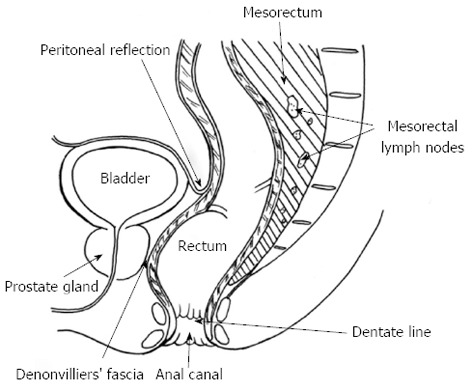
Rectal anatomy.
PRETREATMENT EVALUATION AND STAGING
The process of staging a rectal cancer starts with a careful physical examination. Digital examination of the rectum, along with proctoscopy, should determine degree of tumor fixation, percentage of the circumference involved, distance of the tumor form the anal verge and likelihood of sphincter preservation. Vaginal exam may reveal direct tumoral invasion. Inguinal lymph nodes should be examined if tumor arises from the lower third of the rectum. According to the the American Society of Colon and Rectal Surgeons 2013 guidelines[2], for preoperative staging a complete colonoscopy should be performed, if the tumor is not obstructive, for histologic confirmation of the diagnosis and to rule out proximal synchronous lesions. In cases in which a full colonoscopy cannot be performed, a preoperative double-contrast barium enema or a computed tomography (CT) colonography may be used. Alternatively, for patients with incomplete preoperative colonoscopy, intraoperative colonoscopy may be used as an effective method to detect synchronous lesions[3].
In order to complete pretreatment staging, a CT scan of the thorax and abdomen and pelvis, and the measurement of serum carcinoembryonic antigen are recommended[2]. Once distant metastases have been ruled out, the most important factor to define the strategy of treatment to be adopted is the locoregional staging. Tumor node metastasis (TNM) system, as recommended by the American Joint Committee on Cancer, is currently the most widely used system for staging rectal adenocarcinomas (Tables 1 and 2)[4].
Table 1.
Tumor node metastasis clinical classification (colon and rectum cancer)
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades subserosa or into non-peritonealized pericolic or perirectal tissues |
| T4 | Tumor directly invades other organs or structures and/or perforates visceral peritoneum |
| T4a | Tumor perforates visceral peritoneum |
| T4b | Tumor directly invades other organs or structures |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N1a | Metastasis in 1 regional lymph node |
| N1b | Metastasis in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s), i.e., satellites, in the subserosa, or in non-peritonealized pericolic or perirectal soft tissue without regional lymph node metastasis |
| N2 | Metastasis in 4 or more regional lymph nodes |
| N2a | Metastasis in 4-6 regional lymph nodes |
| N2b | Metastasis in 7 or more regional lymph nodes |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to one organ [liver, lung, ovary, non-regional lymph node(s)] |
| M1b | Metastasis in more than one organ or the peritoneum |
AJCC: American joint committee on cancer[4].
Table 2.
Tumor node metastasis stage grouping (colon and rectum cancer)
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1, T2 | N0 | M0 |
| Stage II | T3, T4 | N0 | M0 |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T4a | N0 | M0 |
| Stage IIC | T4b | N0 | M0 |
| Stage III | Any T | N1, N2 | M0 |
| Stage IIIA | T1, T2 | N1 | M0 |
| T1 | N2a | M0 | |
| Stage IIIB | T3, T4a | N1 | M0 |
| T2, T3 | N2a | M0 | |
| T1, T2 | N2b | M0 | |
| Stage IIIC | T4a | N2a | M0 |
| T3, T4a | N2b | M0 | |
| T4b | N1, N2 | M0 | |
| Stage IVA | Any T | any N | M1a |
| Stage IVB | Any T | any N | M1b |
AJCC: American joint committee on cancer[4].
There is considerable controversy on what is the ideal method to evaluate local extent of the tumor. Although CT scan is no longer considered the modality of choice, it can be used in patients with primarily advanced T-stage tumors (accuracy of 79% to 94%). Accuracy fells to 52% to 74% when smaller and less advanced tumors are analyzed[5-9]. Endorectal ultrasound (EUS) and magnetic resonance imaging (MRI) with either endorectal or increasingly phase array coils are currently the modalities of choice for the local staging. EUS is considered more precise (T-stage accuracy ranging from 75% to 95%), as compared to MRI (T-stage accuracy ranging from 59% to 95%). However, the efficacy of EUS is limited in stenotic or large bulky lesions which cannot be traversed by the probe[10-12].
Evaluation of perirectal lymph nodes is still a major controversial matter, particularly because metastases can be found in normal-sized lymph nodes[13,14]. According to a meta-analysis of 90 studies[15], none of the three imaging modalities were significantly superior in defining lymph node involvement. Sensitivities and specificities of the exams were as follows: CT (55% to 74%), EUS (67% to 78%), and MRI (66% to 76%).
TREATMENT CONTROVERSIES
Local excision
Despite limitations of the imaging exams, they are essential to define the strategy of treatment to be adopted. Tumors classified as T1 with no evidence of nodal involvement may be amenable to local excision, depending upon some specific criteria. These include: well to moderately differentiated carcinomas, measuring less than 3 cm in diameter, occupying less than a third of the circumference of the rectal lumen, with no lymphatic, vascular or perineural invasion[16,17]. Using these selection criteria, it can be achieved 10-year overall survival rates of 84% and disease-free survival of 75%[18].
There are currently two main techniques for local excision. The first one is transanal resection which consists of excision of all layers of the bowel wall, including perirectal fat, with disease-free margins of at least one centimeter. It is a procedure that colorectal surgeons are familiar with, however its indication is limited to tumors located within 8 cm from the anal verge[19]. The second method is transanal endoscopic microsurgery (TEM), which utilizes a special proctoscope with a 3D binocular optic and a set of endoscopic surgical instruments that permit resection of tumors located up to 20 cm from the anal verge. To this moment, there are few studies comparing TEM with transanal resection or with radical surgery[20,21]. However, the published trials have suggested the technique is relatively safe, usually with minor complications.
It is important to take into account that even in well-selected patients (cT1N0), the risk of lymph nodes metastases can reach 10%. The most challenging aspect in selecting patients for that sort of treatment is that the preoperative staging remains limited in defining precisely tumor invasion and nodal involvement. Considering that the local recurrence rate may vary from 26% to 47% for T2 lesions, a radical resection must be indicated for patients with these lesions. However, if the patient is a poor surgical candidate, we could alternatively recommend complementary chemoradiation to reduce the risk of local recurrence[18].
NEOADJUVANT TREATMENT
According to the current evidence, tumors classified as TNM stage II or III (T3/T4, N1/N2) should receive neoadjuvant treatment before radical resection[22]. Two main options of preoperative therapy have been proposed. The first is short-course radiotherapy, which is considered the treatment of choice in North-European countries and Scandinavia. The second option is the so-called long-course preoperative chemoradiotherapy, which is the favored treatment in most European countries and in the United States. Characteristics and results of the main trials investigating the neoadjuvant strategies in rectal carcinoma are presented in Table 3.
Table 3.
Major neoadjuvant therapy trials
| Ref. | n | Treatment arms | Local recurrence rate | Overall survival rate |
| Upsala Trial[23] | 471 | Arm 1 (236): preoperative RT (25.5 Gy delivered in 5-7 d) Arm 2 (235): postoperative RT (60 Gy delivered in 8 wk) | 5 yr of follow-up Arm 1: 12% Arm 2: 21% P = 0.02 | 5-yr survival rate Arm 1: 42% Arm 2: 38% P = 0.42 |
| Stockholm I Trial[24] | 849 | Arm 1 (423 patients): 25 Gy during 5-7 d followed by surgery Arm 2 (421 patients): surgery alone | Median follow-up time of 107 mo Arm 1: 14% Arm 2: 28% P < 0.01 | Median follow-up time of 107 mo No significant difference between groups |
| Swedish Rectal Cancer Trial[25] | 1168 | Arm 1 (553 patients): preoperative RT - 25 Gy delivered in five fractions in 1 wk, followed by surgery Arm 2 (557 patients): Surgery alone | 5 yr of follow-up Arm 1: 11% Arm 2: 27% P < 0.001 | 5-yr survival rate Arm 1: 58.0% Arm 2: 48.0% P = 0.004 |
| Dutch TME Trial[26] | 1861 | Arm 1 (924 patients): preoperative RT (5 Gy × 5 d) followed by TME Arm 2 (937): TME alone | 2 yr of follow-up Arm 1: 2.4% Arm 2: 8.2% P < 0.001 | 2-yr survival rate Arm 1: 82.0% Arm 2: 81.8% P = 0.84 |
| Stockholm II Trial[27] | 557 | Arm 1 (272): preoperative radiotherapy (25 Gy in one week) followed by surgery within a week Arm 2 (285): surgery alone | Median follow-up was 8.8 yr Arm 1: 12% Arm 2: 25% P < 0.001 | Median follow-up 8.8 yr Arm 1: 39% Arm 2: 36% P = 0.2 |
| German Rectal Cancer Study Group[28] | 823 | Arm 1 (421 patients): preoperative CHRT: 50.4 Gy/28 fractions/5 fractions weekly and fluorouracil (continuous infusion) in first and fifth week of RT. TME after 6 wk Additional 4 cycles of FU every 4 wk Arm 2 (402 patients): postoperative CHRT (same as in Arm 1 except a 5.4 Gy boost in RT) | 5 yr of follow-up Arm 1: 6.0% Arm 2: 13% P = 0.006 | 5-yr survival rate Arm 1: 76.0% Arm 2: 74.0% P = 0.80 |
| Polish Rectal Cancer Trial[29] | 312 | Arm 1 (155 patients): preoperative RT (5 Gy × 5 d) followed by TME at 7 d after RT Arm 2 (157 patients): preoperative RT (45 Gy/25 fractions/5 wk) + 2 cycles of chemotherapy on weeks 1 and 5 of RT followed by TME 4-6 wk later. The cycle consisted of leucovorin + fluorouracil both administered as rapid infusion on 5 consecutive days | 4 yr of follow-up Arm 1: 9% Arm 2: 14.2% P = 0.170 | 4-yr survival rate Arm 1: 67.2% Arm 2: 66.2% P = 0.960 |
| MRC CR07 and NCIC CTG C016[30] | 1350 | Arm 1 (674 patients): short-course radiotherapy (25 Gy/5 fractions) followed by surgery. Arm 2 (676 patients): initial surgery with selective postoperative chemoradiotherapy (45 Gy in 25 fractions plus 5-fluorouracil) restricted to patients with involvement of the circumferential resection margin. | 3 yr of follow-up Arm 1: 4.0% Arm 2: 10.6% P < 0. 01 | Estimated 5-yr survival rate Arm 1: 70.3% Arm 2: 67.9% P = 0.40 |
RT: Radiotherapy; TME: Total mesorectal excision; CHRT: Chemoradiotherapy; FU: Fluorouracil.
Recently, the German Rectal Cancer Study Group published results of their trial after a median follow-up of 11 years[31]. Overall survival at 10 years was 59.6% in the preoperative arm and 59.9% in the postoperative arm (P = 0.85). The 10-year cumulative incidence of local relapse was 7.1% and 10.1% in the preoperative and postoperative groups, respectively (P = 0.048), representing a small absolute reduction of long-term local recurrence (3%). No significant differences were detected for 10-year incidence of distant metastases (29.8% and 29.6%) and disease-free survival.
A recently published meta-analysis[32] assessed effectiveness and safety of neoadjuvant radiotherapy in rectal cancer. The authors searched several database, analyzing randomized controlled trials comparing either neoadjuvant therapy vs surgery alone (17 trials including 8568 patients) or neoadjuvant chemoradiotherapy vs neoadjuvant radiotherapy (5 trials including 2.393 patients). Neoadjuvant radiotherapy decrease local recurrence (HR = 0.59; 95%CI: 0.48-0.72) compared to surgery alone even after total mesorectal excision. It has marginal benefit in overall survival (HR = 0.93; 95%CI: 0.85-1.00), but was associated with increased perioperative mortality (HR = 1.48; 95%CI: 1.08-2.03). Neoadjuvant chemoradiation improved local control as compared to radiotherapy alone (HR = 0.53; 95%CI: 0.39-0.72), but both treatments had no influence in long-term survival.
One of the potential advantages of the neoadjuvant treatments is the possibility of tumor shrinkage, which, in theory, could increase the chance of performing a sphincter saving surgery. This hypothesis has been investigated in several trials using different regimens of preoperative treatment[30,33]. Recently, Gerard et al[34] published the results of a well-conducted systematic review on the impact of the neoadjuvant treatments in sphincter preservation. Seventeen randomized trials published between 1988 and 2009 were analyzed. The rate of sphincter saving surgery increased from 30% for the patients operated in the 80’s[18] up to 77% in 2008[15]. However, in none of the main trials analyzed (except three trials with low number of patients) it was possible to demonstrate a significant benefit of the neoadjuvant treatment on the rate of sphincter preserving surgery. According to the authors, this increase in sphincter preservation appears to be the result of new technologies and changes in surgical concepts, such as incorporation of total mesorectal excision (TME) and the techniques for very low anastomosis.
These findings are in line with a previous study by Bujko et al[35] in which 10 randomized trials were reviewed. These studies included 4596 patients in whom preoperative chemoradiotherapy resulted in tumor shrinkage in the neoadjuvant arm as compared with the control arm. As acknowledged by the authors, there were several difficulties in comparing the studies, including different preoperative radiochemotherapy schemes, duration of the interval between radiotherapy and surgery and patient populations. Despite these limitations, it was concluded that preoperative radiotherapy does not have a positive impact on the rate of anterior resection and sphincter preservation.
“WAIT AND SEE” APPROACH
In about 10%-20% of patients with rectal cancer who receive preoperative chemoradiation, a pathological complete response (pCR), characterized by absence of viable tumor cells within the surgical specimen, can be expected[36]. According to a systematic review and meta-analysis of the literature, patients with pCR have better oncologic outcomes as compared with those presenting a less marked response to chemoradiation, including better rates of local recurrence, distant metastases, disease-free and overall survival at 5 years[37].
According to some authors, it is therefore valid to think that patients whose tumors have been sterilized by chemoradiation would have no additional benefit from a subsequent radical resection. In 1998, Habr-Gama et al[38] from Brazil proposed the “wait and see” policy for patients who achieve what they called complete clinical response (cCR) after neoadjuvant treatment. They evaluated 118 patients treated by preoperative CRT (50.4 Gy and concurrent 5-FU and leucovorin for 3 consecutive days on the first and last 3 d of radiotherapy). All patients underwent repeat evaluation and biopsy of any suspected residual lesions or scar tissue. Thirty-six patients (30.5%) were classified as being complete responders. In only six of these patients, complete response was confirmed by the absence of tumor in the surgical specimen. In the other 30 patients, a complete response was assumed by the absence of symptoms and negative findings on physical examination, biopsy and imaging tests during a median follow-up of 36 mo. Out of the later group, eight patients presented local failure, demanding salvage resection. The outcome for patients without recurrence was similar to that of patients found at surgery to have achieved a pCR. The authors concluded that about 26% of their patients could be spared from surgical resection using that conservative strategy of management.
In subsequent publications Habr-Gama et al[39] repeatedly reported favorable results. In 2006, they reported the outcome of 361 patients with distal rectal cancer managed by neoadjuvant chemoradiation. One hundred twenty-two patients were considered to have complete clinical response and were not immediately operated on. Of them, 99 patients sustained complete clinical response for at least 12 mo and were considered stage c0 (27.4%). There were 13 recurrences, but only six of these cases had local recurrent disease. Overall and disease-free 5-year survivals were 93% and 85% respectively.
Such good results, however, could not be reproduced by other groups. Nyasavajjala et al[40] reviewed pathologic results of patients operated on for rectal cancer after long course neoadjuvant chemoradiotherapy in two different tertiary British hospitals. One hundred and thirty-two consecutive patients were treated between 2002 and 2007. Only 13 out of 132 (10%) of patients had a complete pathological response, representing one-third of the cCR previously reported. They concluded that nonsurgical therapy for rectal cancer according to Habr-Gama algorithm of treatment may only be effective in a very small proportion of patients and could not be recommended. As shown in Table 4, there is a wide variation among studies in the rates of local recurrence for patients with cCR to chemoradiation who were not submitted to a subsequent rectal resection.
Table 4.
Locoregional recurrence in patients with complete clinical response who did not proceed to rectal resection n (%)
| Ref. | No. of patients | T2 | Radiotherapy | Chemotherapy | Complete clinical response | Locoregional recurrence |
| Nakagawa et al[41] | 52 | No | 45-50.4 Gy, 28 fractions, 38 d | Fluorouracil + leucovorin | 10 (19.2) | 8 (80) |
| Habr-Gama et al[42] | 360 | Yes (14%) | 50.4 Gy, 28 fractions, 5-6 wk | Fluorouracil + leucovorin | 99 (27.5) | 6 (6) |
| Lim et al[43] | 48 | T1/t2 (33%) | Mean 50 Gy, 25 fractions | Fluorouracil | 27 (56) | 11 (23) |
| Hughes et al[44] | 58 | No | 45 Gy, 25 fractions, 33 d | Fluorouracil + leucovorin | 10 (17) | 6 (60) |
| Dalton et al[45] | 49 | No | 45 Gy, 25 fractions, 33 d | Capecitabine | 12 (24) | 6 (50) |
| Maas et al[46] | 192 | Yes (24%) | 50.4 Gy, 28 fractions, 6 wk | Capecitabine | 21 (10.9) | 1 (5) |
Recently, Glynne-Jones et al[36] conducted a systematic review of studies evaluating non-operative treatments and the “wait and see” strategy in rectal cancer. Most studies were retrospective and there were no randomized phase II or phase III trials. In all they could evaluate nine series, including 650 patients: 361 patients from the Habr-Gama series and 289 patients from the eight remaining series. The results in terms of cCR and local recurrence reported in the Brazilian series were clearly superior. However, there were significant heterogeneity among studies in terms of treatment regimen, methods of assessment used to define a cCR (digital exam, biopsy or imaging tests) and the follow-up strategy used. The authors concluded that evidence for the “wait and see” policy comes mainly from a single retrospective series, which included highly selected cases. Results obtained in patients with small rectal cancers cannot be extrapolated at this moment to patients with more advanced tumors where nodal involvement can be anticipated.
The main obstacle to implement the “wait-and-see” policy is the current lack of accuracy of the tests in determining whether there has really been a complete pathological response[36]. Due to changes in pelvic tissues after radiotherapy (edema, inflammation, fibrosis) it is very difficult to assess whether an apparent clinical response will eventually translate into a complete pathological response. Digital exam, proctoscopy and imaging tests (endoanal ultrasound, MRI or positron emission tomography-CT) are still imprecise for detecting microscopic tumor deposits within the rectum and perirectal lymph nodes[47]. Thus, the non-surgical approach remains experimental at this moment. Prospective multi-institutional controlled studies based on uniform inclusion criteria are needed to define the efficacy and risks of this form of treatment.
RADICAL SURGICAL APPROACH
Surgery remains as the cornerstone curative treatment for rectal cancer. Sphincter preservation must be seen as a secondary objective, which should not compromise oncological adequacy of resection. Radical surgical treatment for rectal cancer, which consists of the resection of the rectum and lymphadenectomy, includes two main procedures: low anterior resection (LAR) and abdominoperineal resection (APR). In LAR, the anal sphincter complex is preserved and it is possible to restore intestinal continuity. In APR, the anal sphincters are resected en bloc and it is necessary to construct a definitive colostomy. Currently, LAR is the most commonly used surgery for the rectal cancer, while APR is applied in cases where it is not possible to get free margins without resecting the anal sphincter complex[48].
As a rule, the lower is the level of a rectal cancer, the worse is its prognosis. In a series[49] in which 2136 patients underwent radical surgeries with TME, the local recurrence rate was 15%, 13% and 9% for tumors located in inferior, medium and superior rectum, respectively. The correspondent five-year survival rates were 59%, 62% and 69%. The rate of local recurrence was 10% in patients submitted to LAR compared with 15% for patients submitted to APR. In addition, the five-year survival rate was 68% for LAR and 55% for APR. A similar study[50] analyze patients who underwent radical surgeries without any adjuvant therapy. The mean rate of local recurrence rate was 18.5% (19.3% for APR and 16.2% for LAR). There is currently strong evidence in literature showing that LAR and APR have similar long-term oncological results when appropriate surgical margins can be assured.
A diverting ostomy is strongly recommended for patients undergoing a LAR, particularly when a low anastomosis is constructed. A meta-analysis[51] of 4 randomized controlled trials and 21 non-randomized studies, including 11429 patients, showed more favorable results in patients with a diverting ostomy as compared with those without a protective stoma. Meta-analysis of the randomized trials showed lower rates of clinical anastomotic leak (RR = 0.39; P < 0.001) and reoperation (RR = 0.29; P < 0.001) in the stoma group. Similarly, meta-analysis of the non-randomized studies demonstrated lower rates of clinical anastomotic leak (RR = 0.74; P < 0.001), reoperation (RR = 0.28; P < 0.001) and mortality (RR = 0.42; P < 0.001) in the stoma group. A protective ostomy may be either a colostomy (usually in the transverse colon) or an ileostomy. The latter is more frequently used because it is technically easier to reverse and less associated with stoma prolapse. Usually, the ileostomy reversal is undertaken within 8 to 12 wk after the primary rectal resection[52].
DISTAL MARGIN OF RESECTION
At present, a distal mural margin of 2cm is considered the standard for rectal cancer resections[2]. One cm distal margin is accepted for low rectal tumors, if it is necessary to avoid an APR[2,53-56], because distal intramural spread occurs over 1 cm in only 4%-10% of the cases[57,58]. Ueno et al[59] demonstrated in a retrospective analysis of 80 patients who underwent APR that intra-mural distal spread occurs in 10.6% of the patients. In only 2.3% of the cases tumor cells can be found more than 1cm from the tumor, mainly in poor differentiated carcinomas. There are some authors that accept margins even shorter than the 1 cm[60]. In a recent meta-analysis evaluating 17 studies including 7097 patients the local recurrence rate was only 1% higher in the group of patients with distal margins less than 1 cm when compared to the group with distal margins measuring more than 1 cm (95%CI: 0.6-2.7, P = 0.175). They were not able to find a statistically significant difference in either local control or survival with margins of less than 1 cm. Analysis of a subgroup of patients with negative margins as close as 5 mm to the lower tumor border suggests it can be safely adopted in histologically favorable tumors[60]. To this date, however, there is no study establishing valid criteria for selecting patients to resection with less than 1 cm distal margin.
TOTAL MESORECTAL EXCISION
TME was proposed and made popular by Heald et al[61] in 1982, being recommended for tumors of the mid and lower rectum. It consists of complete excision of all the mesorectal tissue evolved by the visceral layer of endopelvic fascia, which must be kept intact and the circumferential margins not compromised. TME is based on the observation that viable tumor cells can be found within the mesorectum as far as 3 to 4 cm from the tumor lower border[62,63]. The technique consists of sharp dissection without the use of blunt instruments (including fingers) on the natural avascular plane between visceral and the parietal endopelvic fascia layers. That dissection requires the removal of the Denonvilliers fascia, especially when the tumor is anterior. The hypogastric and parasympathetic pelvic nerves must be preserved, avoiding urinary and sexual disfunction. Circumferential margins should be widely resected to reduce rates of local recurrence[64,65].
Since the introduction of TME, the five-year survival rates increased from 45%-50% to 75% and the local recurrence rates decreased from 30% to 5%-8%[66]. It is not necessary, however, to perform a TME in upper rectal tumors. Resecting a 5 cm distal margin of the mesorectum below the inferior tumor edge (partial mesorectal excision) is enough in those cases[62].
SPHINCTER PRESERVATION IN ULTRA-LOW RECTAL TUMORS
Sphincter preservation remains a challenge in low rectal tumors. Whenever safe distal margins cannot be achieved, an APR is still the treatment of choice. However, for tumors located within 6 cm from the anal verge some conservative surgical procedures may be attempted[67,68]. In the ultra-LAR (uLAR), the rectal transection is performed transanally, with straight view to the inferior tumor board, and a manual coloanal anastomosis (CAA). Another alternative is the intersphincteric resection (ISR), in which the internal anal sphincter is partially or totally resected in order to obtain appropriate longitudinal and radial margins[68].
Recently, Rullier et al[68] have tried to standardize surgical treatment for the inferior rectal tumors, proposing a new classification of these tumors according its location and degree of sphincter invasion. They subdivided low rectal tumors into four categories: Type I (supra-anal tumor): inferior tumor board located more than 1 cm from the anal ring; Type II (juxta-anal tumor): inferior tumor board is located ≤ 1 cm distant from the anal ring; Type III (intra-anal tumor): there is internal sphincter invasion; and Type IV (transanal tumor): when there is external sphincter or levator ani muscle invasion. In this study, Type I cancers were treated through a conventional CAA, that was a Park procedure, including anal mucosectomy above the dentate line and preservation of the anal internal sphincter. Type II tumors underwent partial ISR to achieve sphincter-preserving surgery with 1 cm distal resection margin. Type III lesions had a total ISR removing the whole of the internal sphincter. Type IV lesions were treated through APR. Using that classification, the authors operated 404 cases, with local recurrence rates of 6%, 5%, 9% and 17% respectively for the types I, II, III and IV (P = 0.186). Only 50% of the patients had good fecal continence while 11% had a severe fecal incontinence. Besides, 6% of their patients needed definitive colostomy due to postoperative fecal incontinence. As the authors recognized, results of their retrospective series need to be confirmed in prospective clinical trials.
Whenever a very low colorectal anastomosis is performed, the rectal reservoir is lost and it can result in the so-called anterior resection syndrome (soiling, urgency, multiple defecation). In this context, it is interesting to observe that some studies demonstrated that the quality of life of patients who have undergone uLAR may be inferior when compared to the one of patients submitted to APR[69]. Functional results of ISR are recognizably suboptimum[68,70], with only 50% of patients maintaining fecal continence after two years[68,71]. Thus, ISR must be considered for patients with adequate sphincteric function, as demonstrated by manometric evaluation of anal sphincters, and for those that can accept that functional results may be suboptimum.
SURGICAL SPECIALIZATION
The specialization degree of the surgeon is an important prognostic factor for the treatment of colorectal cancer. Patients operated by surgeons specialized in colorectal surgery have better outcome[72]. Regarding the rectal cancer, some studies confirm the influence of surgical specialization on the prognosis. In a population based audit[73] with 8219 cases, patients who have undergone elective proctectomy by colorectal surgeons obtained higher sphincter preservation rates as compared to the ones operated by general surgeons (OR = 1.42; P = 0.018). In a historical cohort study[74] involving five general hospitals with 683 patients operated on with curative intent, cancer-free five-year survival rate was significantly higher in the group operated by colorectal surgeons (HR = 1.5; P = 0.03). Similarly, a recent review and meta-analysis[75] demonstrated that patients operated by colorectal surgeons presented a lower rate of permanent ostomy (RR = 0.7; 95%CI: 0.53-0.94). In addition, in a retrospective study[76] with 384 consecutive rectal cancer patients, the results were significantly better when patients were operated by colorectal surgeons. After multivariate analysis, the five-year survival was 77% for patients operated by specialists and 68% for the patients operated by general surgeons. There was also a better local control as well as a higher rate of sphincter preservation in patients operated by specialists.
MINIMALLY INVASIVE SURGERY
Current evidence indicates that laparoscopic TME has equivalent oncological results as compared to open TME, when performed by experienced laparoscopic surgeons[2]. Most studies reported similar oncological results when comparing both techniques[77-81]. In a meta-analysis[82] involving 17 trials with 3158 patients with rectal cancer submitted to curative operations there was a statistically significant difference in the average number of recovered lymph nodes (laparoscopy = 10, open = 12, P = 0.001). However, it had no impact on the clinical outcome of the patients. Furthermore, there was no difference in the radial, proximal or distal margin status between the groups. In the CLASICC Trial[83], a single-institution clinical trial with 253 patients, the incidence of positive radial margins was 12% in the laparoscopic LAR group vs 6% for the open LAR one. That difference, however, was not statistically significant and there was no difference regarding local recurrence within five years.
Four prospective trials including 886 patients have reported no significant difference in disease-free or overall survival between the laparoscopic and open groups with a follow-up ranging from 37 to 113 mo[77-80]. In the COLOR II trial[84], a multicenter randomized clinical trial with 1103 patients, there was no significant difference between the open surgery group and the laparoscopy group in relation to radial and distal margin status and in the number of recovered lymph nodes. A definitive answer regarding the safety and effectiveness of the rectal cancer laparoscopic surgery, however, still depends on the results of multicenter studies such as ACOSOG-Z6051 trial[85], currently conducted in the United States.
The recent introduction of the robotic surgical system has revolutionized the field of minimally invasive surgery. It provides several technical advances in relation to laparoscopic surgery: better ergonomics, stable camera control, high-definition three-dimensional vision, filter of physiologic tremor and human wrist-like motion of robotic instruments[86]. Robotic technology also eliminates the fatigue associated with conventional laparoscopy and offers more comfort to the surgeon[87]. Another advantage of robotic surgery is a shorter learning curve when compared to laparoscopy, although cost of robotic surgery is significantly higher[88].
Several technical issues of the robotic surgery, however, should be carefully taken into account. There is a loss of tactile sensation with the robotic approach, which results in lack of tensile feedback to the surgeon. It can cause excessive traction of tissues and damage to anatomic structures, particularly during the initial experiences with the technique. Operative time is usually longer using the robotic system as compared with the laparoscopic approach, particularly because docking and separation of the robotic instruments from patient is a time consuming procedure. The patient’s surgical position cannot be modified without undocking the robotic instruments, which may result in prolonged operative time and potential delay in conversion to open surgery if it is eventually necessary[88,89].
Recent studies have compared robotic surgery to laparoscopy for the treatment of rectal cancer. A meta-analysis[89] with 854 patients has demonstrated that the conversion rate to open surgery was significantly lower in the robotic approach when compared to the laparoscopic surgery (OR = 0.26; 95%CI: 0.12-0.57, P = 0.0007). In that study, there was no significant difference between the groups regarding operative length, hospital stay, postoperative complications, number of recovered lymph nodes and positive radial/distal margin status. In another study[90] including 84 patients who have undergone uLAR, the robotic surgery showed a lower conversion rate to the open procedure (robot, 2.1% vs laparoscopy, 16.2%, P = 0.02) and shorter hospital stay (robot, 9 d vs laparoscopy, 11 d, P = 0.011). There was no significant difference between the groups in rates of local recurrence and overall survival within 3 years. The robotic system has been improving rapidly and has been successfully used even in complex surgical procedures such as uLAR with CAA and ISR[91]. Even showing some potential advantages in relation to laparoscopy, the role of the robotic surgery for the rectal cancer treatment has not been defined yet, still requiring further studies with longer follow-up period.
CONCLUSION
Rectal cancer management is currently a multidisciplinary effort, which incorporates new concepts and technologies, resulting in significant improvement in patients’ oncological and functional outcomes. Despite the evolution seen in the last decades, there are still many unanswered questions about the management of rectal cancer. We still await results of large multi-institutional prospective trials to define some of the most important and controversial points, such as safety and efficacy of the “wait and see” approach and the definitive role of laparoscopic and robotic surgery in rectal cancer.
Footnotes
P- Reviewers: Altomare DF, Kim YJ, Vizoso FJ, Zielinski J S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Nivatvongs S, Gordon PH. Surgical anatomy. In: Gordon PH, Nivatvongs S, editors. Principle and Practice of Surgery for the Colon, Rectum and Anus. New York: Informa Healthcare; 2007. pp. 1–28. [Google Scholar]
- 2.Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56:535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Park YJ. Detection and treatment of synchronous lesions in colorectal cancer: the clinical implication of perioperative colonoscopy. World J Gastroenterol. 2007;13:4108–4111. doi: 10.3748/wjg.v13.i30.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MS . AJCC. Colon and Rectum. In: Edge SB, Byrd DR, Compton CC, eds , editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. pp. 143–164. [Google Scholar]
- 5.Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170:319–322. doi: 10.1148/radiology.170.2.2643135. [DOI] [PubMed] [Google Scholar]
- 6.Shank B, Dershaw DD, Caravelli J, Barth J, Enker W. A prospective study of the accuracy of preoperative computed tomographic staging of patients with biopsy-proven rectal carcinoma. Dis Colon Rectum. 1990;33:285–290. doi: 10.1007/BF02055469. [DOI] [PubMed] [Google Scholar]
- 7.Goldman S, Arvidsson H, Norming U, Lagerstedt U, Magnusson I, Frisell J. Transrectal ultrasound and computed tomography in preoperative staging of lower rectal adenocarcinoma. Gastrointest Radiol. 1991;16:259–263. doi: 10.1007/BF01887361. [DOI] [PubMed] [Google Scholar]
- 8.Cova M, Frezza F, Pozzi-Mucelli RS, Ukmar M, Tarjan Z, Melato M, Bucconi S, Dalla Palma L. Computed tomography and magnetic resonance in the preoperative staging of the spread of rectal cancer. A correlation with the anatomicopathological aspects. Radiol Med. 1994;87:82–89. [PubMed] [Google Scholar]
- 9.Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770–775. doi: 10.1007/BF02236933. [DOI] [PubMed] [Google Scholar]
- 10.Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s–6884s. doi: 10.1158/1078-0432.CCR-07-1137. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA, Madoff RD. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10–15. doi: 10.1007/s10350-004-6106-3. [DOI] [PubMed] [Google Scholar]
- 12.Marusch F, Koch A, Schmidt U, Zippel R, Kuhn R, Wolff S, Pross M, Wierth A, Gastinger I, Lippert H. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34:385–390. doi: 10.1055/s-2002-25292. [DOI] [PubMed] [Google Scholar]
- 13.Dworák O. Number and size of lymph nodes and node metastases in rectal carcinomas. Surg Endosc. 1989;3:96–99. doi: 10.1007/BF00590909. [DOI] [PubMed] [Google Scholar]
- 14.Andreola S, Leo E, Belli F, Bufalino R, Tomasic G, Lavarino C, Baldini MT, Meroni E. Manual dissection of adenocarcinoma of the lower third of the rectum specimens for detection of lymph node metastases smaller than 5 mm. Cancer. 1996;77:607–612. doi: 10.1002/(SICI)1097-0142(19960215)77:4<607::AID-CNCR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 16.Bleday R. Local excision of rectal cancer. World J Surg. 1997;21:706–714. doi: 10.1007/s002689900295. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345–1361. doi: 10.1007/BF02234796. [DOI] [PubMed] [Google Scholar]
- 18.Meredith KL, Hoffe SE, Shibata D. The multidisciplinary management of rectal cancer. Surg Clin North Am. 2009;89:177–215, ix-x. doi: 10.1016/j.suc.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Nastro P, Beral D, Hartley J, Monson JR. Local excision of rectal cancer: review of literature. Dig Surg. 2005;22:6–15. doi: 10.1159/000084345. [DOI] [PubMed] [Google Scholar]
- 20.Neary P, Makin GB, White TJ, White E, Hartley J, MacDonald A, Lee PW, Monson JR. Transanal endoscopic microsurgery: a viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol. 2003;10:1106–1111. doi: 10.1245/aso.2003.01.441. [DOI] [PubMed] [Google Scholar]
- 21.Gavagan JA, Whiteford MH, Swanstrom LL. Full-thickness intraperitoneal excision by transanal endoscopic microsurgery does not increase short-term complications. Am J Surg. 2004;187:630–634. doi: 10.1016/j.amjsurg.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Allaix ME, Fichera A. Modern rectal cancer multidisciplinary treatment: the role of radiation and surgery. Ann Surg Oncol. 2013;20:2921–2928. doi: 10.1245/s10434-013-2966-x. [DOI] [PubMed] [Google Scholar]
- 23.Påhlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75:2269–2275. doi: 10.1002/1097-0142(19950501)75:9<2269::aid-cncr2820750913>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 26.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 27.Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B; Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer. 2001;92:896–902. doi: 10.1002/1097-0142(20010815)92:4<896::aid-cncr1398>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 29.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 30.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 32.Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, Weitz J, Koch M. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. doi: 10.1245/s10434-013-3198-9. [DOI] [PubMed] [Google Scholar]
- 33.Valentini V, Coco C, Cellini N, Picciocchi A, Genovesi D, Mantini G, Barbaro B, Cogliandolo S, Mattana C, Ambesi-Impiombato F, et al. Preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation. Int J Radiat Oncol Biol Phys. 1998;40:1067–1075. doi: 10.1016/s0360-3016(97)00918-8. [DOI] [PubMed] [Google Scholar]
- 34.Gerard JP, Rostom Y, Gal J, Benchimol D, Ortholan C, Aschele C, Levi JM. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol. 2012;81:21–28. doi: 10.1016/j.critrevonc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Bujko K, Kepka L, Michalski W, Nowacki MP. Does rectal cancer shrinkage induced by preoperative radio(chemo)therapy increase the likelihood of anterior resection? A systematic review of randomised trials. Radiother Oncol. 2006;80:4–12. doi: 10.1016/j.radonc.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Glynne-Jones R, Hughes R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg. 2012;99:897–909. doi: 10.1002/bjs.8732. [DOI] [PubMed] [Google Scholar]
- 37.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 38.Habr-Gama A, de Souza PM, Ribeiro U, Nadalin W, Gansl R, Sousa AH, Campos FG, Gama-Rodrigues J. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087–1096. doi: 10.1007/BF02239429. [DOI] [PubMed] [Google Scholar]
- 39.Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319–1328; discussion 1328-1329. doi: 10.1016/j.gassur.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Nyasavajjala SM, Shaw AG, Khan AQ, Brown SR, Lund JN. Neoadjuvant chemo-radiotherapy and rectal cancer: can the UK watch and wait with Brazil? Colorectal Dis. 2010;12:33–36. doi: 10.1111/j.1463-1318.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa WT, Rossi BM, de O Ferreira F, Ferrigno R, David Filho WJ, Nishimoto IN, Vieira RA, Lopes A. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002;9:568–573. doi: 10.1007/BF02573893. [DOI] [PubMed] [Google Scholar]
- 42.Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis. 2006;8 Suppl 3:21–24. doi: 10.1111/j.1463-1318.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 43.Lim L, Chao M, Shapiro J, Millar JL, Kipp D, Rezo A, Fong A, Jones IT, McLaughlin S, Gibbs P. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum. 2007;50:2032–2039. doi: 10.1007/s10350-007-9062-x. [DOI] [PubMed] [Google Scholar]
- 44.Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol. 2010;49:378–381. doi: 10.3109/02841860903483692. [DOI] [PubMed] [Google Scholar]
- 45.Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, Daniels IR. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567–571. doi: 10.1111/j.1463-1318.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 46.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 47.Evans J, Patel U, Brown G. Rectal cancer: primary staging and assessment after chemoradiotherapy. Semin Radiat Oncol. 2011;21:169–177. doi: 10.1016/j.semradonc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Mekras A, Michalopoulos A, Papadopoulos VN, Mekras D, Kalles V, Tzeveleki I, Dabakis G, Netta S, Basdanis G. Changes in treatment of rectal cancer: increased use of low anterior resection. Tech Coloproctol. 2011;15 Suppl 1:S51–S54. doi: 10.1007/s10151-011-0731-3. [DOI] [PubMed] [Google Scholar]
- 49.Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O; Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. doi: 10.1007/s10350-003-0012-y. [DOI] [PubMed] [Google Scholar]
- 50.McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 1995;10:126–132. doi: 10.1007/BF00298532. [DOI] [PubMed] [Google Scholar]
- 51.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462–472. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 52.Gessler B, Haglind E, Angenete E. Loop ileostomies in colorectal cancer patients--morbidity and risk factors for nonreversal. J Surg Res. 2012;178:708–714. doi: 10.1016/j.jss.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 53.Rutkowski A, Bujko K, Nowacki MP, Chmielik E, Nasierowska-Guttmejer A, Wojnar A; Polish Colorectal Study Group. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann Surg Oncol. 2008;15:3124–3131. doi: 10.1245/s10434-008-0125-6. [DOI] [PubMed] [Google Scholar]
- 54.Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, Cohen AM, Guillem JG. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80–85. doi: 10.1245/aso.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Guillem JG, Chessin DB, Shia J, Suriawinata A, Riedel E, Moore HG, Minsky BD, Wong WD. A prospective pathologic analysis using whole-mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann Surg. 2007;245:88–93. doi: 10.1097/01.sla.0000232540.82364.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D; National Cancer Institute Expert Panel. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 57.Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes’ B and C rectal carcinoma. A report of the NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. Ann Surg. 1986;204:480–489. doi: 10.1097/00000658-198610000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients’ survival. Br J Surg. 1983;70:150–154. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 59.Ueno H, Mochizuki H, Hashiguchi Y, Ishikawa K, Fujimoto H, Shinto E, Hase K. Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer. Ann Surg. 2004;239:34–42. doi: 10.1097/01.sla.0000103070.13030.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm Rule of Distal Bowel Resection Margin in Rectal Cancer Based on Clinical Evidence? A Systematic Review. Indian J Surg Oncol. 2012;3:139–146. doi: 10.1007/s13193-012-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 62.Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 63.Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K. Lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. J Am Coll Surg. 1997;184:584–588. [PubMed] [Google Scholar]
- 64.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 65.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 66.Enker WE. Total mesorectal excision--the new golden standard of surgery for rectal cancer. Ann Med. 1997;29:127–133. doi: 10.3109/07853899709113698. [DOI] [PubMed] [Google Scholar]
- 67.Saito N, Ito M, Kobayashi A, Nishizawa Y, Sugito M. Sphincter-saving resection for low rectal cancer. Nihon Geka Gakkai Zasshi. 2011;112:318–324. [PubMed] [Google Scholar]
- 68.Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56:560–567. doi: 10.1097/DCR.0b013e31827c4a8c. [DOI] [PubMed] [Google Scholar]
- 69.Grumann MM, Noack EM, Hoffmann IA, Schlag PM. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149–156. doi: 10.1097/00000658-200102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chin CC, Yeh CY, Huang WS, Wang JY. Clinical outcome of intersphincteric resection for ultra-low rectal cancer. World J Gastroenterol. 2006;12:640–643. doi: 10.3748/wjg.v12.i4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamagami R, Istvan G, Cabarrot P, Liagre A, Chiotasso P, Lazorthes F. Fecal continence following partial resection of the anal canal in distal rectal cancer: long-term results after coloanal anastomoses. Surgery. 2000;127:291–295. doi: 10.1067/msy.2000.103487. [DOI] [PubMed] [Google Scholar]
- 72.Rosen L, Stasik JJ, Reed JF, Olenwine JA, Aronoff JS, Sherman D. Variations in colon and rectal surgical mortality. Comparison of specialties with a state-legislated database. Dis Colon Rectum. 1996;39:129–135. doi: 10.1007/BF02068065. [DOI] [PubMed] [Google Scholar]
- 73.Borowski DW, Kelly SB, Bradburn DM, Wilson RG, Gunn A, Ratcliffe AA; Northern Region Colorectal Cancer Audit Group. Impact of surgeon volume and specialization on short-term outcomes in colorectal cancer surgery. Br J Surg. 2007;94:880–889. doi: 10.1002/bjs.5721. [DOI] [PubMed] [Google Scholar]
- 74.Porter GA, Soskolne CL, Yakimets WW, Newman SC. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227:157–167. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Archampong D, Borowski D, Wille-Jørgensen P, Iversen LH. Workload and surgeon’s specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. 2012;3:CD005391. doi: 10.1002/14651858.CD005391.pub3. [DOI] [PubMed] [Google Scholar]
- 76.Read TE, Myerson RJ, Fleshman JW, Fry RD, Birnbaum EH, Walz BJ, Kodner IJ. Surgeon specialty is associated with outcome in rectal cancer treatment. Dis Colon Rectum. 2002;45:904–914. doi: 10.1007/s10350-004-6327-5. [DOI] [PubMed] [Google Scholar]
- 77.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 78.Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 79.Hillingsø JG, Wille-Jørgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer--a systematic review. Colorectal Dis. 2009;11:3–10. doi: 10.1111/j.1463-1318.2008.01625.x. [DOI] [PubMed] [Google Scholar]
- 80.Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]
- 81.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 82.Anderson C, Uman G, Pigazzi A. Oncologic outcomes of laparoscopic surgery for rectal cancer: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1135–1142. doi: 10.1016/j.ejso.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 84.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 85.Fleshman J; American College of Surgeons Oncology Group (ACOSOG)-Z6051. A Phase III prospective randomized trial comparing laparoscopic-assisted resection vs open resection for rectal cancer. Available from: http://clinicaltrials.gov/ct2/show. Accessed on september 23, 2013.
- 86.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alasari S, Min BS. Robotic colorectal surgery: a systematic review. ISRN Surg. 2012;2012:293894. doi: 10.5402/2012/293894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aly EH. Robotic colorectal surgery: summary of the current evidence. Int J Colorectal Dis. 2014;29:1–8. doi: 10.1007/s00384-013-1764-z. [DOI] [PubMed] [Google Scholar]
- 89.Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–e156. doi: 10.1111/j.1463-1318.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 90.Baek SJ, Al-Asari S, Jeong DH, Hur H, Min BS, Baik SH, Kim NK. Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg Endosc. 2013;27:4157–4163. doi: 10.1007/s00464-013-3014-4. [DOI] [PubMed] [Google Scholar]
- 91.Kang J, Hur H, Min BS, Lee KY, Kim NK. Robotic coloanal anastomosis with or without intersphincteric resection for low rectal cancer: starting with the perianal approach followed by robotic procedure. Ann Surg Oncol. 2012;19:154–155. doi: 10.1245/s10434-011-1952-4. [DOI] [PubMed] [Google Scholar]


