Abstract
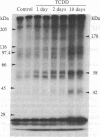
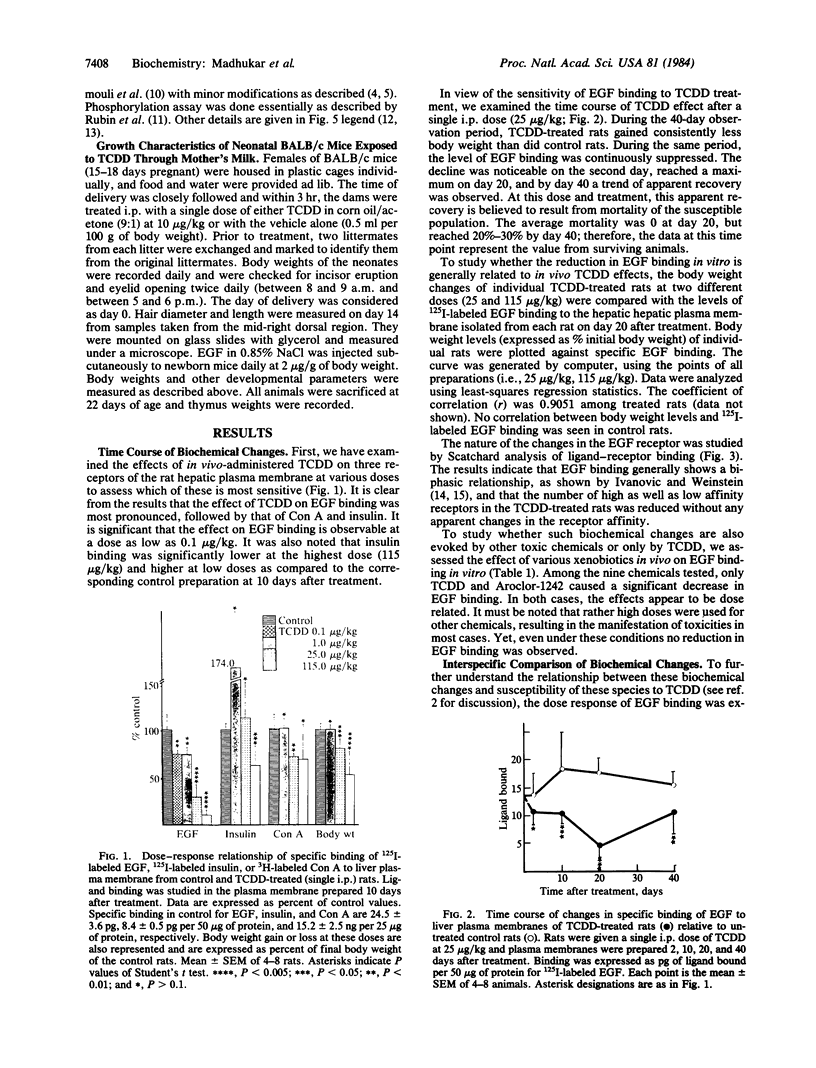
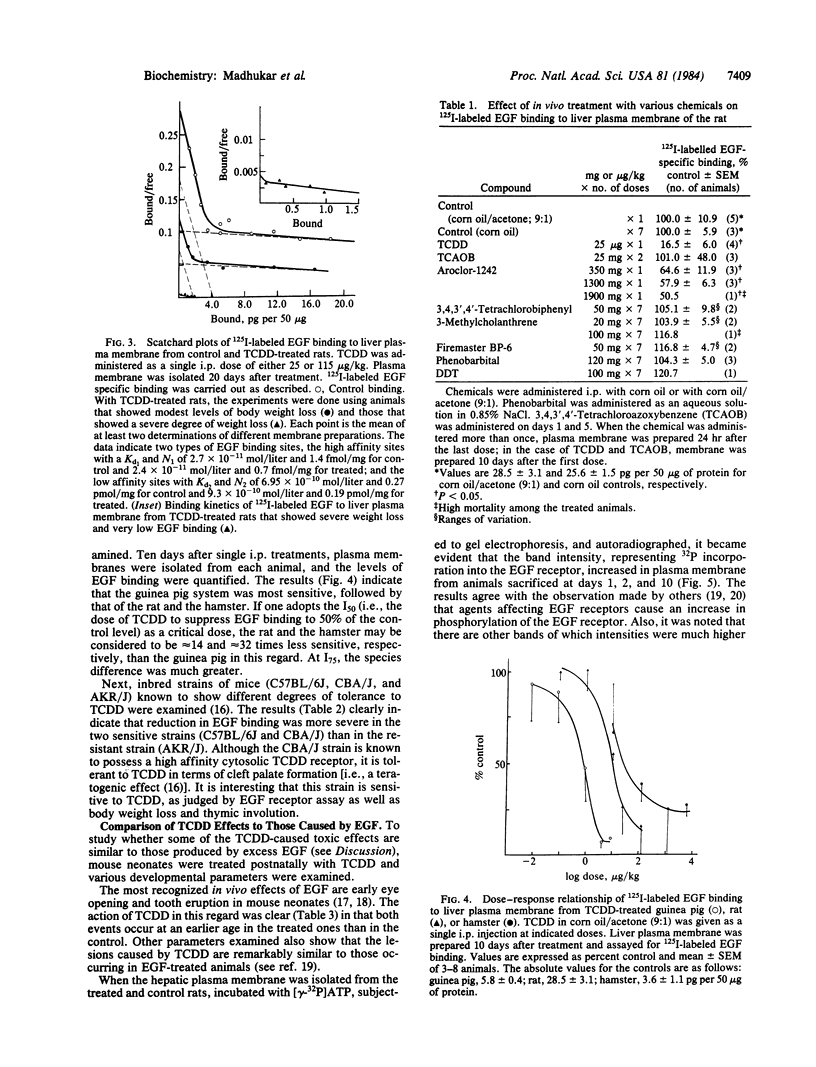
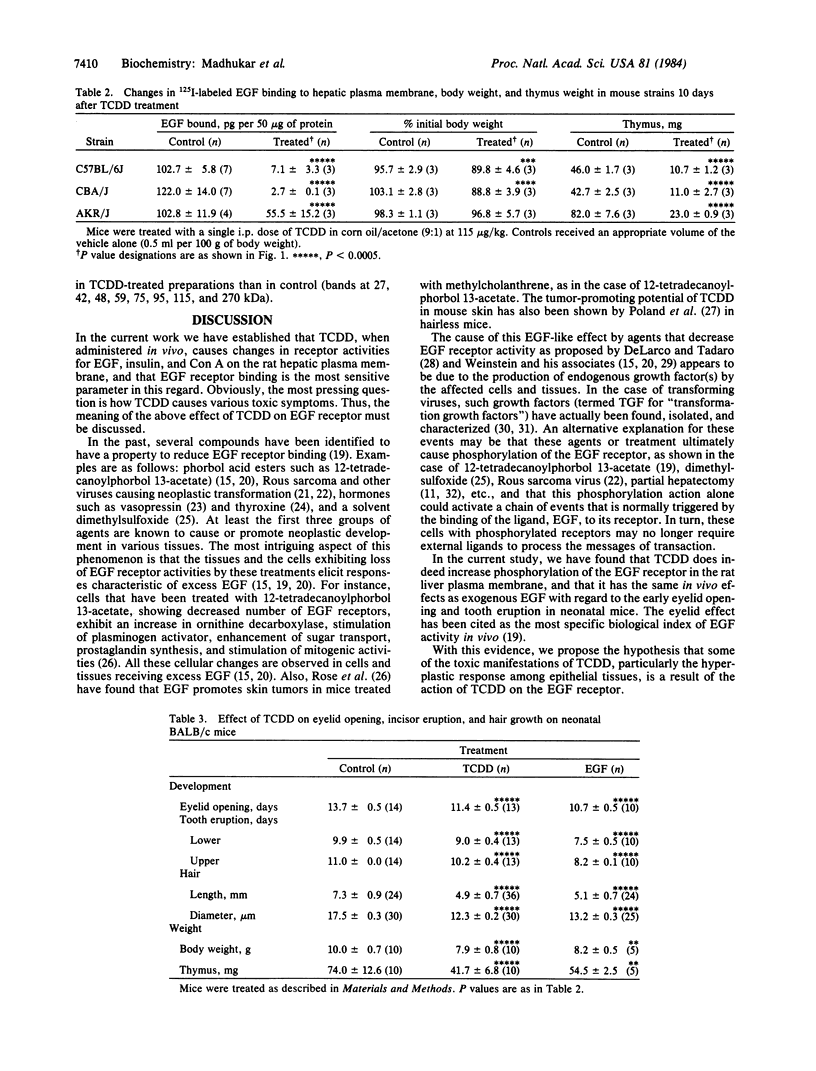
The effect of in vivo-administered 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on epidermal growth factor (EGF) receptor activity of the rat hepatic plasma membrane was studied. TCDD causes a significant reduction in EGF binding at an early stage of toxicity (day 2) and at very low doses (1 microgram/kg, single i.p., rat). This reduction appears to be due to a decline in the number of receptors. There is a good correlation between levels of decline in EGF binding and loss of body weight among TCDD-treated rats. The reduction in EGF binding occurs at a relatively low dose in the guinea pig (a very sensitive species) and at high doses in the hamster (a tolerant species). Among three mice strains, TCDD (115 micrograms/kg, single i.p.) caused 98% reduction in EGF binding in the sensitive strains (C57BL/6J and CBA/J) but only a 50% reduction in the tolerant strain (AKR/J). To relate the above biochemical changes to in vivo effects, TCDD was postnatally administered (through mother's milk) to mouse neonates. The most prominent toxic manifestations were early eye opening and incisor eruption, loss in body weight gain, and retardation of hair growth. All of these symptoms have been ascribed to EGF effects. TCDD was also found to stimulate phosphorylation of the EGF receptor in the rat hepatic plasma membrane. This phosphorylation effect was observed at day 1 and persisted until the end of the test (day 10). It has long been recognized that agents causing reduction in number of EGF receptors (e.g., phorbol esters) elicit in vivo cellular responses that are similar to those caused by exposure to excess doses of growth factors. Accordingly, a hypothesis has been proposed to ascribe some of the EGF-like effects of TCDD, such as fatty infiltration of the liver and hyperplastic proliferation of gastric epithelia and epidermal cells to its action on the EGF receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., Corbett J. T., Harris M., Lawson L. D. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on lipid profiles in tissue of the Fischer rat. Chem Biol Interact. 1978 Dec;23(3):315–330. doi: 10.1016/0009-2797(78)90093-5. [DOI] [PubMed] [Google Scholar]
- Bower J. M., Camble R., Gregory H., Gerring E. L., Willshire I. R. The inhibition of gastric acid secretion by epidermal growth factor. Experientia. 1975 Jul 15;31(7):825–826. doi: 10.1007/BF01938488. [DOI] [PubMed] [Google Scholar]
- Brewster D. W., Madhukar B. V., Matsumura F. Influence of 2,3,7,8-TCDD on the protein composition of the plasma membrane of hepatic cells from the rat. Biochem Biophys Res Commun. 1982 Jul 16;107(1):68–74. doi: 10.1016/0006-291x(82)91670-9. [DOI] [PubMed] [Google Scholar]
- COHEN S., ELLIOTT G. A. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J Invest Dermatol. 1963 Jan;40:1–5. doi: 10.1038/jid.1963.1. [DOI] [PubMed] [Google Scholar]
- Chandramouli V., Williams S., Marshall J. S., Carter J. R., Jr Cell surface changes in diabetic rats. Studies of lectin binding to liver cell plasma membranes. Biochim Biophys Acta. 1977 Feb 14;465(1):19–33. doi: 10.1016/0005-2736(77)90352-2. [DOI] [PubMed] [Google Scholar]
- Earp H. S., O'Keefe E. J. Epidermal growth factor receptor number decreases during rat liver regeneration. J Clin Invest. 1981 May;67(5):1580–1583. doi: 10.1172/JCI110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Shealy D. J., Erikson R. L. Evidence that viral transforming gene products and epidermal growth factor stimulate phosphorylation of the same cellular protein with similar specificity. J Biol Chem. 1981 Nov 25;256(22):11381–11384. [PubMed] [Google Scholar]
- Gupta B. N., Vos J. G., Moore J. A., Zinkl J. G., Bullock B. C. Pathologic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals. Environ Health Perspect. 1973 Sep;5:125–140. doi: 10.1289/ehp.7305125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden L. J., Severson D. L. Correlation of membrane phosphorylation and epidermal growth factor binding to hepatic membranes isolated from triiodothyronine-treated rats. Biochim Biophys Acta. 1983 May 5;730(2):226–230. doi: 10.1016/0005-2736(83)90337-1. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., LeQuire V. S., Cohen S. The induction of fatty liver in neonatal animals by a purified protein (EGF) from mouse submaxillary gland. Life Sci. 1965 Sep;4(17):1625–1633. doi: 10.1016/0024-3205(65)90206-7. [DOI] [PubMed] [Google Scholar]
- Ivanovic V., Weinstein I. B. Benzo[a]pyrene and other inducers of cytochrome P1-450 inhibit binding of epidermal growth factor to cell surface receptors. Carcinogenesis. 1982;3(5):505–510. doi: 10.1093/carcin/3.5.505. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., Poland A. Keratinization of mouse teratoma cell line XB produced by 2,3,7,8-tetrachlorodibenzo-p-dioxin: an in vitro model of toxicity. Cell. 1980 Nov;22(1 Pt 1):27–36. doi: 10.1016/0092-8674(80)90151-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Mechanism of tumor promoter inhibition of cellular binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5168–5172. doi: 10.1073/pnas.76.10.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Matsumura F. Biochemical aspects of action mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related chemicals in animals. Pharmacol Ther. 1982;19(2):195–209. doi: 10.1016/0163-7258(82)90062-6. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Brewster D. W., Madhukar B. V., Bombick D. W. Alteration of rat hepatic plasma membrane functions by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Arch Environ Contam Toxicol. 1984 Sep;13(5):509–515. doi: 10.1007/BF01056330. [DOI] [PubMed] [Google Scholar]
- Norback D. H., Allen J. R. Biological responses of the nonhuman primate, chicken, and rat to chlorinated dibenzo-p-dioxin ingestion. Environ Health Perspect. 1973 Sep;5:233–240. doi: 10.1289/ehp.7305233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E., Hollenberg M. D., Cuatrecasas P. Epidermal growth factor. Characteristics of specific binding in membranes from liver, placenta, and other target tissues. Arch Biochem Biophys. 1974 Oct;164(2):518–526. doi: 10.1016/0003-9861(74)90062-9. [DOI] [PubMed] [Google Scholar]
- Olson J. R., Holscher M. A., Neal R. A. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the golden Syrian hamster. Toxicol Appl Pharmacol. 1980 Aug;55(1):67–78. doi: 10.1016/0041-008x(80)90221-5. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Hamada N., Yang K. H., Madhukar B. V., Matsumura F. Reversal of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced depression of ouabain biliary excretion by pregnenolone-16 alpha-carbonitrile and spironolactone in isolated perfused rat livers. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):407–416. doi: 10.1016/0041-008x(79)90393-4. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Madhukar B. V., Yang K. H., Matsumura F. Depression of adenosine triphosphatase activities in isolated liver surface membranes of 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: correlation with effects on ouabain biliary excretion and bile flow. J Pharmacol Exp Ther. 1979 Aug;210(2):275–282. [PubMed] [Google Scholar]
- Poland A., Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol. 1974 Mar;10(2):349–359. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Potter C. L., Sipes I. G., Russell D. H. Hypothyroxinemia and hypothermia in rats in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin administration. Toxicol Appl Pharmacol. 1983 Jun 15;69(1):89–95. doi: 10.1016/0041-008x(83)90123-0. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rose S. P., Stahn R., Passovoy D. S., Herschman H. Epidermal growth factor enhancement of skin tumor induction in mice. Experientia. 1976;32(7):913–915. doi: 10.1007/BF02003764. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rubin R. A., Earp H. S. Dimethyl sulfoxide stimulates tyrosine residue phosphorylation of rat liver epidermal growth factor receptor. Science. 1983 Jan 7;219(4580):60–63. doi: 10.1126/science.6294827. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., O'Keefe E. J., Earp H. S. Alteration of epidermal growth factor-dependent phosphorylation during rat liver regeneration. Proc Natl Acad Sci U S A. 1982 Feb;79(3):776–780. doi: 10.1073/pnas.79.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Schreiber A. B., Levi A., Lax I., Libermann T., Yarden Y. Regulation of cell proliferation by epidermal growth factor. CRC Crit Rev Biochem. 1983;14(2):93–111. doi: 10.3109/10409238309102791. [DOI] [PubMed] [Google Scholar]
- Schwetz B. A., Norris J. M., Sparschu G. L., Rowe U. K., Gehring P. J., Emerson J. L., Gerbig C. G. Toxicology of chlorinated dibenzo-p-dioxins. Environ Health Perspect. 1973 Sep;5:87–99. doi: 10.1289/ehp.730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny M., Cohen S. Epidermal growth factor. IV. The induction of ornithine decarboxylase. Biochim Biophys Acta. 1970 Apr 15;204(2):578–589. [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]