Abstract
Single-port laparoscopic surgery (SPLS) is implemented through a tailored minimal single incision through which a number of laparoscopic instruments access. Introduction of operation-customized port system, utilization of a camera without a separate external light, and instruments with different lengths has brought the favorable environment for SPLS. However, performing SPLS still creates several hardships compared to multiport laparoscopic surgery; a single-port system inevitably leads to clashing of surgical instruments due to crowding. To overcome such difficulties, investigators has developed novel concepts and maneuvers, including the concept of inverse triangulation and the maneuvers of pivoting, spreading out dissection, hanging suture, and transluminal traction. The final destination of SPLS is expected to be a completely seamless operation, maximizing the minimal invasiveness. Specimen extraction through the umbilicus can undermine cosmesis by inducing a larger incision. Therefore, hybrid laparoscopic technique, which combined laparoscopic surgical technique with natural orifice specimen extraction (NOSE) - i.e., transvaginal or transanal route-, has been developed. SPLS and NOSE seemed to be the best combination in pursuit of minimal invasiveness. In the near future, robotic SPLS with natural orifice transluminal endoscopic surgery’s way of specimen extraction seems to be pursued. It is expected to provide a completely or nearly complete seamless operation regardless of location of the lesion in the abdomen.
Keywords: Colorectal neoplasms, Colectomy, Laparoscopy, Natural orifice endoscopic surgery, Single-port laparoscopic surgery
Core tip: Single-port laparoscopic surgery (SPLS) has clear-cut benefits in terms of cosmesis and reduced wound morbidity. The technical difficulties have been overcome by novel concepts and maneuvers, including the concept of inverse triangulation and the maneuvers of pivoting, spreading out dissection, hanging suture, and transluminal traction. Cosmetic demerits, caused by the specimen extraction through the single-port site, can be selectively overcome by natural orifice specimen extraction, such as using transvaginal or transanal route. In the near future, robotic SPLS with natural orifice transluminal endoscopic surgery’s way of specimen extraction seems to be pursued.
PAST: EMERGING AS A RISING HOPE
Laparoscopic surgery did not only cosmetically satisfy patients but also led to improvement in parameters related with short-term operative outcomes, such as reduction in postoperative pain and duration of ileus, quicker postoperative recovery, shorter hospital stay, and earlier return to normal activity[1-7]. Furthermore, a randomized clinical trial reported a reduction in tumor relapse following laparoscopic surgery, suggesting long-term oncologic benefits[8]. The reasons were attributed to various potential mechanisms, including a lower stress response after surgical trauma, an attenuated cytokine response, minimal tumor handling, accurate application of the no-touch technique, and lower complication rates.
Conventional laparoscopic surgery (CLS) usually requires 3-6 small incisions for ports. These incisions are not only cosmetically unappealing, but also increase the wound pain and potential wound morbidity, such as abdominal wall bleeding, port-site hernia, and internal organ damage. The ardent pursuit of minimal invasiveness and the increasing recognition of patient’s satisfaction has led to the ultimate form of laparoscopic surgery, single port laparoscopic surgery (SPLS). Ever since the first attempt of SPLS hysterectomy in 1992[9], SPLS was adopted by general surgery in procedures such as appendectomy[10], cholecystectomy[11], and adrenalectomy[12]. Colorectal surgeons were also eager to employ the novel SPLS technique in right hemicolectomy[13,14], sigmoidectomy[15,16], and total colectomy[17,18]. The spectrum of SPLS applications has extended from benign diseases to malignant colorectal cancers[13,16,19] and the safety and feasibility of SPLS in colorectal surgery is supported by many reports and comparative studies[14,20-22].
PRESENT: EXCLAMATION AND FRUSTRATION
SPLS nomenclature
The exact nomenclature of laparoscopic surgery, which is performed through only on minimal incision, has not been determined. The surgical procedure has been variously referred depending on the continent, country, hospital, department, and even individual operator (Table 1). In this paper, we referred to it as SPLS, which is the most widely used terminology in South Korea.
Table 1.
Acronyms of single port laparoscopic surgery
| Acronym | Details |
| eNOTES | Embryonic natural orifice transluminal endoscopic surgery |
| LESS | Laparo-endoscopic single site surgery |
| NOTUS | Natural orifice trans-umbilical surgery |
| OPUS | One port umbilical surgery |
| SPAS | Single port access |
| SPL | Single port laparoscopy |
| SIPLS | Single instrument port laparoscopic surgery |
| SIMPL | Single incision multi-port laparoscopic-endoscopic |
| SILS | Single incision laparoscopic surgery |
| SLIT | Single laparoscopic incision transabdominal |
| SLAPP | Single laparoscopic port procedure |
| SSL | Single site laparoscopy |
| TUES | Trans-umbilical endoscopic surgery |
| TULA | Trans-umbilical laparoscopic assisted |
| TUSPLS | trans-umbilical single port laparoscopic surgery |
Beneficial effects of SPLS
SPLS is implemented through a tailored minimal single incision through which a number of laparoscopic instruments access. This single incision site usually functions as (1) an access port entering into abdominal cavity; (2) a specimen-extracting orifice; and (3) a pathway for a drain. The preferred single incision site is the umbilicus. Umbilicus is the thinnest part of the abdomen; has no vessel or nerve; and can be regarded as predetermined, ready-made scar which can hide artificial scar effectively. Furthermore, centrally located, it can provide a shortcut to various intra-abdominal organs in all abdominal quadrants. Other sites besides umbilicus, however, can be utilized as a single incision for various reasons, including abandoning the umbilicus due to possible adhesion and making incision at predetermined ileostomy site. We experienced several cases of abdominoperineal resection and low anterior resection using SPLS other than transumbilical route due to the latter reason, and found it to be acceptable in terms of operative proficiency and cosmesis[15]; no wound was identified postoperatively except for the ileostomy site, simulating an even more “scar-less” operation than using the umbilicus.
Besides cosmetic superiority, the potential benefits of SPLS is to reduce wound morbidity. The number and overall size of the wound directly affect wound morbidity, such as injuries of vessels, bowel, and other intra-abdominal organs, and trocar site hernia[23]. Weiss et al[24], in their analysis of 1145 consecutive series of SPLS, reported that SPLS reduced wound complication more than CLS (2.38% vs 8.45%, P = 0.015).
Other benefits of SPLS over CLS have not determined yet. Until now, a series of comparative studies suggested a number of potential benefits of SPLS, including pain reduction and fastened postoperative recovery[20,25-27], and others did not[28-31]. The severity and duration of pain after an operation influences postoperative recovery, which is reflected by duration before re-initiation of a diet, return to normal activity, and the length of hospital stay. Therefore, the effect of SPLS on postoperative pain needs to be determined first. Tsimoyiannis et al[25], in a randomized controlled trial comparing outcomes following cholecystectomies either by CLS (n = 20) or SPLS (n = 20), showed that SPLS more reduced postoperative pain scores. However, prospective, large-scaled clinical trials of the short- and long-term outcomes are essential to determine the precise effects of SPLS.
SPLS is particularly useful in operations which are aimed at more than two target organs in different quadrants; for the umbilicus provides a shortcut to reach all intra-abdominal organs. Combined appendectomy and cholecystectomy is one of examples. Colorectal surgery involves the most extensive area in the abdomen because the colorectum is extensively distributed. Therefore, the merit of SPLS is pronounced in colorectal surgery. Furthermore, SPLS may be the optimal choice in selected patients with a history of multiple abdominal operations. Open or laparoscopic surgery can equally put such patients in the risk of iatrogenic bowel perforation. In these situations, SPLS can be attempted because a single minimal incision provides a safe settlement point from which the dissection can be initiated cautiously.
SPLS poses several challenges, such as the handling of straight instruments in parallel with the laparoscope through a small single incision. Technical limitations of instrumentation in SPLS has led to advancement in techniques to overcome the limitations. Such technical advancements are unique to SPLS; difficult or unable to apply to CLS; and therefore show the potential of SPLS to outperform CLS.
Instrument for SPLS
Ports: In the beginning, a homemade glove port, which combines a wound retractor and a surgical glove, has been utilized. More recently, commercial single ports, including the OCTO port (Dalim medical Co., South Korea) and the SILS port (single-incision laparoscopic surgery port, Covidien, United States) have also been developed and introduced (Figure 1). For convenience, we categorized the single ports into two subtypes depending on detachability; one-piece (SPLS port, R-port etc.) type and two-piece (Glove port, OCTO port etc.) type. We think that the two-piece type is more convenient in colorectal surgery considering the comfortability of specimen extraction through the port site. We also classified the single ports into the terminal type and preoccupied type according to the presence of a common channel; it can be called as terminal type when laparoscopic instruments share a common channel in the single port except for their entrance, and called as preoccupied type when each individual laparoscopic instrument has its own independent access to the abdominal cavity. We preferred the terminal type because it can be used with smaller incisions and evokes less instrumental clinching.
Figure 1.

Ports designed for single-port laparoscopic surgery. A: Materials for making homemade glove port (two-piece, terminal type); B: OCTO port (Dalim medical Co., South Korea) (two-piece, terminal type); C: Single incision laparoscopic surgery port (Covidien, United States) (one-piece, preoccupied type); D: Commercial glove port (Sejong medical Co., South Korea) (one-piece, terminal type).
Camera: Utilization of a camera without a separate external light not only provides more space externally but also reduces the chance of it being knocked out of place by a surgeon. We prefer a camera with 5-mm diameter due to various reasons, such as taking up lesser space and leaving small incision. The 30-degree telescope provides an extensive vision, especially in the deepest portion of the pelvic cavity.
Working instruments: As SPLS is based on well-established laparoscopic foundation, SPLS can be reproduced using conventional laparoscopic instruments. Fixed straight instruments are usually preferred in SPLS because they can transmit constant force and maintain throughout retraction. Numerous articulating devices, however, have been developed to actively manipulate and fulfill tasks regardless of instrument position. The practical utility of their flexibility raises controversy. Judging from our experience, articulating devices were particularly convenient when utilized with one hand rather than with both hands and/or when applied to the patients with a prominent pelvic promontory. Instruments with longer (44-45 cm) shaft lengths than conventional devices (33-34 cm) are advantageous in the procedures in the left upper quadrant (LUQ), such as splenic flexure dissection. Considerable instrumental clinches occurred outside rather than inside of abdominal cavity. We have overcome the clinches to a large extent using different-length instruments and a reduced bulk camera.
An instrument placed through a single port divides the hole in the port into two. Therefore, when an instrument cannot, or is difficult to reach the targeted organ, we recommend to draw the instrument completely out of the abdomen and re-insert it into another compartment bordered by the instrument.
Challenge and response
Performing SPLS is more strenuous than CLS. The environment provided by SPLS inevitably results in motion limitations and clashing of surgical instruments due to crowding. Furthermore, SPLS significantly increases the difficulty of colonic exposure and dissection due to inability of triangular dissection which has been considered a cornerstone of laparoscopic surgery. Such difficulties prompted the development of instruments and maneuvers to overcome the limitations. Herein, some of these attempts, including the maneuvers which were ingenuously developed at our institution, will be discussed.
Inverse triangulation: Laparoscopic surgeons have performed convenient traction and dissection using the concept of triangulation. SPLS, however, provides the unfavorable surroundings for triangulation, often resulting in the chopsticks or sword fighting effect due to parallel alignment of instruments. We have attempted to overcome the limitations using a new concept of “inverse triangulation” (Figure 2). Inverse triangulation refers to the formation of an inverted triangle viewed from the operator; one single-incision port site and two instrumental ends which are positioned in a crossing-over pattern comprise three triangles. The two instrumental ends do not encounter, but assist each other by creating tension. The operation is carried out with the two instruments crossed-over. The surgeon’s right hand holds the left-sided instrument and vice versa. Inverse-triangulation makes it convenient to perform various kinds of laparoscopic procedure, including dissection, traction, and resection. And, inverse triangulation does not increase the umbilical pain because the range of motion of the instruments is restricted within the umbilical port.
Figure 2.
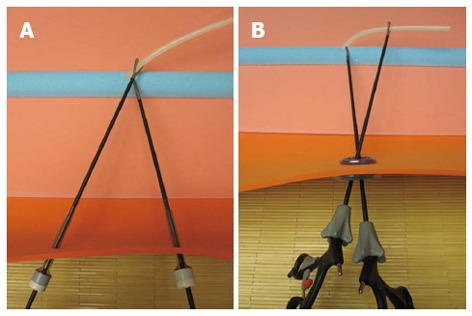
Concept of triangulation vs inverse triangulation. Triangulation in multiport laparoscopic surgery allows traction on tissues to improve dissection along anatomical planes (A). In inverse triangulation of single-port laparoscopic surgery, the two instrumental ends do not encounter, but assist each other by creating tension (B). Therefore, the operation is carried out with the two instruments crossed-over.
Pivoting: Colorectum is located extensively in four quadrants of the abdomen, and the umbilicus is located in the center of four quadrants. Therefore, a pan-abdominal approach without additional incisions is possible through the umbilicus. Furthermore, SPLS is advantageous in the operation which includes more than two target organs in different quadrants, such as combining splenectomy and appendectomy. The only requirements in such a situation are positional changes of the patient and shifts of operation members.
Spreading out dissection: Whether it is laparoscopy or open surgery, the operation of the patient with multiple adhesions demands a great deal of hard works. In CLS, even if a port for camera is successfully entered, insertion of an additional port far apart from the camera port can be threatening due to potential risk of intestinal injuries. However, SPLS has an advantage over CLS in that it does not require risky additional port insertion; only after securement of single-port access, dissection of adherent tissue can be expanded from the single-port site with safety.
Hanging suture: Application of a hanging suture is helpful when sustained maintenance of the visual field overcoming an obstacle is required. For example, the practice of total mesorectal excision (TME) for rectal cancer is limited during SPLS due to narrow pelvic cavity and hindering structures. To facilitate TME, the peritoneal fold (in males) or the uterus (in females) can be elevated by placing an intracorporeal stitch through the low abdominal wall (Figure 3). Thereafter, adjusting patient’s position according to the procedure can further optimize operative field.
Figure 3.
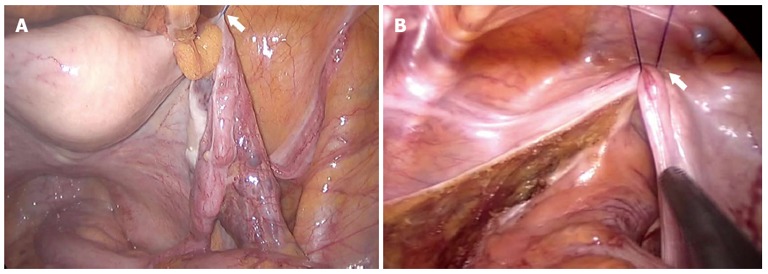
Hanging suture. To facilitate operative field during total mesorectal excision, the uterus in female (A) or the peritoneal fold in male (B) were elevated by placing an intracorporeal stitch through the low abdominal wall.
Transluminal traction: In the low colorectal surgery, the support of a colorectum, which is determined to be dissected, can facilitate dissection by way of adjusting the organ’s direction. This support can be provided by transrectal application of instruments, such as PPH (Procedure for Prolapsed and Hemorrhoid Endo-Surgery, Ethicon, United States), a circular stapler, an anal trocar, or colonoscopy (Figure 4).
Figure 4.
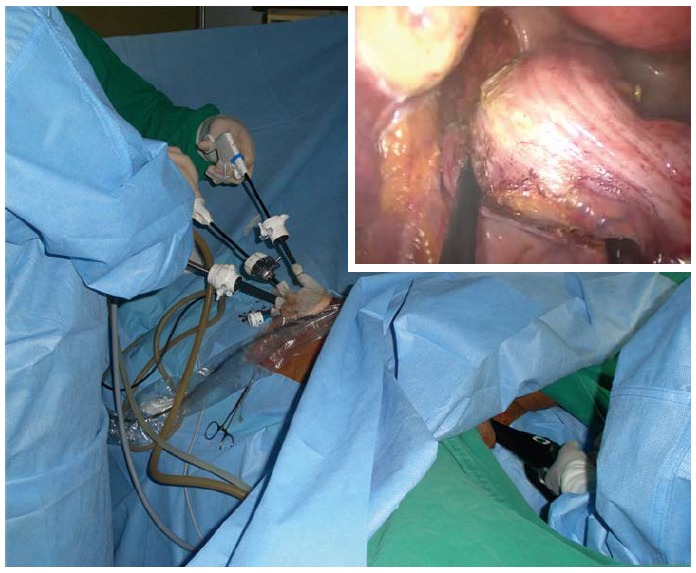
Transluminal traction. A PPH (procedure for Prolapsed and Hemorrhoid Endo-Surgery, Ethicon, United States) was utilized to support the colorectum during dissection and to facilitate dissection by shifting the colorectum’s location as well.
FUTURE: WAY TO ULTIMITE SCARLESS SURGERY
SPLS is mainly accomplished by two persons. The operator both holds an organ structure and dissects it with bimanual manipulation. Therefore, the operator’s contribution is more substantial than any other procedures. An assistant’s role is, however, usually to steer a laparoscope. Therefore, the assistant’s role can be replaced by an instrument, such as a camera holder (laparoscopic instrument holder, Sejong medical Co., South Korea) (Figure 5). If the instrument replaces an assistant surgeon, the surgical team is only comprised of a surgeon and a scrub nurse. Surgery department often lacks manpower; therefore such instrument-dependent SPLS can overcome the personal defect. We found it is particularly advantageous in the operations of which target organ is localized in a single quadrant, such as appendectomy, cholecystectomy, and herniorrhaphy.
Figure 5.
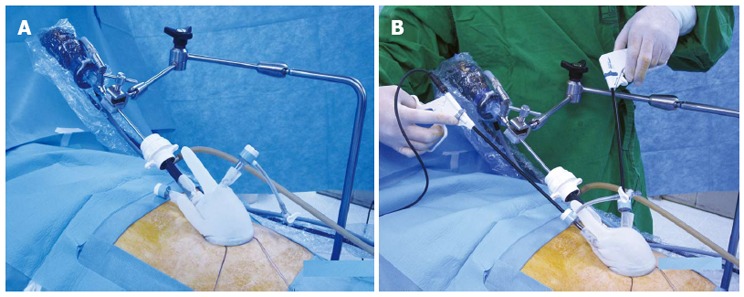
Laparoscopic instrument holder. An installation of a laparoscopic instrument holder in operation bed (A). Application of a laparoscopic instrument holder during single-port laparoscopic surgery (B).
SPLS was initially designed to achieve a seamless operation. Reaching a “completely seamless operation” is the final destination, while maintaining comparable therapeutic outcomes as CLS. In spite of attempts to reduce the number and size of the skin incision, the bulk of the specimen inevitably affects the length of incision, mostly the umbilical incision. Laparoscopic surgeons attempted to solve this problem by borrowing idea from natural orifice transluminal endoscopic surgery (NOTES). Consequently, hybrid laparoscopic technique, which combined laparoscopic surgical technique with natural orifice specimen extraction (NOSE), has been developed[32-41]. Of NOSE, transvaginal[32-37] or transanal[38-41] route of specimen retrieval has been preferred in laparoscopic surgery. SPLS and NOSE are the best combination in pursuit of minimal invasiveness. In operations which include the formation of a stoma, such as abdominoperineal resection or low anterior resection with diverting ileostomy, SPLS can be initiated through a predetermined stoma site and the specimen can be extracted through the stoma site (Figure 6). Therefore, the ideal seamless operation can be accomplished by this way.
Figure 6.
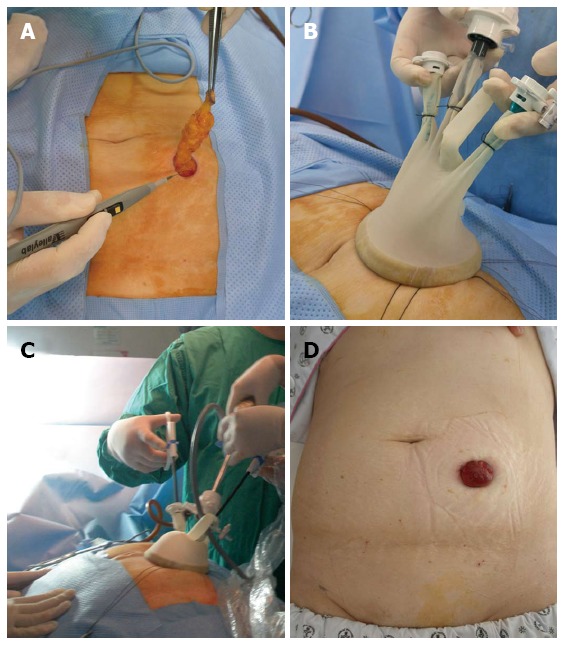
Umbilicus-sparing single-port laparoscopic surgery. After making a incision for single-port to the predetermined enterostomy site (A), a single-port was inserted (B), and operation was accomplished through the enterostomy site (C). Postoperatively, no scar, except for enterostomy, remained (D).
Transanal endoluminal laparoscopic surgery (TELS) is displayed in the rectal lumen using a port established in the anus[42-44]. And, laparoscopic assisted transanal transabdominal proctosigmoidectomy is a combined approach to remove low rectal cancer via the anus and abdominal cavity[45-48]. Ideal seamless operation can be designed by combining these two operative procedures. First, after making an incision in anus, the dissection proceeds forward, and then the specimen is extracted via anus, and colo-anal anastomosis is achieved through the anus. Such an accomplishment can be remarked as one of the most advanced forms of SPLS[49,50].
The advent of robotic surgery should be addressed when discussing the future of minimally invasive surgery. Because the robotic surgery is performed using a laparoscopic approach, an upgraded version of robotic surgery will be single-port robotic surgery[51]. It seemed that robotic SPLS combined with NOTE’s way of specimen extraction would be attempted in the near future[52]. It is expected to provide a completely or nearly complete seamless operation regardless of location of the lesion in the abdomen.
Footnotes
P- Reviewers: Altomare DF, Bridoux V, Ruffolo C S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759–766; disscussion 767. doi: 10.1097/01.SLA.0000036269.60340.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662; discussion 662-664. doi: 10.1097/SLA.0b013e318155a762. [DOI] [PubMed] [Google Scholar]
- 4.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 5.Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728–738. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 6.Ng KH, Ng DC, Cheung HY, Wong JC, Yau KK, Chung CC, Li MK. Laparoscopic resection for rectal cancers: lessons learned from 579 cases. Ann Surg. 2009;249:82–86. doi: 10.1097/SLA.0b013e31818e418a. [DOI] [PubMed] [Google Scholar]
- 7.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 8.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 9.Pelosi MA, Pelosi MA. Laparoscopic supracervical hysterectomy using a single-umbilical puncture (mini-laparoscopy) J Reprod Med. 1992;37:777–784. [PubMed] [Google Scholar]
- 10.Ateş O, Hakgüder G, Olguner M, Akgür FM. Single-port laparoscopic appendectomy conducted intracorporeally with the aid of a transabdominal sling suture. J Pediatr Surg. 2007;42:1071–1074. doi: 10.1016/j.jpedsurg.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Podolsky ER, Rottman SJ, Poblete H, King SA, Curcillo PG. Single port access (SPA) cholecystectomy: a completely transumbilical approach. J Laparoendosc Adv Surg Tech A. 2009;19:219–222. doi: 10.1089/lap.2008.0275. [DOI] [PubMed] [Google Scholar]
- 12.Hirano D, Minei S, Yamaguchi K, Yoshikawa T, Hachiya T, Yoshida T, Ishida H, Takimoto Y, Saitoh T, Kiyotaki S, et al. Retroperitoneoscopic adrenalectomy for adrenal tumors via a single large port. J Endourol. 2005;19:788–792. doi: 10.1089/end.2005.19.788. [DOI] [PubMed] [Google Scholar]
- 13.Bucher P, Pugin F, Morel P. Single port access laparoscopic right hemicolectomy. Int J Colorectal Dis. 2008;23:1013–1016. doi: 10.1007/s00384-008-0519-8. [DOI] [PubMed] [Google Scholar]
- 14.Remzi FH, Kirat HT, Kaouk JH, Geisler DP. Single-port laparoscopy in colorectal surgery. Colorectal Dis. 2008;10:823–826. doi: 10.1111/j.1463-1318.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunner W, Schirnhofer J, Waldstein-Wartenberg N, Frass R, Weiss H. Single incision laparoscopic sigmoid colon resections without visible scar: a novel technique. Colorectal Dis. 2010;12:66–70. doi: 10.1111/j.1463-1318.2009.01894.x. [DOI] [PubMed] [Google Scholar]
- 16.Bucher P, Pugin F, Morel P. Transumbilical single incision laparoscopic sigmoidectomy for benign disease. Colorectal Dis. 2010;12:61–65. doi: 10.1111/j.1463-1318.2009.01825.x. [DOI] [PubMed] [Google Scholar]
- 17.Leblanc F, Makhija R, Champagne BJ, Delaney CP. Single incision laparoscopic total colectomy and proctocolectomy for benign disease: initial experience. Colorectal Dis. 2011;13:1290–1293. doi: 10.1111/j.1463-1318.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 18.Cahill RA, Lindsey I, Jones O, Guy R, Mortensen N, Cunningham C. Single-port laparoscopic total colectomy for medically uncontrolled colitis. Dis Colon Rectum. 2010;53:1143–1147. doi: 10.1007/DCR.0b013e3181dd062f. [DOI] [PubMed] [Google Scholar]
- 19.Mufty H, Hillewaere S, Appeltans B, Houben B. Single-incision right hemicolectomy for malignancy: a feasible technique with standard laparoscopic instrumentation. Colorectal Dis. 2012;14:e764–e770. doi: 10.1111/j.1463-1318.2012.03175.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Ryu GO, Choi BJ, Kim JG, Lee KJ, Lee SC, Oh ST. The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg. 2011;254:933–940. doi: 10.1097/SLA.0b013e318237826b. [DOI] [PubMed] [Google Scholar]
- 21.Napolitano L, Waku M, De Nicola P, Di Bartolomeo N, Cotellese R, D’Aulerio A, Innocenti P. Laparoscopic colectomy in colon cancer. A single-center clinical experience. G Chir. 2007;28:126–133. [PubMed] [Google Scholar]
- 22.Singh J, Podolsky ER, Castellanos AE, Stein DE. Optimizing single port surgery: a case report and review of technique in colon resection. Int J Med Robot. 2011;7:127–130. doi: 10.1002/rcs.378. [DOI] [PubMed] [Google Scholar]
- 23.Shabanzadeh DM, Sørensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg. 2012;256:934–945. doi: 10.1097/SLA.0b013e318269a46b. [DOI] [PubMed] [Google Scholar]
- 24.Weiss HG, Brunner W, Biebl MO, Schirnhofer J, Pimpl K, Mittermair C, Obrist C, Brunner E, Hell T. Wound complications in 1145 consecutive transumbilical single-incision laparoscopic procedures. Ann Surg. 2014;259:89–95. doi: 10.1097/SLA.0b013e31827b7818. [DOI] [PubMed] [Google Scholar]
- 25.Tsimoyiannis EC, Tsimogiannis KE, Pappas-Gogos G, Farantos C, Benetatos N, Mavridou P, Manataki A. Different pain scores in single transumbilical incision laparoscopic cholecystectomy versus classic laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2010;24:1842–1848. doi: 10.1007/s00464-010-0887-3. [DOI] [PubMed] [Google Scholar]
- 26.Tugcu V, Ilbey YO, Mutlu B, Tasci AI. Laparoendoscopic single-site surgery versus standard laparoscopic simple nephrectomy: a prospective randomized study. J Endourol. 2010;24:1315–1320. doi: 10.1089/end.2010.0048. [DOI] [PubMed] [Google Scholar]
- 27.Yim GW, Jung YW, Paek J, Lee SH, Kwon HY, Nam EJ, Kim S, Kim JH, Kim YT, Kim SW. Transumbilical single-port access versus conventional total laparoscopic hysterectomy: surgical outcomes. Am J Obstet Gynecol. 2010;203:26.e1–26.e6. doi: 10.1016/j.ajog.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Cassera MA, Spaun GO, Hammill CW, Hansen PD, Aliabadi-Wahle S. Randomized controlled trial comparing single-port laparoscopic cholecystectomy and four-port laparoscopic cholecystectomy. Ann Surg. 2011;254:22–27. doi: 10.1097/SLA.0b013e3182192f89. [DOI] [PubMed] [Google Scholar]
- 29.Lai EC, Yang GP, Tang CN, Yih PC, Chan OC, Li MK. Prospective randomized comparative study of single incision laparoscopic cholecystectomy versus conventional four-port laparoscopic cholecystectomy. Am J Surg. 2011;202:254–258. doi: 10.1016/j.amjsurg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Pedraza R, Aminian A, Nieto J, Faraj C, Pickron TB, Haas EM. Single-incision laparoscopic colectomy for cancer: short-term outcomes and comparative analysis. Minim Invasive Surg. 2013;2013:283438. doi: 10.1155/2013/283438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang J, Bae BN, Gwak G, Park I, Cho H, Yang K, Kim KW, Han S, Kim HJ, Kim YD. Comparative study of a single-incision laparoscopic and a conventional laparoscopic appendectomy for the treatment of acute appendicitis. J Korean Soc Coloproctol. 2012;28:304–308. doi: 10.3393/jksc.2012.28.6.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aron M, Berger AK, Stein RJ, Kamoi K, Brandina R, Canes D, Sotelo R, Desai MM, Gill IS. Transvaginal nephrectomy with a multichannel laparoscopic port: a cadaver study. BJU Int. 2009;103:1537–1541. doi: 10.1111/j.1464-410X.2009.08612.x. [DOI] [PubMed] [Google Scholar]
- 33.Awad ZT, Qureshi I, Seibel B, Sharma S, Dobbertien MA. Laparoscopic right hemicolectomy with transvaginal colon extraction using a laparoscopic posterior colpotomy: a 2-year series from a single institution. Surg Laparosc Endosc Percutan Tech. 2011;21:403–408. doi: 10.1097/SLE.0b013e31823945ac. [DOI] [PubMed] [Google Scholar]
- 34.Benhidjeb T, Stark M. An innovative technique for colorectal specimen retrieval: a new era of “Natural Orifice Specimen Extraction” (N.O.S.E.) Dis Colon Rectum. 2010;53:502–503; author reply 503. doi: 10.1007/DCR.0b013e3181ca7dd7. [DOI] [PubMed] [Google Scholar]
- 35.Diana M, Perretta S, Wall J, Costantino FA, Leroy J, Demartines N, Marescaux J. Transvaginal specimen extraction in colorectal surgery: current state of the art. Colorectal Dis. 2011;13:e104–e111. doi: 10.1111/j.1463-1318.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 36.Faller E, Albornoz J, Messori P, Leroy J, Wattiez A. A new technique of laparoscopic intracorporeal anastomosis for transrectal bowel resection with transvaginal specimen extraction. J Minim Invasive Gynecol. 2013;20:333. doi: 10.1016/j.jmig.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Fan JK, Tong DK, Law S, Law WL. Transvaginal cholecystectomy with endoscopic submucosal dissection instruments and single-channel endoscope: a survival study in porcine model. Surg Laparosc Endosc Percutan Tech. 2009;19:29–33. doi: 10.1097/SLE.0b013e3181902ba7. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs KH, Breithaupt W, Varga G, Schulz T, Reinisch A, Josipovic N. Transanal hybrid colon resection: from laparoscopy to NOTES. Surg Endosc. 2013;27:746–752. doi: 10.1007/s00464-012-2534-7. [DOI] [PubMed] [Google Scholar]
- 39.Gaujoux S, Bretagnol F, Au J, Ferron M, Panis Y. Single port access proctectomy with total mesorectal excision and intersphincteric resection with a primary transanal approach. Colorectal Dis. 2011;13:e305–e307. doi: 10.1111/j.1463-1318.2011.02676.x. [DOI] [PubMed] [Google Scholar]
- 40.Hara M, Takayama S, Sato M, Imafuji H, Takahashi H, Takeyama H. Laparoscopic anterior resection for colorectal cancer without minilaparotomy using transanal bowel reversing retrieval. Surg Laparosc Endosc Percutan Tech. 2011;21:e235–e238. doi: 10.1097/SLE.0b013e3182297667. [DOI] [PubMed] [Google Scholar]
- 41.Wolthuis AM, Penninckx F, D’Hoore A. Laparoscopic sigmoid resection with transrectal specimen extraction has a good short-term outcome. Surg Endosc. 2011;25:2034–2038. doi: 10.1007/s00464-010-1472-5. [DOI] [PubMed] [Google Scholar]
- 42.Khoo RE. Transanal excision of a rectal adenoma using single-access laparoscopic port. Dis Colon Rectum. 2010;53:1078–1079. doi: 10.1007/DCR.0b013e3181ddf589. [DOI] [PubMed] [Google Scholar]
- 43.Léonard D, Colin JF, Remue C, Jamart J, Kartheuser A. Transanal endoscopic microsurgery: long-term experience, indication expansion, and technical improvements. Surg Endosc. 2012;26:312–322. doi: 10.1007/s00464-011-1869-9. [DOI] [PubMed] [Google Scholar]
- 44.Lorenz C, Nimmesgern T, Back M, Langwieler TE. Transanal single port microsurgery (TSPM) as a modified technique of transanal endoscopic microsurgery (TEM) Surg Innov. 2010;17:160–163. doi: 10.1177/1553350610370751. [DOI] [PubMed] [Google Scholar]
- 45.Choi BJ, Lee SC, Kang WK. Single-port laparoscopic total mesorectal excision with transanal resection (transabdominal transanal resection) for low rectal cancer: initial experience with 22 cases. Int J Surg. 2013;11:858–863. doi: 10.1016/j.ijsu.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 47.Tuech JJ, Bridoux V, Kianifard B, Schwarz L, Tsilividis B, Huet E, Michot F. Natural orifice total mesorectal excision using transanal port and laparoscopic assistance. Eur J Surg Oncol. 2011;37:334–335. doi: 10.1016/j.ejso.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Zhou JN, Wang DZ, Huang XE, Xu FP, Shang JQ, Gu RM. Transabdominal transanal resection of distal rectal cancer after high dose preoperative radiotherapy: a Chinese experience in preserving sphincter function. Isr Med Assoc J. 2006;8:675–678. [PubMed] [Google Scholar]
- 49.Co CS, Cheung HY, Yau KK, Chung CC, Li M. Combined single-port and endoluminal technique for laparoscopic anterior resection. Surg Laparosc Endosc Percutan Tech. 2010;20:253–256. doi: 10.1097/SLE.0b013e3181e21b33. [DOI] [PubMed] [Google Scholar]
- 50.Hompes R, Mortensen N, Cahill RA. Transanal endoscopic surgery using single access and standard laparoscopic instrumentation. Minerva Gastroenterol Dietol. 2012;58:273–281. [PubMed] [Google Scholar]
- 51.Ragupathi M, Ramos-Valadez DI, Pedraza R, Haas EM. Robotic-assisted single-incision laparoscopic partial cecectomy. Int J Med Robot. 2010;6:362–367. doi: 10.1002/rcs.346. [DOI] [PubMed] [Google Scholar]
- 52.Choi GS, Park IJ, Kang BM, Lim KH, Jun SH. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc. 2009;23:2831–2835. doi: 10.1007/s00464-009-0484-5. [DOI] [PubMed] [Google Scholar]


