Abstract
Messenger RNA of rat ornithine carbamoyltransferase (EC 2.1.3.3), a mitochondrial matrix enzyme, was enriched by immunoprecipitation of rat liver free polysomes, and recombinant plasmids were prepared from the enriched mRNA by a vector-primer method. The cDNA clones for ornithine carbamoyltransferase were identified by hybrid-arrested translation and hybrid-selected translation. One of the clones, designated pOTC-1, contained a 1.6-kilobase insert and hybridized to a mRNA of approximately equal to 1.8 kilobases in rat liver. The cDNA clone was subjected to nucleotide sequence analysis. The deduced amino acid sequence indicates that the ornithine carbamoyltransferase precursor consists of the mature enzyme of 322 amino acid residues and an NH2-terminal peptide extension (presequence) of 32 amino acid residues. The presequence contains 8 basic amino acid residues, no acidic residues, and no hydrophobic amino acid stretch. The amino acid sequence of the rat ornithine carbamoyltransferase was compared with the recently reported sequence of the human enzyme [Horwich, A. L., Fenton, W. A., Williams, K. R., Kalousek, F., Kraus, J. P., Doolittle, R. F., Konigsberg, W. & Rosenberg, L. E. (1984) Science 224, 1068-1074]. The sequences of the mature enzyme portion are 93% identical, whereas those of the presequences are 69% identical. There are two highly conserved segments in the presequences of the rat and human enzymes. One of the two conserved segments is significantly similar to a segment of the presequence of yeast mitochondrial elongation factor EF-Tu. These results suggest that the homologous segments are important for the proteins that are synthesized in the cytosol to be transported into the mitochondrial matrix.
Full text
PDF



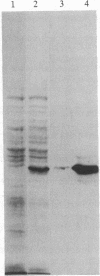
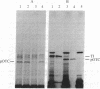
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argan C., Lusty C. J., Shore G. C. Membrane and cytosolic components affecting transport of the precursor for ornithine carbamyltransferase into mitochondria. J Biol Chem. 1983 Jun 10;258(11):6667–6670. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Adams J. M. Immunoprecipitation of specific polysomes using Staphylococcus aureus: purification of the immunoglobulin k chain messenger RNA from the mouse myeloma MPC11. Biochemistry. 1978 Dec 12;17(25):5560–5566. doi: 10.1021/bi00618a036. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kraus J. P., Williams K., Kalousek F., Konigsberg W., Rosenberg L. E. Molecular cloning of the cDNA coding for rat ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4258–4262. doi: 10.1073/pnas.80.14.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Kolansky D. M., Conboy J. G., Fenton W. A., Rosenberg L. E. Energy-dependent translocation of the precursor of ornithine transcarbamylase by isolated rat liver mitochondria. J Biol Chem. 1982 Jul 25;257(14):8467–8471. [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Tatibana M. Transport of ornithine carbamoyltransferase precursor into mitochondria. Stimulation by potassium ion, magnesium ion, and a reticulocyte cytosolic protein(s). J Biol Chem. 1983 Jun 10;258(11):6671–6674. [PubMed] [Google Scholar]
- Mori M., Miura S., Morita T., Takiguchi M., Tatibana M. Ornithine transcarbamylase in liver mitochondria. Mol Cell Biochem. 1982 Nov 26;49(2):97–111. doi: 10.1007/BF00242488. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free translation of carbamyl phosphate synthetase I and ornithine transcarbamylase messenger RNAs of rat liver. Effect of dietary protein and fasting on translatable mRNA levels. J Biol Chem. 1981 Apr 25;256(8):4127–4132. [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Ikeda F., Amaya Y., Tatibana M., Cohen P. P. Synthesis, intracellular transport, and processing of the precursors for mitochondrial ornithine transcarbamylase and carbamoyl-phosphate synthetase I in isolated hepatocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6056–6060. doi: 10.1073/pnas.78.10.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Morita T., Miura S., Tatibana M. Uptake and processing of the precursor for rat liver ornithine transcarbamylase by isolated mitochondria. Inhibition by uncouplers. J Biol Chem. 1981 Aug 25;256(16):8263–8266. [PubMed] [Google Scholar]
- Morita T., Miura S., Mori M., Tatibana M. Transport of the precursor for rat-liver ornithine carbamoyltransferase into mitochondria in vitro. Eur J Biochem. 1982 Mar 1;122(3):501–509. doi: 10.1111/j.1432-1033.1982.tb06465.x. [DOI] [PubMed] [Google Scholar]
- Nagata S., Tsunetsugu-Yokota Y., Naito A., Kaziro Y. Molecular cloning and sequence determination of the nuclear gene coding for mitochondrial elongation factor Tu of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6192–6196. doi: 10.1073/pnas.80.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson D. L., Cox S. L., Gilbert B. E. Human ornithine transcarbamylase. Purification and characterization of the enzyme from normal liver and the liver of a Reye's syndrome patient. J Biol Chem. 1977 Sep 25;252(18):6464–6469. [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. Differences in size, structure and function of free and membrane-bound polyribosomes of rat liver. Evidence for a single class of membrane-bound polyribosomes. Biochem J. 1977 Oct 15;168(1):1–8. doi: 10.1042/bj1680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]