Abstract
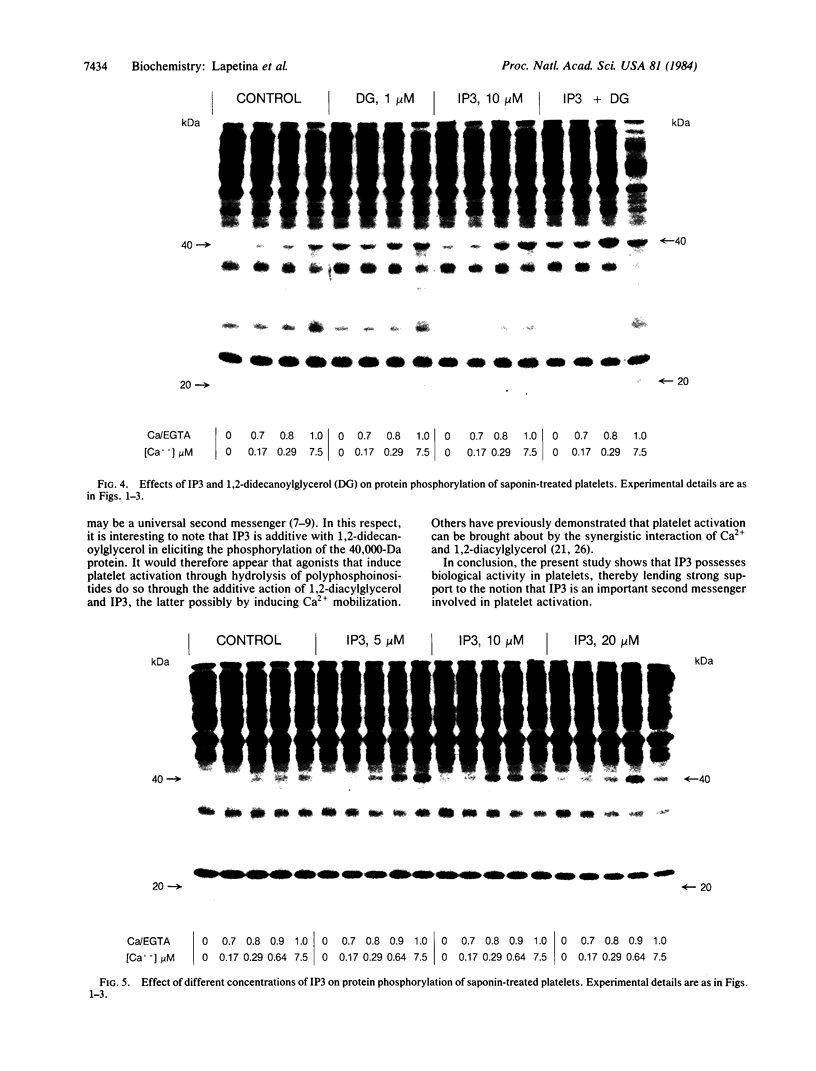
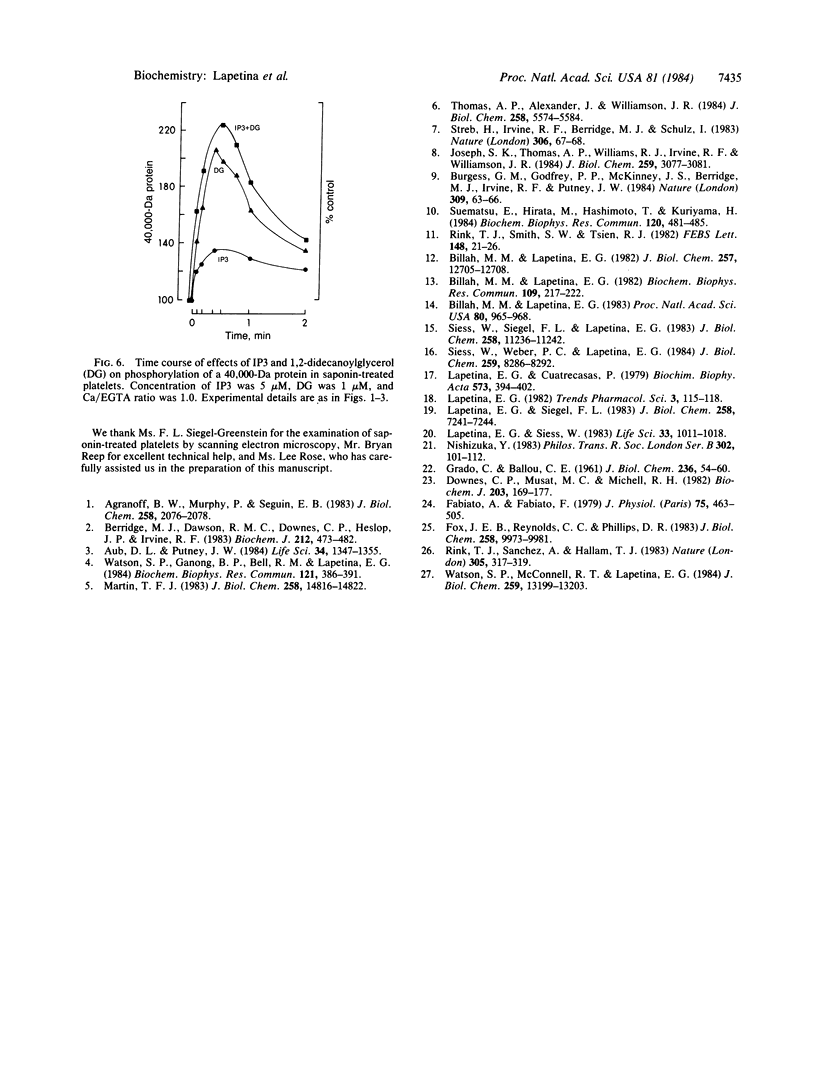
In an attempt to establish a system with physiological substrates and phospholipid surfaces to investigate Ca2+- and 1,2-diacylglycerol-dependent protein kinase C activation, saponized platelets were used. Saponin, through interaction with plasma membrane cholesterol, makes cells permeable without major disruption of organelles. Washed platelets, prelabeled with 32P, were treated with 1-50 micrograms of saponin per ml. Permeabilization was evident at a concentration of 10 micrograms of saponin per ml, as indicated by the action of extracellular Ca2+ on the phosphorylation of the 20,000- and 40,000-Da proteins. These proteins are, respectively, the substrates for myosin light chain kinase and protein kinase C. Activation of these enzymes occurred when the estimated free [Ca2+] was changed from approximately equal to 80 nM to 300 nM. The effect of Ca2+ on kinase C-induced phosphorylation was potentiated by 1,2-didecanoylglycerol (1 microM). myo-Inositol 1,4,5-trisphosphate (5-20 microM) increased phosphorylation of the 20,000- and 40,000-Da proteins. This action was time and concentration dependent. The effect of myo-inositol 1,4,5-trisphosphate on the activation of kinase C was additive with 1,2-didecanoylglycerol. The action of myo-inositol 1,4,5-trisphosphate could be due to mobilization of Ca2+ from platelet organelles and/or to a direct effect on protein kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Aub D. L., Putney J. W., Jr Metabolism of inositol phosphates in parotid cells: implications for the pathway of the phosphoinositide effect and for the possible messenger role of inositol trisphosphate. Life Sci. 1984 Apr 2;34(14):1347–1355. doi: 10.1016/0024-3205(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Degradation of phosphatidylinositol-4,5-bisphosphate is insensitive to CA2+ mobilization in stimulated platelets. Biochem Biophys Res Commun. 1982 Nov 16;109(1):217–222. doi: 10.1016/0006-291x(82)91587-x. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Platelet-activating factor stimulates metabolism of phosphoinositides in horse platelets: possible relationship to Ca2+ mobilization during stimulation. Proc Natl Acad Sci U S A. 1983 Feb;80(4):965–968. doi: 10.1073/pnas.80.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Siegel F. L. Shape change induced in human platelets by platelet-activating factor. Correlation with the formation of phosphatidic acid and phosphorylation of a 40,000-dalton protein. J Biol Chem. 1983 Jun 25;258(12):7241–7244. [PubMed] [Google Scholar]
- Lapetina E. G., Siess W. The role of phospholipase C in platelet responses. Life Sci. 1983 Sep 12;33(11):1011–1018. doi: 10.1016/0024-3205(83)90654-9. [DOI] [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Nishizuka Y. Calcium, phospholipid turnover and transmembrane signalling. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):101–112. doi: 10.1098/rstb.1983.0043. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Siess W., Siegel F. L., Lapetina E. G. Arachidonic acid stimulates the formation of 1,2-diacylglycerol and phosphatidic acid in human platelets. Degree of phospholipase C activation correlates with protein phosphorylation, platelet shape change, serotonin release, and aggregation. J Biol Chem. 1983 Sep 25;258(18):11236–11242. [PubMed] [Google Scholar]
- Siess W., Weber P. C., Lapetina E. G. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984 Jul 10;259(13):8286–8292. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Watson S. P., Ganong B. R., Bell R. M., Lapetina E. G. 1,2-Diacylglycerols do not potentiate the action of phospholipases A2 and C in human platelets. Biochem Biophys Res Commun. 1984 May 31;121(1):386–391. doi: 10.1016/0006-291x(84)90734-4. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]