Abstract
Background:
Insomnia is one of the most common complaints faced by primary care practitioners after pain. Non-pharmacological management of Insomnia that is noninvasive is gaining interest among patients with insomnia.
Purpose:
To determine the feasibility of recruiting and retaining participants in a clinical trial on shirodhara, Ayurvedic oil dripping therapy, for insomnia in the United States and also to investigate the therapeutic usefulness of Shirodhara for insomnia using standardized outcome measures.
Design:
Case series.
Study Intervention and Data Collection:
Shirodhara with Brahmi oil was done for 45 minutes on each participant for 5 consecutive days. Insomnia Severity Index (ISI) was used to evaluate the severity of insomnia as well as to determine the response to Shirodhara therapy. Data were collected at baseline, end of the treatment (day 5) and 1 week after the treatment ended (follow-up).
Results:
Two males and eight females with a mean age of 40 years (range 23 to 72), SD ± 14.2, were enrolled in the study. One dropped out of the study, but all remaining nine participants experienced improvement at the end of treatment. The percentage of improvement range varied from 3.85% to 69.57%. At follow-up, most participants continued to improve. Comparison of means between baseline and day 5 indicated an overall significant improvement (P < .005), but in a comparison of baseline vs 1 week posttreatment the improvement was not significant (P < .089). No adverse events were reported during the study.
Conclusion:
Shirodhara with Brahmi oil may be beneficial for moderate to severe insomnia. It is feasible to recruit and retain participants for such therapies in the United States. It is important to validate these findings and investigate the mechanism of action using a larger sample and rigorous research design.
Key Words: Shirodhara, oil dripping therapy, insomnia, Ayurveda, case series
摘要
背景: 失眠是继痛症之后,基础护理执业医生所面临的最常见抱怨之一 针对失眠进行非侵入式的非药理性治疗,越来越受到失眠患者的青睐。
目的: 确定招募和挽留参与者参加在美国进行的一项额头滴油疗法(阿育吠陀滴油疗法)治疗失眠临床试验的可行性,并采用标准化结果衡量指标研究额头滴油疗法在治疗失眠方面的治疗效果。
设计: 案例系列。
研究干预和数据收集: 连续 5天,每天用 Brahmi 油对每名患者施行 45 分钟的额头滴油疗法。 采用失眠严重程度指数 (ISI) 评价失眠的严重程度,并确定对额头滴油疗法的应答情况。 在基线、治疗结束(第 5 天)和治疗结束后 1 周(跟进)时收集数据。
结果: 共有两名男性和八名女性参与该研究,其平均年龄为 40 岁(年龄介于 23 岁至 72 岁之间),SD± 14.2。 一名受试者退出研究,但所有剩余的 9 名受试者在治疗结束时均出现改善。 改善百分数范围介于 3.85% 至 69.57% 之间。跟进时,大多数参与者持续出现改善现象。 对基线与第 5 天时的平均数进行比较发现,整体出现显著改善 (P < 0.005),但在对基线与治疗后 1 周时的平均数进行比较发现,改善并不显著 (P < 0.089)。研究期间未报告任何不良事件。
结论: 采用 Brahmi 油进行的额头滴油疗法可能对中度至重度失眠有益。 招募并挽留参与者参加在美国进行的该等治疗是可行的。 验证该等发现结果并采用更大的样本和严谨的研究设计对其作用机制进行研究,至关重要。
SINOPSIS
Antecedentes:
Después del dolor, el insomnio es una de las quejas más habituales a las que se enfrentan los médicos de atención primaria. El tratamiento no farmacológico y no invasivo del insomnio está ganando interés entre los pacientes con insomnio.
Propósito:
Determinar la viabilidad de reclutar y conservar a participantes en un ensayo clínico sobre shirodhara, la terapia de goteo de aceite ayurvédico, para el insomnio en Estados Unidos e investigar también la utilidad terapéutica de Shirodhara para el insomnio utilizando mediciones de resultados estandarizadas.
Diseño:
Serie de casos.
Intervención del estudio y recogida de datos:
Se realizó shirodhara con aceite de Brahmi durante 45 minutos en cada participante durante 5 días consecutivos. Se utilizó el Índice de Gravedad del Insomnio (Insomnia Severity Index, ISI) para evaluar la gravedad del insomnio así como para determinar la respuesta a la terapia de shirodhara. Se recogieron los datos en el inicio, final del tratamiento (día 5) y 1 semana después de la finalización del tratamiento (seguimiento).
Resultados:
Se inscribió en el estudio a dos varones y ocho mujeres con una edad media de 40 años (intervalo de 23 a 72), DE ± 14,2. Uno abandonó el estudio, aunque los 9 sujetos restantes experimentaron mejoría al final del tratamiento. El porcentaje del intervalo de mejoría varió del 3,85 % al 69,57 %. En el seguimiento, la mayoría de los participantes continuaron mejorando. La comparación de promedios entre el inicio y el día 5 indicó una mejora significativa total (P < 0,005), aunque en una comparación entre el inicio y 1 semana después del tratamiento, la mejoría no fue significativa (P < 0,089). No se informó de acontecimientos adversos durante el estudio.
Conclusión:
El shirodhara con aceite de Brahmi podría ser beneficioso para el insomnio moderado a grave. Es factible reclutar y conservar a participantes para dichas terapias en Estados Unidos. Es importante validar estos hallazgos e investigar el mecanismo de acción usando una muestra más grande y un diseño de investigación riguroso.
BACKGROUND
Insomnia is a common sleep disorder that affects an estimated 30% of the general population.1 It is characterized by difficulty with sleeping, which may include falling asleep, maintaining sleep, or a combination of the two. It often leads to fatigue, lack of energy, difficulty concentrating, and irritability. Women are affected more commonly than men, and it increases in both sexes with age.2 Additionally, studies have found that insomnia is more prevalent in divorced, separated, and widowed adults than in married adults. It also has been observed that several psychiatric and physical illnesses have a strong correlation with insomnia. Insomnia in elderly people results in deterioration of social and/or physical functioning.3 From previous studies in Canada, it has been shown that in addition to affecting the lives of individuals, insomnia may prove to be indirectly detrimental to the economy, costing the province of Quebec 6.6 billion CD each year due to missed work days.4 While the exact cause of insomnia is debatable, there appear to be numerous factors that may lead to insomnia. Insomnia may be induced by stress, anxiety, depression, tumors, asthma, cancers, hormonal cycles, or lifestyle factors.1 Diagnosis of insomnia is made primarily through patient interviews and sleep diaries. Both pharmacological and non-pharmacological approaches are used in the treatment of insomnia.5 Current treatments and medications for insomnia are costly and often harmful due to side effects. Many over-the-counter sleeping tablets, in addition to the commonly doctor-prescribed benzodiazepines, non-benzodiazepines, melatonin, anti-depressant, and antihistamine medications.,6 may lead to tolerance, dependence on the pills in order to fall asleep, or abuse of the medication.7 As the number of people who suffer from insomnia rises, there is an increased interest in complementary and alternative medicine (CAM). Recent studies have shown that roughly 1.6 million Americans (2002 National Health Interview Survey) turned to CAM therapies within the previous 12 months to treat their conditions.8 Some of these include herbal supplements, Ayurveda, acupuncture, yoga, hypnosis, meditation, and exercise. The amount of evidence regarding the effectiveness of these treatments is currently lacking, and due to the nature of how individuals obtain CAM treatments through atypical means, an estimation of the prevalence of CAM treatment is difficult to ascertain. Ayurveda, the Indian system of medicine, uses externally applied medicated herbal oils in addition to internal herbal remedies to balance the doshas (biological humors) and treat ailments. According to Ayurveda, the three doshas (Vata, Pitta, Kapha) regulate the internal physiological activity.9
Shirodhara, oil dripping on the forehead in a steady stream or flow, is a widely practiced complementary treatment (upakarma) of Ayurveda in both India and the United States. It is usually indicated to treat stress, anxiety, and insomnia and to relax the nervous system.10 Generally, sesame oil processed with various Indian herbs is used for Shirodhara. However, milk and buttermilk processed with herbs also are used in this treatment depending upon the patient's condition. A typical Shirodhara session would last 30 to 60 minutes and is done for 3, 7, 14, or 28 days. The duration of a session may vary. The Ayurvedic practitioner determines both the duration and the number of days according to the patient's age, constitution, and dosha status and the severity of the disease. Despite its wide use in the United States, usefulness of Shirodhara treatment for insomnia has not been scientifically investigated in the West. The purpose of this case series was to determine the feasibility of recruiting and retaining participants in a clinical trial on Shirodhara for insomnia in the United States and also to investigate the therapeutic usefulness of Shirodhara for insomnia using standardized outcome measures.
MATERIALS AND METHODS
Sample Recruitment
A prospective case series design was adopted. Ten consecutive volunteers who responded to community-wide recruitment efforts were enrolled in the study between September 2009 and August 2010. Participants between ages 18 and 75 years of either sex with a duration of insomnia of at least 1 year who were willing to sign an informed consent and who had a minimum score of 14 on the Insomnia Severity Index (ISI) were included in the study. It was decided to exclude people with comorbidities such as depression or any other psychological conditions that require medications and those who were on prescription medication for insomnia. Additionally, people with serious medical conditions such as uncontrolled hypertension, uncontrolled diabetes, or any other acute condition that disturbed sleep were excluded. Participants who were unwilling to comply with the study protocol were considered unsuitable for this study. A local institutional review board approved the study, and the study was registered at clinicaltrials.gov. Written informed consent was obtained from all participants prior to their participation in the study (NCT00606658).
Study Intervention
Brahmi oil (coconut oil processed with Hydrocotyle asiatica primarily and less than 20% of Triphala) manufactured by Bazaar of India Imports, Berkeley, California, was used to perform Shirodhara. The current blend of Brahmi oil was chosen because of its local availability and cost. Each participant was treated for 40 minutes for 5 consecutive days. Equipment manufactured by ShiroPlus (Homer, Alaska) that automatically warms (99.0° F) and pumps the oil was used in this study for oil dripping. During each treatment, 400 mL to 500 mL oil was used for each participant depending upon their scalp size and quantity of hair. Dry, hairy scalps consumed more oil than cleanly shaven or bald ones. The oil was changed after 3 days, and fresh oil was used to perform the procedure over the next 2 days. It is believed that the initial oil loses its efficacy after it is heated and dripped over a forehead for 2 to 3 days for 40 minutes each day. The oil dripping was done by two research assistants who were trained by an Ayurvedic practitioner trained in India who had experience performing Shirodhara treatments before. The same assistant performed all five treatments on a participant to maintain uniformity in the treatment and interaction with the participants.
Outcome Measure
The ISI was used to evaluate the nature, severity, and impact of each participant's insomnia and response to the oil-dripping therapy. The ISI is a seven-item patient self-report questionnaire assessing the different dimensions of insomnia, including severity of sleep onset, sleep maintenance, early morning awakening, sleep dissatisfaction, interference of sleep difficulties with daytime functioning, noticeability of sleep problems by others, and distress caused by the sleep difficulties.11 Each question is rated on a scale from 1 to 4 with a total score ranging from 0 to 28; the higher the score, the more severe the insomnia.12 The ISI has been shown to be valid and reliable in previous studies.11 and validated against both polysomnographic and prospective sleep diary measures.13 Additionally, participants were asked to fill out an adverse event form if necessary. Data were collected at baseline, day 5 (end of treatment), and 1 week after the treatment was over. Participants also were asked to give their overall impressions about the therapy at the end of their study.
Data Analysis
Descriptive statistics were used to describe the demographic data of all the participants. Total (mean) scores and percentage of improvement from baseline to the end of the study were calculated to report the differences in outcomes. Paired t-tests were used to compare means between baseline, end of treatment, and follow-up.
RESULTS
Nine participants completed the study successfully. The sample included two males and seven females with a mean age of 40 years (range 23 y-72 y; SD ± 14.2). Given the small sample size, individual case data were examined. The coding of the ISI scale is such that high positive numbers are indicative of high severity and low positive numbers are indicative of lower severity. Comparing baseline to day 5 (end of treatment), all nine participants experienced improvement. For six participants, the percentage of improvement ranged from 25.93% to 69.57%. For the other three participants, there was slight improvement with a range of 3.85% to 8.33%. Comparing baseline to 1 week posttreatment, the percentage of improvement ranged from 8.33% to 86.96% with one exception: one study participant's insomnia worsened by −66.67% (Table 1, Figure 1).
Table 1.
Insomnia Severity Index Total Scores of Participants at Baseline, Day 5, and 1 Week Follow-up and Percentage Improvement
| Subject | Screening | Baseline | Day 5 (End of Treatment) | 1 Wk Posttreatment | Percentage Improvement Baseline vs End of Treatment (Day 5) | Percentage Improvement Baseline vs 1 Wk Posttreatment |
|---|---|---|---|---|---|---|
| 1 | 17 | 17 | 10 | 14 | 41.18 | 17.65 |
| 2 | 23 | 17 | 7 | 6 | 58.82 | 64.71 |
| 3 | 14 | 19 | 12 | 17 | 36.84 | 10.53 |
| 4 | 15 | 12 | 11 | 11 | 8.33 | 8.33 |
| 5 | 14 | 12 | 11 | 20 | 8.33 | −66.67 |
| 6 | 25 | 26 | 25 | 23 | 3.85 | 11.54 |
| 7 | 19 | 22 | 16 | 19 | 27.27 | 13.64 |
| 8 | 25 | 23 | 7 | 3 | 69.57 | 86.96 |
| 9 | 27 | 27 | 20 | 16 | 25.93 | 40.74 |
Figure 1.
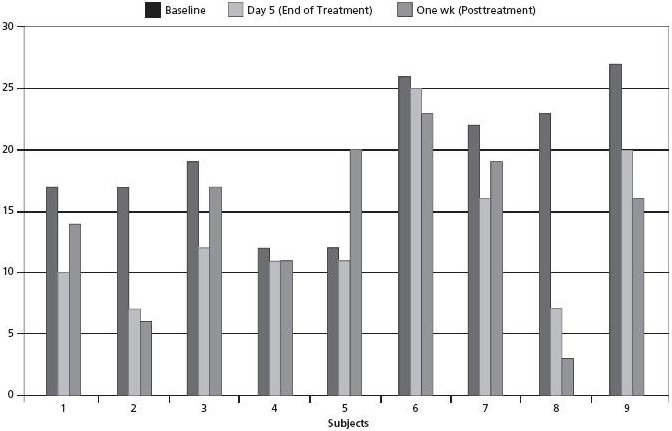
Insomnia Severity Index total scores.
Table 2 and Figure 2 report the ISI mean scores. At baseline, the mean ISI score was 19.44, which decreased over time—indicative of improvement. At day 5, the corresponding score was 13.22, and at 1 week post-baseline, the mean ISI score was 14.33. Comparison of means was also done to determine if there were any statistically significant changes between pretest and posttest. Paired sample t-test was used for comparing baseline ISI mean values with 5-day and 1-week posttreatment mean ISI scores. For the baseline and day 5 comparison, there was an overall improvement (significant at P < .005), and for the baseline vs 1-week posttreatment comparison the improvement was not significant (P < 0.089) (Table 3). However, these results have to be interpreted carefully.
Table 2.
Insomnia Severity Index Mean Values (SD) at Screening, Baseline, Day 5, and 1 week Follow-up (N = 9)
| Screening | Baseline | Day 5 | 1 Wk Posttreatment |
|---|---|---|---|
| 19.89 (5.18) | 19.44 (5.50) | 13.22 (6.04) | 14.33 (6.60) |
Figure 2.
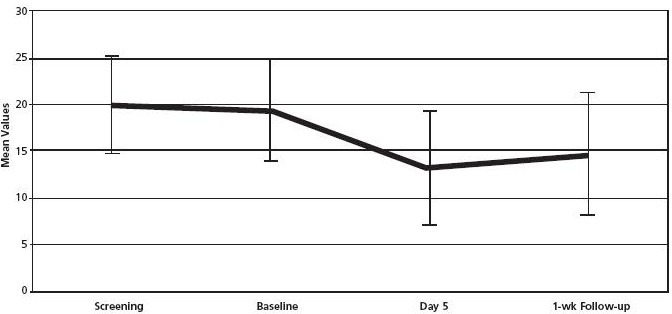
Insomnia Severity Index mean values and standard deviation.
Table 3.
Mean Comparison Between Baseline and Day 5 and Between Baseline and Follow-up Using Paired Samples t-test
| Time Points | Mean (SD) | 95% Confidence Intervals | P value |
|---|---|---|---|
| Baseline vs day 5 | 6.22 (4.92) | 2.44 - 10.00 | 0.005a |
| Baseline vs F/U | 5.11 (7.92) | −0.98 - 11.20 | 0.089 |
Degrees of freedom = 8;
Abbreviation: F/U, follow-up.
DISCUSSION
Insomnia is the most common sleep problem seen in healthcare practices that may present as a symptom or clinical syndrome.11 The clinical guidelines for the evaluation and management of chronic insomnia in adults indicate insomnia as a public health problem and recommend accurate diagnosis and effective treatment.14 Therapeutic approaches are aimed primarily at improving sleep quality and reducing insomnia-related daytime impairments. Non-pharmacological treatments such as cognitive behavior therapy and relaxation therapy.15 have been shown to be equally good or even better than pharmacological treatments in terms of long-term benefits. Shirodhara has been shown to be effective in attention deficit/hyperactivity disorder in children16, hypertension17, meno-pause,18 cerebral ataxia,19 general anxiety disorder,20 chronic headache,21 and insomnia.22 In a study by Pokahrel and Sharma (n = 30),22 Shirodhara done with warm milk (for 15 days) and combined with an Ayurvedic herbal formula, Insomrid (National Institute of Ayurveda, Jaipur, India), was compared to Insomrid alone. The authors reported that the combination group showed better improvement than participants who received Shirodhara or Isomrid alone (P < .001). The authors did not conduct a between-group analysis and also had only 10 participants in each group. Furthermore, the authors did not use valid and reliable outcome measures.22 The purpose of our study is to determine the feasibility of recruiting and retaining participants because we believe that this is the first clinical trial investigating this unique Ayurvedic therapy in the West. Secondarily, the study also intended to understand the usefulness of Shirodhara for insomnia.
The results suggest that there was a moderate improvement of insomnia with Shirodhara with Brahmi oil at the end of the fifth day in most patients. These results are similar to those in the study by Pokharel and Sharma.22 Although all of the participants but one reported improvement in sleep at the end of the fifth day, the improvement was not sustained in all of the participants. No adverse events or side effects were reported by the participants during the entire study period. One participant dropped out after one treatment because she did not want to wash her hair every day after the oil treatment.
The moderate improvements reported in this study could be due to lying on a table with eyes closed, ambience, or music. Nevertheless, psychoneuroimmunological effects of this therapy, such as a decrease in noradrenaline, exhibiting a sympatholytic effect, and resulting in the activation of peripheral foot skin circulation and increase in natural killer cells, have been demonstrated by Japanese researchers.23 These researchers also predicted that the effects of Shirodhara in reducing anxiety could be attributed to the somato-autonomic reflex through thermosensors or pressure sensors in the skin or hair follicles via the trigeminal cranial nerve.24
The overall impressions of the participants that were collected through an informal interview at the end of the study also supported the improvements noticed in the ISI scores. The following quotes indicate some of the impressions of participants in this study:
I was very surprised that I was able to sleep until 5:55 am after the fifth day of treatment! And I was shocked that I was able to take 2 (lack of break) 3.5-hour naps! (usually, no matter how sleep deprived, I cannot nap—or if I do fall asleep, I immediately awaken.) It's nice to sleep past 2:00, 3:00, or 4:00 am. The long naps, however, kind of messed up the days/evening and getting to sleep at a decent hour. Thank you for letting me be part of your insomnia study.
The first few days, I slept through the night. Now on and off through the night.
During the treatment I felt very relaxed, especially after the first 15 minutes or so. I did experience some emotional symptoms during two of the treatments but felt better afterwards. I think my sleeping patterns got a little better toward the end of the week and the following weekend. However, the next week they resumed to how they were before the treatment began. I think that if I had done the study during a period of time that was not as stressful in my life, I may have benefited more.
The effects of the treatment lasted noticeably for about 2 to 3 weeks. I'm not sure if it may be due to my change of schedule. I was feeling less irritable and fatigued. If I was [experiencing] insomnia again and I had a chance to undergo this therapeutic treatment, I would do so again.
LIMITATIONS
Despite the positive outcomes and encouraging comments of the participants, this study has several limitations. While interpreting the results, one must account for the nonrandom sample, participant selection bias, and the effects of regression to the mean. Furthermore, due to the small sample size, the study results will lack generalizability outside the study setting. Additionally, lack of a control group limits the internal validity of this study, and we cannot rule out biases that are associated with single group studies. The therapy duration and type of oil were not individualized per the severity and type of insomnia and constitution (ie, prakriti/vikriti) of the participants. Prakriti is the original constitution of a person that determines what keeps a person in balance with nature. Vikriti is the current state of imbalance of a person's doshas. Typically, both prakriti and vikriti are taken into account while choosing an Ayurvedic treatment. The duration and length of the therapy was arbitrarily decided as 45 minutes duration for only 5 days contrary to usual Ayurvedic practice that advocates 7 to 28 days of oil dripping and duration ranging from 30 to 60 minutes per day.
CONCLUSION
Shirodhara done with Brahmi oil for 45 minutes may be beneficial for moderate to severe insomnia. The results and the impressions of the participants encourage the investigators to pursue further rigorous research on this modality using a larger sample and adding a comparison group. Additionally, we would like to understand the mechanism of action of Shirodhara using brain functional magnetic resonance imaging and specifically note if a specific area within the brain is getting activated after Shirodhara. Any positive findings from these studies would establish Shirodhara as a noninvasive approach to the management of insomnia.
Acknowledgments
The authors would like to thank Aum Mahan, Rancho Cucamonga, California, for its financial support in the form of a mini-grant and Bazaar of India Imports for donating some of the oil used in the study. The authors would also like to acknowledge Julia Wu and Nina La for their support in performing the treatments and collecting the data.
Disclosures The authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and have no relevant conflicts to disclose.
Contributor Information
Sivarama Prasad Vinjamury, Southern California University of Health Sciences, United States.
Manjusha Vinjamury, Southern California University of Health Sciences, United States.
Claudia der Martirosian, Southern California University of Health Sciences, United States.
Judith Miller, Southern California University of Health Sciences, United States.
REFERENCES
- 1.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007; 3(5 Suppl): S7–10 [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002; 6(2): 97–111 [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo JL-T, Gras CB, García YD, Lapeira JT, del Campo JM, Verdejo MAL. Functional status in the elderly with insomnia. Qual Life Res. 2007; 16(2): 279–86 [DOI] [PubMed] [Google Scholar]
- 4.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009; 32(1): 55–64 [PMC free article] [PubMed] [Google Scholar]
- 5.Zammit GK. The prevalence, morbidities, and treatments of insomnia. CNS Neurol Disord Drug Targets. 2007; 6(1): 3–16 [DOI] [PubMed] [Google Scholar]
- 6.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med. 2006; 166(16): 1775–82 [DOI] [PubMed] [Google Scholar]
- 7.Rosekind MR, Gregory KB. Insomnia risks and costs: health, safety, and quality of life. Am J Manag Care. 2010; 16(8): 617–26 [PubMed] [Google Scholar]
- 8.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med. 2006; 166(16): 1775–82 [DOI] [PubMed] [Google Scholar]
- 9.Hankey A. Ayurvedic physiology and etiology: Ayurvedo Amritanaam. The doshas and their functioning in terms of contemporary biology and physical chemistry. J Altern Complement Med. 2001; 7(5): 567–74 [DOI] [PubMed] [Google Scholar]
- 10.Uebaba K, Xu FH, Tagawa M, et al. Using a healing robot for the scientific study of shirodhara. Altered states of consciousness and decreased anxiety through Indian dripping oil treatments. IEEE Eng Med Biol Mag. 2005; 24(2): 69–78 [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34(5): 601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2(4): 297–307 [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Wegener ST. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthrit Care Res. 2003; 49(S5): S184–96 [Google Scholar]
- 14.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008; 4(5): 487–504 [PMC free article] [PubMed] [Google Scholar]
- 15.Petit L, Azad N, Byszewski A, Sarazan FF-A, Power B. Non-pharmacological management of primary and secondary insomnia among older people: review of assessment tools and treatments. Age Ageing. 2003; 32(1): 19–25 [DOI] [PubMed] [Google Scholar]
- 16.Chawla J. Insomnia treatment & management. http://emedicine.medscape.com/article/1187829-treatment AccessedDecember4, 2013
- 17.Kundu C, Shukla VD, Santwani MA, Bhatt NN. The role of psychic factors in pathogenesis of essential hypertension and its management by Shirodhara and Sarpagandha Vati. Ayu. 2010; 31(4): 436–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santwani K, Shukla VD, Santwani MA, Thaker G. An assessment of Manasika Bhavas in menopausal syndrome and its management. Ayu. 2010; 31(3): 311–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sriranjini SJ, Pal PK, Devidas KV, Ganpathy S. Improvement of balance in progressive degenerative cerebellar ataxias after Ayurvedic therapy: a preliminary report. Neurol India. 2009; 57(2): 166–71 [DOI] [PubMed] [Google Scholar]
- 20.Tubaki BR, Chandrashekar CR, Sudhakar D, Prabha TNS, Lavekar GS, Kutty BM. Clinical efficacy of Manasamitra Vataka (an Ayurveda medication) on generalized anxiety disorder with comorbid generalized social phobia: a randomized controlled study. J Altern Complement Med. 2012; 18(6): 612–21 [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Singh RH. A clinical study on Ayurvedic management of chronic daily headache with special reference to sirodhara and sirovirecana. J Res Ayurveda Siddha. 2001; 12: 81–94 [Google Scholar]
- 22.Uebaba K, Xu F-H, Ogawa H, et al. Psychoneuroimmunologic effects of Ayurvedic oil-dripping treatment. J Altern Complement Med. 2008; 14(10): 1189–98 [DOI] [PubMed] [Google Scholar]
- 23.Pokharel S, Sharma AK. Evaluation of Insomrid tablet and shirodhara in the management of Anidra (insomnia). Ayu. 2010; 31(1): 40–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu F, Uebaba K, Ogawa H, et al. Pharmaco-physio-psychologic effect of Ayurvedic oil-dripping treatment using an essential oil from Lavendula angustifolia. J Altern Complement Med. 2008; 14(8): 947–56 [DOI] [PubMed] [Google Scholar]


