Abstract
Alpha lipoic acid (ALA, thioctic acid), among other actions, is an essential coenzyme in the conversion of pyruvate to acetyl co-enzyme A. Therefore, it is necessary for the production of energy for aerobic organisms. Scientists have found that it can be used medically to help regenerate liver tissue, reverse the complications of diabetes mellitus, slow or stop the growth of cancer cells, and chelate heavy metals, among other actions. In this article, the authors describe the cellular mitochondrial damage from excessively high doses of this beneficial agent.
Key Words: Extremely high doses, alpha-lipoic acid, mitochondrial structural damage, liver regeneration, acute hepatic necrosis, diabetes, cancer
摘要
在丙酮酸盐转化成乙酰辅酶 A 这类反应中,α 硫辛酸(ALA,硫辛酸)是一种必需的辅酶。 科学家们已经发现,这种辅酶可以应用在医疗领域,从而帮助肝组织再生,逆转糖尿病并发症,减缓或终止癌细胞生长以及螯合重金属,诸如此类。 在本文中,作者叙述了以过高剂量施用这种有益药剂对细胞线粒体所造成的伤害。
SINOPSIS
El alfa-ácido lipoico (ALA, ácido tióctico), entre otras acciones, es una coenzima esencial en la conversión del piruvato en acetil coenzima A. Por lo tanto, es necesario para la producción de energía para los organismos aerobios. Los científicos han descubierto que puede ser utilizado médicamente para ayudar a regenerar el tejido hepático, invertir las complicaciones de la diabetes mellitus, ralentizar o detener el crecimiento de células cancerígenas y frenar la toxicidad de los metales pesados, entre otras acciones. En este artículo, los autores describen el daño mitocondrial celular causado por dosis excesivamente altas de este agente beneficioso.
INTRODUCTION
The mitochondrion converts nutrients into the energy-generating molecule adenosine triphosphate (ATP) in order to fuel aerobic respiration. Hepatocytes have exceptionally high energy demands, so they have particularly high numbers of mitochondria.
Any disruption of mitochondrial structure and/or function can lead to a wide assortment of medical disorders because the resulting oxidative stress interferes with the generation of ATP molecules. In this way, tissues are starved of the energy from electrons that normally result from the oxidation of food.
Alpha-lipoic acid (ALA, thioctic acid) was first characterized by Reed et al in 1951.1 ALA was found to play a most important role in the pyruvate dehydrogenase enzyme complex (PDC) in mitochondrial function. The PDC is composed of three enzymes: E1 (pyruvate dehydrogenase), E2 (dihydrolipoyl transacetylase), and E3 (dihydrolipoyl dehydrogenase).
As one can see, because lipoic acid is such an essential cofactor in the conversion of pyruvate to acetyl coenzyme A, it is thought to be the rate-limiting factor for the production of energy in the eukaryotic cell. The PDC cannot operate without ALA, and all aerobic organisms would die without it. In addition, ALA is essential for several other metabolic reactions including thyroid function,2 glycine cleavage,3 and the tricarboxcylic acid cycle,4 among others. Many of these reactions occur within the mitochondrion.
Bartter et al and Berkson reported in three articles, in the first large-scale human clinical study out of the National Institutes of Health (NIH), that 75 of 79 patients survived severe acute hepatic necrosis as a result of hepatotoxic mushroom poisoning on just intravenous ALA.5–7 Those articles described the original NIH protocol. The source of the intravenous ALA in the study by Bartter et al was Italian prescription-grade thioctic acid (Merrill Richardson ALA, Merrill Richardson Pharmaceuticals, Milan, Italy). In addition, encouraging results were published using oral and intravenous ALA as a component of a treatment regimen for hepatitis C,8 various cancers,9–13 and the reversal of diabetic neuropathies.14,15
Recently, ALA was listed in the Physicians' Desk Reference as a nutraceutical rather than as an investigational drug, as it was originally listed, and many medical and naturopathic doctors have been administering ALA intravenously as a treatment for various disorders such as diabetic neuropathies, acute and chronic liver disease, autoimmune disease, blood vessel disease, and cancer.
It has come to the attention of the authors anecdotally that some clinicians have used extremely high doses of this agent or have not diluted it properly as the NIH protocol describes, which can lead to adverse reactions. If numerous physicians use ALA therapeutically for various disorders, it would be beneficial to know what dose might be toxic to humans.
Some clinicians follow the original NIH protocol of Bartter et al, and others have created their own variations. Drs Bartter and Berkson were originators of the US Food and Drug Administration (FDA) Investigation New Drug Application (9957), and Dr Berkson was the FDA principal investigator for 23 years. When administered properly, ALA should have only beneficial effects.
MATERIALS AND METHODS
In 1996, a group of researchers at Animal Research Laboratories, White Sands Missile Range, New Mexico, led by one of the authors (MV), were concerned that physicians might inadvertently administer this normally harmless agent in excess amounts to their patients. His research group was interested in determining what dose would be excessive and harmful.16 The source of their intravenous ALA was Sigma-Aldrich (St Louis, Missouri). The workers performed LD50 studies on six rhesus monkeys and found that approximately 90 mg/kg to 100 mg/kg of intravenous ALA was lethal to three of the six trial specimens. Consequently, the workers considered that dose to be the LD50 (median lethal dose) for primates. The details of this study will be presented in an upcoming publication.
The authors of this article do not normally administer more than 10 mg/kg—very well below any potential lethal dose—to any patient. However, even much smaller doses might elicit hypoglycemia, so physicians must be prepared to administer intravenous sugar solution or a sweet beverage. The treated primates, including the animals that died from the lethal dose and the ones that did not die, were sacrificed and subjected to postmortem examination. All animals displayed evidence of acute hepatic necrosis. Their livers were enlarged, turgid, fragile, and markedly discolored in a pale and dark red reticulated pattern. Large waxy gray infarcts were also seen.
Other organs also were affected by the lethal and sub-lethal doses of intravenous ALA. Large necrotic areas were observed in the large muscles of their thighs, the liver, the heart, and the kidneys. At a later date, lab technicians prepared sections of the damaged liver tissues for transmission electron microscopy in order to determine the cytological basis of injury. Tissues from the necrotic regions of the liver were excised and prepared according to a generally used 2% glutaraldehyde immersion fixation preparation scheme for transmission electron microscopy. The fixed tissue was embedded and sectioned and then observed by the authors.
RESULTS
Typical rhesus monkey liver tissue demonstrates normally appearing mitochondria with numerous intact christae and other healthy membranes (Figure 1). The normal hepatocyte mitochondrion exhibits a double membrane wall with an infolding of the inner wall to form an extensive inner surface of christae on the surface of which ATP is generated.
Figure 1.
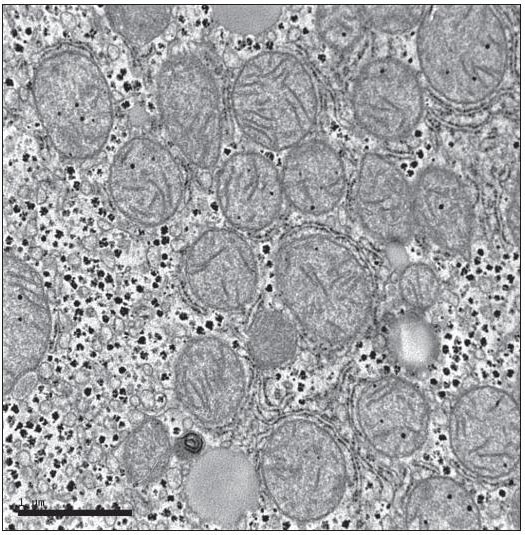
Healthy primate mitochondria. Note intact membranes and cristae.
Mitochondria from animals that had received excessively high doses of ALA became extremely edematous and demonstrated a disruption of all of the crucial membranes (Figure 2). These mitochondria did not exhibit the regular double membrane wall structure but showed a coalescence of these structures with a deliquescence of other structures, thus exhibiting a complete disruption of normal ultrastructure.
Figure 2.
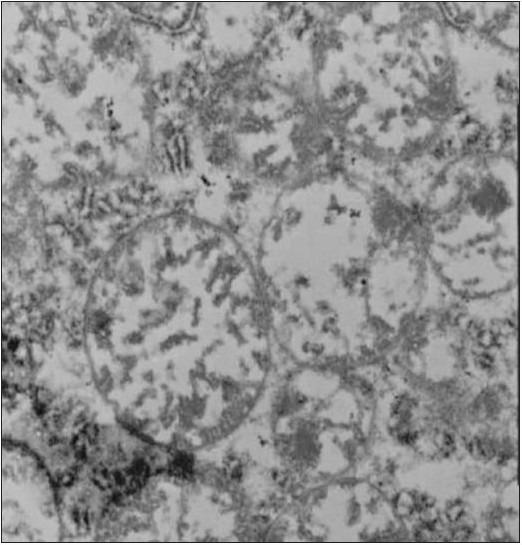
Primate mitochondria after exposure to extremely high doses of intravenous alpha lipoic acid. Note gross swelling and damage to cristae.
DISCUSSION
In normal doses, intravenous ALA prevents oxidative stress–induced damage to the membranous structures of the cell including the mitochondrion. Von Zglinicki et al confirmed this by showing that rat liver cells damaged by a high iron/ascorbate solution were resuscitated by the early administration of ALA.17 And Li CJ et al reported that myocardial apoptosis can be suppressed by ALA through the inhibition of mitochondrial oxidative stress. ALA, if administrated early, prevented this mitochondrial cardiac damage.18
One plausible mechanism for ALA's effect in enormously high doses involves the lipids in the mitochondrial membrane. When excess amounts of the reduced intravenous ALA comes in contact with diatomic oxygen in the mitochondrion, it might form superoxide anions, a damaging free radical with a negative charge. The unsaturated double bonds in lipids of the mitochondrial membranes are prime sites for reactions with superoxide radicals.
Lipid peroxidation is common in mitochondria due to this reaction. The peroxidation of the lipids destroys the mitochondrial membranes cascading the system into apoptosis. This sequence of events is well known among the biochemical consequences of reactive oxygen species (ROS).
CONCLUSION
In this article, we show that exceptionally high doses of intravenous ALA can produce the same symptoms that smaller doses prevent. To prove this, the Couch and Vigil group demonstrated that the LD50 dose for primates was about 90 mg to 100 mg for IV ALA.
Korotchkina et al reported that ALA inhibits pyruvate dehydrogenase kinase.19 This is the enzyme that disables the pyruvate dehydrogenase complex. Consequently, ALA speeds up the conversion of pyruvate to acetyl Co A.
It is our hypothesis that ALA is the rate-limiting agent in the production of energy from carbohydrate and protein in aerobic eukaryotes. With appropriate ALA levels, the mitochondrion functions normally. If the mitochondrion does not obtain sufficient ALA, it suffers and the organism lacks energy.
Conversely, if the mitochondrion is supplied with excessive amounts of ALA, it accelerates aerobic respiration and the process runs ahead of the other necessary constituents. The mitochondrion then heats up, and its membranous components break down. Severe damage to the mitochondrion is first seen by gross swelling and then severe damage to the cristae and matrix material.
It is interesting to note that therapeutic doses of intravenous ALA help a liver regenerate, but extremely high doses of the same agent cause liver necrosis. Of course, excessive and unreasonable amounts of any substance, including water and salt, given intravenously can be lethal; however, until intravenous ALA is better studied, the authors suggest that it not be used by doctors who are not completely skilled in its chemistry and its administration. In addition, even much lower doses of intravenous ALA may cause hypoglycemia in certain individuals, so the clinician must always be prepared to counter this potentially hazardous problem.
Disclosures The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and had no potential conflicts to disclose.
Contributor Information
Michael Vigil, Department of Biochemistry, New Mexico State University, Las Cruces (Dr Vigil).
Burton M. Berkson, Integrative Medical Center of New Mexico, Las Cruces, Department of Entomology, Plant Pathology, and Weed Science, New Mexico State University (Dr Berkson).
Ana Patricia Garcia, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia (Dr Garcia)..
REFERENCES
- 1.Reed LJ, DeBusk BG, Gunsalus IC, et al. Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. 1951; 114(2952): 93–4 [DOI] [PubMed] [Google Scholar]
- 2.Segermann J, Hotze A, Ulrich H, Rao GS. Effect of alpha-lipoic acid on the peripheral conversion of thyroxine to triiodothyronine and on serum lipid-, protein- and glucose levels. Arzneimittelforschung. 1991; 41(12): 1294–8 [PubMed] [Google Scholar]
- 3.Fujiwara K, Okamura-Ikeda K, Motokawa Y. Expression of mature bovine H-protein of the glycine cleavage system in Escherichia coli and in vitro lipoylation of the apoform. J Biol Chem. 1992; 267(28): 20011–6 [PubMed] [Google Scholar]
- 4.Reed LJ, Debusk BG. A conjugate of alpha-lipoic acid required for oxidation of pyruvate and alpha-ketoglutarate by an Escherichia coli mutant. J Biol Chem. 1952; 199(2): 873–80 [PubMed] [Google Scholar]
- 5.Bartter FC, Berkson BM, Gallelli J, Hiranaka P. Thioctic acid in the treatment of poisoning with alpha-amanitin. : Falstich H, Kommerell B, Wieland TH.editors Amanita toxins and poisonings. Baden-Baden, Germany: Verlag Gerhard Witzstrock; 1980: 197–202 [Google Scholar]
- 6.Berkson BM. Treatment of four delayed mushroom poisoning patients with thioctic acid. : Falstich H, Kommerell B, Wieland TH.editors Amanita toxins and poisonings. Baden-Baden, Germany: Verlag Gerhard Witzstrock; 1980: 203–7 [Google Scholar]
- 7.Berkson BM. Thioctic acid in treatment of hepatotoxic mushroom (Phalloides) poisoning. N Engl J Med. 1979; 300(7): 371. [DOI] [PubMed] [Google Scholar]
- 8.Berkson BM. A conservative triple antioxidant approach to the treatment of hepatitis C. Combination of alpha lipoic acid (thioctic acid), silymarin, and selenium: three case histories. Med Klin (Munich). 1999October15; 94 Suppl 3: 84–9 [DOI] [PubMed] [Google Scholar]
- 9.Wenzel U, Nickel A, Daniel H. Alpha-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis. 2005; 10(2): 359–68 [DOI] [PubMed] [Google Scholar]
- 10.Berkson BM, Rubin D, Berkson A. The long term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous alpha-lipoic acid/low dose naltrexone (ala-n) protocol. Integr Cancer Ther. 2006; 5(1): 83–9 [DOI] [PubMed] [Google Scholar]
- 11.Berkson BM, Rubin D, Berkson A. Revisiting the ALA/N (alpha-lipoic acid/ low-dose naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: a report of three new cases. Integr Cancer Ther. 2009; 8(4): 416–22 [DOI] [PubMed] [Google Scholar]
- 12.Van de Mark K, Chen JS, Steliou K, Perrine SP, Faller DV. Alpha-lipoic acid induces p27Kip-dependent cell cycle arrest in non-transformed cell lines and apoptosis in tumor cell lines. J Cell Physiol. 2003; 194(3): 325–4 [DOI] [PubMed] [Google Scholar]
- 13.Mantovani G, Maccio A, Madeddu C, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003; 81(10): 664–73 [DOI] [PubMed] [Google Scholar]
- 14.Schreiber FK. On the treatment of diabetic neuropathy. Dtsch Med Wochenschr. 1961March24; 86: 531–2 [DOI] [PubMed] [Google Scholar]
- 15.Sladki E, Maldyk H, Prusinski A. Treatment of polyneuritic complications in diabetes with thioctic acid]. Pol Tyg Lek. 1963January1; 18: 16–8 [PubMed] [Google Scholar]
- 16.Couch RC, Vigil M., Thomas MA, Sibert G. A dose escalation toxicity study of DL-6-8 thioctic acid (lipoic acid) in Rhesus monkeys. Annual Meeting of Toxicology; Cincinnati, Ohio; March12, 1997 [Google Scholar]
- 17.von Zglinicki T, Wiswedel I, Trümper L, Augustin W. Morphological changes of isolated rat liver mitochondria during Fe2+/ascorbate-induced peroxidation and the effect of thioctacid. Mech Ageing Dev. 1991; 57(3): 233–46 [DOI] [PubMed] [Google Scholar]
- 18.Li CJ, Zhang QM, Li MZ, Zhang JY, Yu P, Yu DM. Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy. Chin Med J (Engl). 2009; 122(21): 2580–6 [PubMed] [Google Scholar]
- 19.Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004; 38(10): 1083–92 [DOI] [PubMed] [Google Scholar]


