Abstract
Mast cells are critical effectors in inflammatory diseases, including cardiovascular and metabolic diseases and their associated complications. These cells exert their physiological and pathological activities by releasing granules containing histamine, cytokines, chemokines, and proteases, including mast cell-specific chymases and tryptases. Several recent human and animal studies have shown direct or indirect participation of mast cell-specific proteases in atherosclerosis, abdominal aortic aneurysms, obesity, diabetes, and their complications. Animal studies have demonstrated the beneficial effects of highly selective and potent chymase and tryptase inhibitors in several experimental cardiovascular and metabolic diseases. In this review, we summarize recent discoveries from in vitro cell-based studies to experimental animal disease models, from protease knockout mice to treatments with recently developed selective and potent protease inhibitors, and from patients with preclinical disorders to those affected by complications. We hypothesize that inhibition of chymases and tryptases would benefit patients suffering from cardiovascular and metabolic diseases.
Keywords: Mast cell, chymase, tryptase, atherosclerosis, abdominal aortic aneurysms, obesity, diabetes
INTRODUCTION
Mast cells, like other inflammatory cells such as macrophages and T cells, are essential in mediating the inflammatory process. When activated, mast cells rapidly release characteristic granules and various hormonal mediators into the interstitium. These mediators include histamine, proteoglycans, cytokines (e.g., interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α], and interferon-γ [IFN-γ]), mast cell-specific proteases (tryptase, chymase, and carboxypeptidase A), and other proteases (cysteinyl cathepsins and matrix metalloproteinases [MMPs]) [1, 2] that participate in the pathogenesis of atherosclerosis, abdominal aortic aneurysms (AAAs), obesity, and diabetes [3–15]. As mast cell-specific proteases, both chymase and tryptase belong to the serine protease family, while carboxypeptidase A is a zinc-dependent metalloproteinase. Their three-dimensional structures, substrate cleavage specificity, common substrates, and in vivo functions are well reviewed elsewhere [16]. Chymase can mediate the conversion from angiotensin-I (Ang-I) into angiotensin-II (Ang-II) [17–19]; generate fibronectin and transforming growth factor-β (TGF-β) from extracellular matrix (ECM) [20, 21]; process collagen for fibril formation from type I procollagen [22]; activate MMPs from their zymogens [23, 24]; and activate IL-1β, IL-18, endothelin-1, and endothelin-2 from their latent forms [25–27]. Chymase also degrades lipoproteins, thereby promoting macrophage foam cell formation [28]. Tryptase activates pro-MMPs [29] and degrades chemokines [30], lipoproteins [31], and fibronectin [32]. Mast cell-derived chymase and tryptase also are implicated in collagen synthesis and tissue fibrosis [33–35], angiogenesis [36, 37], and immunoglobulin molecule synthesis [38] — all of which associate closely with the pathophysiology of cardiovascular and metabolic disorders [39–44].
In this review, we briefly summarize our current understanding of the functions of mast cell chymases and tryptases in cardiovascular and metabolic diseases, mainly in atherosclerosis, AAA, obesity, and diabetes, including protease functions and drug developments against these proteases.
CHYMASES AND TRYPTASES IN CARDIOVASCULAR DISEASES
Mast cells’ participation in cardiovascular diseases was first implicated more than half a century ago [45–47]. Great progress has been made over the past decades, and much of our basic knowledge regarding these cells has come from studies led by Dr. Petri Kovanen and his colleague Dr. Ken Lindstedt. Their work provided a basic understanding of how mast cells, as a whole or as individual components, may affect different arterial cells or lipid proteins, thereby contributing to atherogenesis. For example, mast cells release heprin proteoglycan to bind to apolipoprotein B (apoB) from low-density lipoprotein (LDL) or release neutral proteases to degrade apoB, thereby facilitating LDL accumulation in macrophages and finally, foam cell formation [48, 49]. The same heprin proteoglycan also mediates LDL accumulation in smooth-muscle cells (SMCs) and promotes SMC foam cell formation [50] or inhibits SMC proliferation [51], which contribute importantly to media SMC loss and arterial wall thinning. Mast cells are also rich sources of growth factors. By releasing pro-angiogenic factor basic fibroblast growth factor (bFGF), mast cells may contribute to neovascularization. Indeed, in human atherosclerotic lesions, bFGF-positive mast cells are localized to macrovesssels in both intima and adventitia [52]. Direct participation of mast cells in cardiovascular diseases, however, has only recently been established using experimental animals. Using mast cell-deficient KitW-sh/W-sh mice and atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr−/−) mice on a C57BL/6 background, and aortic elastase perfusion-induced experimental AAA, we demonstrated that mast cells participate in both atherosclerosis and AAA by releasing proinflammatory cytokines, chemokines, and proteases to induce inflammatory cell recruitment, arterial cell apoptosis, angiogenesis, and matrix protein remodeling [53, 54]. Pharmacological stabilization of mast cells with their inhibitor, cromolyn (disodium cromoglycate, DSCG), reduced mast cell activation-induced atherosclerotic plaque intraplaque hemorrhage, macrophage apoptosis, vascular leakage, and CXCR2/VLA-4 (Very Late Antigen-4)-mediated recruitment of leukocytes in apolipoprotein E-deficient (Apoe−/−) mice [55]. Mast cell stabilizers MY-1250 and cromolyn also can prevent mast cell-mediated macrophage foam cell formation in vitro [56]. We showed that mast cell stabilization with cromolyn reduced elastase perfusion-induced AAA [54], which suggests that mast cell-derived cytokines, growth factors, proteoglycans, chymases and tryptases, or other proteases — such as cysteinyl cathepsins and MMPs — participate directly and indirectly in the pathogenesis of atherosclerosis and AAA. Several recent articles summarize these findings [57–59].
CHYMASES AND TRYPTASES IN ATHEROSCLEROSIS
Atherosclerosis is a chronic inflammatory disease of the arterial wall, caused largely by the accumulation of macrophages, or foam cells, that are enriched with intracellular cholesterol and lipid promoted by low-density lipoprotein (LDL) without adequate removal of fats and cholesterol from the macrophages by functional high-density lipoprotein (HDL). In addition to macrophages, monocytes, neutrophils, lymphocytes, and mast cells also reside in the arterial intima, many of them in close proximity to foam cells [60] — suggesting that these inflammatory cells are involved in the transformation of macrophages into foam cells.
Ihara and colleagues found high levels of Ang-II forming activity and chymase expression in human atherosclerotic lesions [47]. Several pieces of pioneering work demonstrated important effects of mast cell chymase and tryptase on arterial wall macrophages, SMCs, and endothelial cells (ECs). By degrading apoE or HDL3 components, such as apolipoprotein AI (apoAI), apoA2, preβ1LpA1, and LpA4, mast cell chymase abolishes HDL3 activities in cholesterol efflux from macrophage foam cells [60–63]. Mast cell tryptase also degrades HDL3 and impairs cholesterol reverse transport, a process facilitated by proteoglycan [31]. By degrading SMC matrix protein fibronectin and disrupting SMC focal adhesion [64] and by disrupting the NF-κB-mediated survival-signaling pathway [65], mast cell chymase induces SMC apoptosis [66], providing a mechanistic explanation of chymase contribution to aortic wall media SMC loss and thinning during atherogenesis. In addition, chymase inhibits SMC growth and collagen synthesis [67]. Mast cells release chymase (and carboxypeptidase A) and degrade endothelin-1 from ECs [68], and therefore affect normal vasodilation. Both chymase and TNF-α contribute to EC apoptosis. While TNF-α triggers EC apoptosis by translocating cytochrome C from mitochondria into cytoplasm [69], chymase induces EC apoptosis by degrading EC matrix protein vitronectin and fibronectin and inactivating focal adhesion kinase (FAK)-mediated cell survival signaling [70]. Therefore, mast cell proteases affect the pathobiology of macrophages, SMCs, and ECs. To demonstrate a direct role of mast cell proteases in atherosclerosis, we used bone marrow-derived mast cells (BMMCs) from chymase-deficient [71] and tryptase-deficient [72] mice, and proved that both proteases promote aortic SMC apoptosis. While BMMCs from wild-type (WT) mice induced mouse aortic SMC apoptosis, those from chymase-deficient or tryptase-deficient mice showed no activity in inducing SMC death. Although not tested in our studies due to technical difficulties, BMMCs from chymase-deficient or tryptase-deficient mice may also have impaired activities in inducing EC apoptosis. Therefore, mast cell proteases may contribute to plaque erosion and complications of atherosclerosis by inducing vascular cell apoptosis. In vitro, treatment of human coronary arteries intraluminally with recombinant tryptase or chymase induced endothelial damage, as characterized by disruption of EC adhesion followed by retraction and desquamation [73].
Chymase- and tryptase-mediated bioactivation of pro-enzyme and latent cytokines also appears important to atherogenesis. While chymase mediates pro-MMP-9 activation [24, 74], tryptase activates MMP-1, −2, and −3 [75–77]. All of these metalloproteinases have been implicated in promoting the pathogenesis of atherosclerosis and AAA [78–82]. Chymase also activates latent TGF-β1 from mast cells themselves, and those exogenously added to the mast cell culture [83] or from extracellular matrix of cultured epithelial cells or ECs [21]. Active TGF-β1 may then cause endothelial dysfunction via stimulation of reactive oxygen species (ROS) production by the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase. Transgenic overexpression of TGF-β1 accelerates atherosclerosis and hypertension in Apoe–/– mice [84], and adenovirus-mediated overexpression of TGF-β1 induces formation of cellular and matrix-rich intima in mouse carotid arteries, due to enhanced SMC migration and matrix deposition [85].
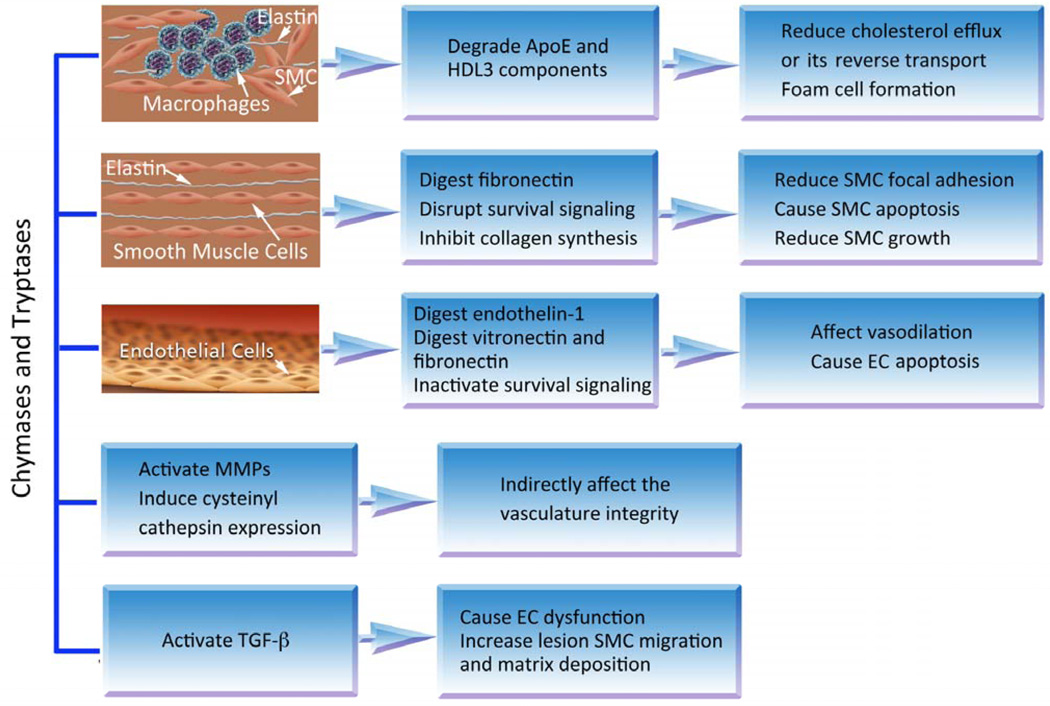
Several studies, including our own [86], have established an association of blood tryptase levels with atherosclerotic plaque instability [87, 88]. Patients with acute myocardial infarction (MI) or unstable angina pectoris have significantly higher serum tryptase levels than those without significant coronary heart disease or with stable angina pectoris. Serum chymase levels were also higher in patients with acute MI or unstable angina pectoris than in patients with stable angina pectoris or those without significant coronary heart disease [86]. In mice, both chymases and tryptases control vascular wall atherosclerosis-pertinent cathepsin expression [71, 72]. Monocytes from mouse tryptase mMCP (mouse mast cell protease)-6-deficient mice [72] and SMCs from mouse chymase mMCP-4-deficient mice [71] have significantly reduced expression and activities of cathepsins B, S, K, and L. Mast cell chymases and tryptases therefore may directly and indirectly participate in atherogenesis. Some selected activities of chymase and tryptase in atherogenesis are summarized in (Fig. 1).
Fig. (1).
Role of mast cell chymase and tryptase in atherogenesis. Besides acting on macrophages, SMCs, and ECs, these proteases also regulate the expression and activation of other proteases (MMPs and cysteinyl cathepsins) or growth factors (e.g., TGF-β), thereby indirectly affecting atherogenesis.
CHYMASES AND TRYPTASES IN AAA
The arterial wall histopathological changes of AAA include accumulation of lipids in foam cells, extracellular free cholesterol crystals, calcifications, thrombosis, angiogenesis, adventitial inflammatory cell infiltration, and ulcerations and ruptures of the endothelium layers. Inflammatory cells — including macrophages, lymphocytes, neutrophils, and mast cells — produce cytokines and proteases to promote inflammatory reactions, aortic medial SMC apoptosis, extracellular matrix degradation, and neovascularization [89], all of which associate closely with AAA pathogenesis.
We found that mast cells induce vascular cell — SMC and EC — protease expression by releasing their inflammatory cytokines (IL-6, TNF-α, and IFN-γ), thereby enhancing angiogenesis, vascular cell apoptosis, and ECM degradation [54]. Mäyränpää and colleagues investigated the relationship between mast cells and inflammation, neovascularization, and intraluminal thrombus in human AAA, and their results support the direct participation of mast cells in the pathogenesis of AAA — particularly regarding neovascularization of the aortic wall [90].
We demonstrated significant correlations of serum chymase [71] and tryptase levels with AAA annual expansion rate [72]. More interesting discoveries include that high serum tryptase levels significantly increased the risks of later surgical repair and overall mortality in a patient follow-up study [69]. Both mast cell proteases were expressed highly in the media and adventitia of human AAA, but were negligible in normal aortas, as determined by immunohistology and immunoblot analysis. In an elastase perfusion-induced mouse AAA model, mice lacking connective tissue chymase mMCP-4 [71] or tryptase mMCP-6 [72] developed significantly smaller AAA lesions than did WT control mice. Mechanistically, we found that chymase contributed to microvessel growth, vascular SMC apoptosis, and vascular cell cysteinyl cathepsin expression and activities. Compared with mast cells from WT mice, those from chymase mMCP-4-deficient mice had significantly impaired induction of microvessel sprouting in an aortic ring assay, aortic SMC apoptosis, and cathepsin expression and activities in ECs and SMCs [71]. In contrast, tryptase contributed to SMC apoptosis, vascular EC and SMC cathepsin expression, and monocyte transmigration, but showed no effect on microvessel growth [72]. These animal experiments supported a direct participation of mast cell chymase and tryptase in AAA formation and progression. But both chymase and tryptase may also contribute indirectly to AAA pathogenesis. As discussed above, chymase-deficient SMCs have reduced expression and activities of cathepsins B, L, K, and S [71]. Monocytes from tryptase-deficient mice also demonstrate reduced expression of the same set of cathepsins, as determined by real-time polymerase chain reaction [72]. Mast cells from tryptase-deficient mice show reduced activities of cathepsins B, K, L, and S, as determined by cathepsin active site labeling assay [72]. Our recent studies using aortic elastase perfusion-induced experimental AAA or Ang-II infusion-induced experimental AAA proved important roles of cathepsins K [4], L [3], and S [91] in AAA pathogenesis. Absence of any one of these proteases significantly suppressed AAA formation and progression. Therefore, reduced AAA in chymase- or tryptase-deficient mice [71, 72] may be due partially to reduced expression or activities of these AAA-pertinent cathepsins. Although not published, we detected great reduction of intracellular cytokine/chemokine levels in mast cells from chymase-deficient mice. Therefore, reduced activities of chymase-deficient mast cells in inducing neovascularization and SMC apoptosis could also be partially due to impaired production of pro-inflammatory cytokines from these mast cells — a hypothesis that merits further investigation.
Conversely, mast cell chymase and tryptase activities may also explain AAA formation studied in other classes of proteases or inflammatory molecules. For example, cathepsin C participates in AAA formation by activating neutrophil proteases, and thereby controlling neutrophil chemokine CXCL2 production and neutrophil recruitment [10]. Although this study did not test whether mast cells or mast cell proteases participate in cathepsin C-mediated AAA formation, we know that cathepsin C activates chymase from its pro-enzyme in mast cell granules [92], thereby enhancing mast cell Ang-II production, which ultimately induces vascular wall expression of MMP-9, another well-known protease involved in AAA pathogenesis [82]. Reduced AAA formation in cathepsin C-deficient mice therefore also may result from reduced activities of MMP-9 and chymases.
MAST CELL CHYMASES AND TRYPTASES IN METABOLIC DISEASES
Although direct participation of chymases or tryptases in obesity or diabetes has not been tested in any experimental models, cysteinyl protease cathepsins — which are indirectly regulated by chymase and tryptase [71, 72] — have been confirmed in diet-induced and genetically generated experimental obesity and diabetes. Mice deficient in cathepsins L or K, or WT mice treated with cathepsin L- or K-selective small molecule inhibitors, are leaner than control mice or have significantly improved glucose sensitivity [5, 6]. Our recent studies demonstrated that mast cells participate directly in obesity and diabetes [93]. Very few studies have studied mast cells in obesity. White adipose tissue (WAT) contains high numbers of tryptase-positive mast cells [93] or mast cell progenitor cells [7]. Mast cell-derived prostaglandins (PGs) metabolite 15-deoxy-delta-12,14-PGJ(2) (15-deoxy-delta PGJ(2)) is essential to mast cell-induced adipogenesis of 3T3-L1 cells [94]. Although not reported, we found that mast cells from chymase mMCP4-deficient mice had impaired induction of 3T3-L1 cell differentiation (Shi, unpublished observation). Chymase and tryptase therefore may participate directly or indirectly in the development of obesity. In contrast, mast cell functions in diabetes mellitus have been much more thoroughly investigated. Placentae from pregnant women who had class C diabetes (diabetes developed between 10–19 years of age without vascular complications) contain significantly higher numbers of mast cells and associated vascular endothelial growth factor (VEGF) expression than do those from gestationally matched controls [95]. As we will discuss further, mice lacking mast cells or receiving mast cell stabilizers demonstrated enhanced glucose and insulin sensitivities in diet-induced obese mice. In non-obese diabetic (NOD) mice, antibody against Fcε receptor-1, the high affinity receptor of immunoglobulin E (IgE), delayed type 1 diabetes onset [96].
Vascular hypertrophy is a feature of experimental and human diabetes. Streptozotocin-induced diabetic rats showed significant increases in mesenteric artery weight, wall-to-lumen ratio, arterial wall ECM deposition, and gene expression of epidermal growth factor (EGF) and TGF-β1 [97]. In those rats, diabetes also associated with an increase of mast cell numbers to the tongues [98] and in mesenteric vessels [97] — often associated with fibrosis, a common feature of diabetic microvascular complications [35]. In those experimental diabetic rats, treatment with the mast cell degranulation inhibitor tranilast (N-[3,4-demethoxycinnamoyl]-anthranilic acid) reduced mesenteric vessel fibrosis, artery weight, wall-to-lumen ratio, and matrix deposition, although it did not influence tryptase-positive mast cell infiltration, plasma glucose level, or systolic blood pressure [35, 99]. In vitro, tranilast greatly suppressed glucose-induced insulin secretion from cultured rat pancreatic β-cell line INS-1E cells and rat islets, and enhanced INS-1E cell glucose uptake [100]. Conversely, insulin and insulin-like growth factor-1 (IGF-1) promote mast cell survival in the absence of IL-3 in a PI3K–dependent manner [101], and high glucose increases mast cell intracellular ROS levels and expression of β-hexosaminidase and proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-13) [102] — among which, IL-6 is a well-known inducer of mast cell chymases and tryptases [103–105]. These observations support strongly an essential role of mast cells, and possibly of their proteases, in obesity and diabetes.
CHYMASES AND TRYPTASES IN OBESITY
Like atherosclerosis and AAA, obesity is an inflammatory disease. In addition to adipocytes, inflammatory cells — including macrophages, T cells, neutrophils, and mast cells — play essential roles in the pathogenesis of obesity.
WAT may represent an important source of mast cells in physiological and pathological situations [7]. Staining human WAT sections with a mast cell tryptase monoclonal antibody revealed high numbers of mast cells in WAT from obese subjects, compared with that of lean subjects. Higher numbers of mast cells were also found in epididymal fat tissue from obese mice compared with that of lean subjects, by staining with a monoclonal antibody against mouse mast cell protease-6 (mMCP6) [106], a human tryptase homologue. In our study of 80 obese patients and 32 lean controls, we found that mast cell-specific serum tryptase concentration was significantly higher in obese subjects than in lean individuals (P = 0.001) [93]. In a more recent study of a general population of 1,216 people, Fenger et al. demonstrated that serum tryptase levels associated strongly with age (P < 0.0001, P < 0.0001), male sex (P = 0.0012, P = 0.012), and body-mass index (BMI) (P < 0.0001, P = 0.037) before and after adjustment for age, sex, BMI, serum HDL, alcohol consumption, smoking, and atopy status [107]. While subjects with BMI < 25 had serum tryptase levels at 3.3 µg/L, those with BMI > 30 had serum tryptase levels at 4.4 µg/L, P < 0.0001 [107]. These observations suggest a possible association between mast cells or mast cell proteases with obesity [93, 107].
Direct participation of mast cells in obesity was established using mast cell-deficient KitW-sh/W-sh mice. These mice, after consuming a Western diet for 12 weeks, gained significantly less body weight and had improved glucose intolerance and reduced adipose tissue inflammation, with reduced leptin and insulin levels in the circulation, compared with congenic WT controls. Consistently, WT mice receiving a daily intraperitoneal injection of the mast cell stabilizer DSCG also had attenuated body weight gain. Adoptive transfer experiments of different cytokine deficient mast cells into KitW-sh/W-sh mice demonstrated that mast cells contributed to diet-induced obesity by producing the inflammatory cytokines IL-6 and IFN-γ. KitW-sh/W-sh mice receiving BMMCs from Il6−/− mice and Ifnγ mice, but not WT mice or Tnf−/− mice, had significantly reduced body weight gain and improved glucose tolerance. In addition to releasing cytokines, mast cells may contribute to obesity by promoting angiogenesis. Mast cells are often localized next to the microvessels in WAT. The numbers of microvessels correlated positively with the increases in mast cell numbers during the development of obesity. WAT and muscle tissues from WT obese mice showed substantial immunostaining of CD31 (microvessels) and KIT (mast cells) — significantly more than those from WT lean mice [93]. Although not tested, high numbers of mast cells in WAT from obese mice may result from enhanced mast cell proliferation, reduced mast cell apoptosis, or increased mast cell recruitment — all of which may contribute to angiogenesis in WAT.
Indeed, we detected significantly higher levels of monocyte chemoattractant protein-1 (MCP-1) in WAT from obese mice than in WAT from lean mice [93]. KitW-sh/W-sh mice fed a Western diet, or those receiving DSCG, had smaller CD31-positive areas, similar to those from chow diet-fed lean mice, compared with those in WT control mice not receiving medication. Reduced angiogenesis in KitW-sh/W-sh mice or those receiving DSCG resulted in high numbers of apoptotic cells in WAT and muscle tissues. Mast cells contain cell type-specific chymases and tryptases. Although no data suggest that these mast cell proteases participate directly in obesity, the findings in our recent AAA study indicate that mast cell chymase plays an important role in angiogenesis. While BMMCs from WT mice promoted microvessel sprouting in an aortic ring assay, those from chymase-deficient mice showed significantly reduced potency to microvessel sprouting [71]. Besides mast cell-specific chymases and tryptases, mast cells are important reservoirs of MMPs and cysteine proteases, such as cathepsins S, K, and L [2] — all of which can regulate neovascularization, cell survival [108], and ECM remodeling essential for WAT growth. Both chymase and tryptase may also regulate the expression of these cysteinyl cathepsins in WAT [71, 72] (Fig. 1). Indeed, WAT protein extracted from obese WT mice contained higher activities of cathepsins B, S, and L than that from KitW-sh/W-sh mice [93].
MAST CELL CHYMASES AND TRYPTASES IN TYPE 1 DIABETES
Diabetes mellitus is a metabolic syndrome characterized by hyperglycemia and associated with microvascular and macrovascular complications. Type 1 and type 2 are the most common types of diabetes. Although mast cells have been implicated in diabetes and its associated complications, few studies have focused on mast cell proteases. In a streptozotocin (STZ)-induced type 1 diabetes in hamsters, Maeda and colleagues [109] found that STZ induced renal chymase expression, accompanied by increased intrarenal Ang-II levels, overexpression of TGF-β and fibronectin in glomeruli, and renal mesangial expansion and deteriorated proteinuria. A selective chymase inhibitor, TEI-F00806, completely ameliorated the pathological changes of diabetic nephropathy, independent of blood pressure levels. The ACE inhibitor ramipril, which also inhibits Ang-II formation, did not show such therapeutic effects, supporting the participation of chymase in SZT-induced diabetic nephropathy. Shinji and colleagues [110] used the same experimental type 1 diabetes model and showed increased blood glucose levels and pancreatic chymase and total Ang-II-forming activities. Chymase inhibition with TY-51469 significantly reduced blood glucose levels and pancreatic chymase and total Ang-II-forming activities, along with significantly more pancreatic islets in the TY-51469 group than in the placebo group. Chymase activity therefore contributes to STZ-induced pancreatic islet disorganization.
MAST CELL CHYMASES AND TRYPTASES IN TYPE 2 DIABETES
We have shown that mast cells participate importantly in the pathogenesis of diet-induced type 2 diabetes. Glucose and insulin tolerance assays confirmed that deficiency or inhibition of mast cells improved these parameters, whereas diabetic mice had high serum insulin and glucose levels and high KIT positive mast cells in WAT [93]. In our recent Pre-diabetes Intervention Project involving 80 patients with confirmed diabetes, 189 patients with pre-diabetes, and 71 normal controls selected from 3163 volunteers, we found that both chymase and IgE levels in the blood were significantly higher in diabetic patients, followed by those with pre-diabetes, than those from controls with normal blood glucose levels. In an ordinal logistic model, interactions between IgE and chymase greatly increased the risk (odds ratio, OR) of developing diabetes before (OR: 2.479 [1.079–5.778], P = 0.033) and after (OR: 2.594 [1.118–6.018], P = 0.026) adjustment for age, sex, hypertension, waist circumference, waist-to-hip ratio, BMI, total cholesterol, triglyceride, HDL, LDL, hyperinsulinemia, and homeostatic model assessment indexes [111]. Although not statistically significant, interactions between IgE and tryptase also increased the risk of developing diabetes by 2.091 (P = 0.068) and 2.167 (P = 0.057) folds, before and after the same adjustment [111]. Among the same population, when patients with pre-diabetes — including isolated impaired fasting glucose (I-IFG), isolated impaired glucose tolerance (I-IGT), and mixed IFG/IGT — were considered, we found that plasma chymase levels associated with an increased risk of I-IGT (OR: 2.862 [1.186–6.907], P = 0.019) and mixed IFG/IGT (OR: 3.142 [1.310–7.541], P = 0.010). After adjustment for age, sex, and BMI, high plasma chymase levels remained significantly associated with an increased risk of I-IGT (OR: 3.057 [1.231– 7.590], P = 0.016) and mixed IFG/IGT (OR: 2.127 [1.218–8.030], P = 0.018) (Shi, unpublished data). These data suggest that mast cells and mast cell chymase and tryptase are potential drug targets for human diabetes or pre-diabetes — a hypothesis currently being tested in human clinical trial.
MAST CELL CHYMASES AND TRYPTASES IN DIABETIC COMPLICATIONS
Mast cells, which localize to various organs including the lungs, heart, and kidneys [112–114], also play a central role in the pathogenesis of diabetic complications. Mast cells infiltrate the kidney and got degranulated in renal diseases [115]. The degranulation of mast cells releases pathological substances like TGF-β, chymase, tryptase, cathepsin G, histamine, renin, and various inflammatory cytokines [116–119], which may play a detrimental role in the pathogenesis of diabetic nephropathy. The level of chymase increases in diabetic nephropathy, and this increase associates with glomerulosclerosis, tubulointerstitial fibrosis, and vascular fibrosis in patients with diabetic nephropathy [120, 121]. Chymase converts Ang-I into Ang-II, which may play a critical role in diabetic vascular disease [122]. Increased chymase associates with the accumulation of advanced glycation end products (AGEs) in diabetic renal vasculature [122]. Moreover, chymase participates in the conversion of pro-MMP-9 to MMP-9 [123], a protease implicated in diabetic nephropathy and diabetic retinopathy [124, 125]. All these activities suggest that mast cell chymase participates in the pathogenesis of diabetic complication [126], although direct evidence is currently not available.
Tryptase enhances the production of VEGF [127], which is implicated in the development of diabetic nephropathy [128]. Notably, mast cells are a major source of tryptase [129], and renal mast cell degranulation-mediated release of tryptase may play a detrimental role in the pathogenesis of diabetic nephropathy. This contention is supported by the participation of mast cell tryptase in the development of renal interstitial fibrosis, by increasing the production of ECM proteins [130].
CHYMASE-GENERATED ANG-II IN CARDIOVASCULAR DISEASES
Ang-II, an end product of the renin-angiotensin system (RAS), exerts a wide range of physiological and pathological effects on the cardiovascular, renal, endocrine, and nervous systems. Ang-II regulates blood pressure through its action on multiple target receptors, mainly Ang-II receptor type 1 (AT1) and type 2 (AT2) on the vascular wall, kidney, and adrenal gland. Octapeptide Ang-II is converted from Ang I through the cleavage of the Phe8–His9 bond by angiotensin-converting enzyme (ACE) both within the circulatory system and within tissues. But chymase is an important alternative pathway of Ang-II conversion within the cardiovascular tissues [131]. The activities of Ang-II from the chymase-depend pathway are almost the same as those from the ACE-dependent pathway, except that Ang-II from the chymase-dependent pathway may not regulate blood pressure. Unlike ACE, chymase may generate and degrade Ang-II, depending on the species. For example, human chymase generates Ang-II without further degradation, where as chymase from other species — including dog, hamster, rat, and mouse — also generates Ang-II, but degradation follows in a time-dependent manner [132].
Ang-II from the chymase-dependent pathway has been implicated in vascular proliferation and the pathogenesis of aortic valve diseases, MI, heart failure, and AAA. In a dog model of percutaneous coronary intervention, chymase activity — but not that of ACE — increased after balloon catheter injury [44]. In this model, angiotensin-receptor blockers (ARB; candesartan) and chymase inhibitor (NK3201), but not ACE inhibitor (enalapril), prevented vascular proliferation [44, 131]. In experimental coronary artery bypass grafting, Ang-II formation in the grafted veins seemed to depend mainly on chymase activity. Chymase-produced Ang-II induced post-grafting expression of fibronectin, and collagen types I and III. Chymase inhibitor Suc-Val-Pro-Phep(OPh)2 greatly suppressed these matrix protein expressions and post-grafting vascular proliferation [133]. Chymase-generated Ang-II may also exert proinflammatory and profibrotic effects. With its activities in inducing vascular cell MCP-1 expression [134, 135] and in inducing TGF-β expression and consequent collagen synthesis [136], Ang-II may potentially contribute to the pathogenesis of calcific aortic valve disease. Indeed, stenotic aortic valves from patients undergoing valve replacement surgery contain greatly increased mRNA and protein levels of AT1 receptor and chymase [137].
In tachycardia-induced heart failure in dogs, the left ventricle (LV) showed increased chymase-positive mast cells. Chymase inhibitor SUNC8257 reduced LV mast cell numbers and cardiac Ang-II levels, suppressed LV expression of TGF-β and collagen types I and III, and decreased LV fibrosis and LV end-diastolic pressure [138]. Similar observations were obtained in experimental MI animals. Chymase-activated TGF-β is a major stimulator of myocardial fibrosis. In cultured fibroblasts, addition of chymase increased fibroblast proliferation and media TGF-β levels, all of which can be suppressed by a chymase inhibitor [139]. Cardiac tissue chymase and associated Ang-II levels are increased in hamsters 1 day after MI [140]. Chymase inhibitor [141, 142] and ARB [140] both attenuated cardiac dysfunction and extended survival.
In elastase infusion-induced AAA in hamsters and mice, chymase and Ang-II-forming activities were increased [41, 143], and were reduced by the chymase inhibitor NK3201 [143]. In Apoe−/− mice, either Ang-II or chymase infusion induced AAA. Ang-II-induced AAA had increased expression of chymase and MMP-9, and their activities were inhibited by NK3201 [144].
CHYMASE-GENERATED ANG-II IN METABOLIC DISEASES
Ang-II activities in obesity have been throughly studied [145]. The expression of renin, ACE, and AT1 genes significantly increased in adipocytes from obese hypertensive patients [146] and WAT from obese patients contain increased numbers of mast cells [93]. These observations suggest a role of chymase in Ang-II production in WAT. How important chymase-generated Ang-II is in WAT as compared with that from the ACE pathway, however, remains unknown. In contrast, several studients have implicated chymase-generated Ang-II directly and indirectly in diabetes in several studies.
In cultured vascular SMCs, high glucose and AGEs — a key molecule accumulated in the microvasculature in the development of diabetic retinopathy [147, 148] — induced chymase-dependent, but not ACE-dependent, Ang-II formation via ERK1/2 MAP kinase activation [149], suggesting that in vascular tissues from diabetic patients, high glucose and AGEs may induce chymase-dependent Ang-II formation. Ang-II promotes retina VEGF expression [26]. In diabetic patients, VEGF is important in the initiation and development of diabetic retinopathy, by regulating vascular permeability, angiogenesis, and EC proliferation. The vitreous of eyes from patients with diabetic retinopathy contains increased levels of ACE, VEGF, and MMP9 [150]. ABR treatment inhibited AGE and VEGF expression in diabetic rats [151], indirectly suggesting that chymase inhibition also prevents diabetic retinopathy. In STZ-induced type 1 diabetes in hamsters, chymase and Ang-II–forming activities are increased in the pancreas [110]. After STZ injection, pancreatic islets were reduced. Chymase inhibitor TY-51469 reduced glucose levels, inhibited the chymase and total Ang-II–forming activities, suppressed oxidative stress, and maintained pancreatic islet numbers [110].
In type 2 diabetic animals [152] and human patients with evidence of vascular diseases or diabetes [153, 154], Ang-II contributes to islet disorganization and high risk for cardiovascular events. ACE inhibitors or AT1 receptor antagonists improved islet fibronogenesis, apoptosis, and oxidative stress; diabetes-associated complications; and cardiovascular morbidity and death. Chymase inhibitors, therefore, may be useful in preventing, or lowering the risk for, type 2 diabetes [155].
CHYMASE AND TRYPTASE INHIBITORS IN CARDIOVASCULAR DISEASES
Although both chymases and tryptases are essential to mast cell biology and to the pathogenesis of atherosclerosis, AAA, and associated complications, most current studies focus on the development of chymase inhibitors (Table 1) in experimental cardiovascular diseases in animals. Tryptase inhibitors receive much less attention, but this does not mean that tryptase is less important than chymase. We demonstrated that mouse tryptase mMCP-6-null mice are resistant to elastase perfusion-induced AAA [72]. In THP-1-derived macrophages and primary human monocyte-derived macrophages, the tryptase inhibitor APC-366 blocked ox-LDL-induced foam cell formation. Mechanistically, APC-366 inhibited tryptase activities can block reduction of nuclear receptor LXRa (regulates lipid homeostasis) expression, can increase the expression of ATP-binding cassette transporters A1 and G1 (ABCA1, ABCG1; involved in the cholesterol efflux pathway and macrophage foam cell formation), and can increase sterol regulatory element binding protein-1c (SREBP-1c) (regulates gene for de novo lipogenesis) expression [156].
Table 1.
Chymase and Tryptase Inhibitors in Cardiovascular Diseases
| Inhibitor (inhibitor category) [Experimental model] (reference) |
Major findings | Mechanisms |
|---|---|---|
| NK3201 (chymase inhibitor) [Dog carotid artery bypass /injury neointima models] [44, 157] [Elastase-induced AAA in dog and hamster] [41, 143] [Ang-II perfusion-induced AAA in Apoe−/− mice] [144] |
Reduced neointimal thickening Reduced aortic diameter, media thinning and luminal area Reduced arterial wall infiltration of mast cells, neutrophils, monocytes, and macrophage |
Reduced lesion chymase activity and associated Ang-II forming activity and MMP activity Reduced stem cell factor activation Reduced lesion vascular cell proliferation and inflammatory cell infiltration |
| R05066852 (chymase inhibitor) [Apoe−/− carotid plaque model] [160] |
Reduced atherosclerotic plaque size and progression Reduced lesion necrotic core size Enhanced lesion collagen content Reduced intraplaque hemorrhage frequency and size |
Reduced chymase activity and possibly tryptase activity |
| SUN-C8257 (chymase inhibitor) [Hamster diet-induced atherosclerosis model] [158, 159] |
Suppressed aortic lipid deposition in hamster atherosclerotic lesions No effect on blood pressure or blood cholesterols |
Suppressed Ang-II forming activity |
| APC-366 (tryptase inhibitor) [Cultured human macrophages] [156] |
Reduced numbers of separated lipid vacuoles in human macrophages Reduced intracellular neutral lipid in human macrophages |
Increased ABCG1/ABCA1/SREBP-1c expression and blocked reduction of nuclear receptor LXRa expression in human macrophages, in addition to tryptase activity inhibition |
CHYMASE INHIBITORS IN ATHEROSCLEROSIS
As summarized in (Table 1), at least three chymase inhibitors have been tested in atherosclerosis and related conditions. In a dog vein graft disease experimental model [157], each animal underwent right common carotid artery bypass grafting with the ipsilateral external jugular vein. Grafting increased vascular cell proliferation, Ang-II forming activity, chymase activity, ACE activity, and intimal thickening (intima/media ratio) 28 days after operation. When dogs received chymase inhibitor NK3201 orally, however, all such phenotypes were significantly suppressed. The same group performed a similar experiment using a balloon catheter-induced carotid artery injury model in dogs to test the role of NK3201 in intimal hyperplasia [44]. Carotid injury significantly increased artery chymase activity. NK3210 suppressed artery chymase activity, but had no effect on artery ACE activity, plasma renin or ACE activity, or plasma Ang-II concentration. Injury-induced carotid artery intimal thickening also decreased significantly after dogs received NK3201 treatment [44]. These experiments suggest that chymase contributes to arterial wall thickening and neointima formation, likely by promoting inflammatory cell migration and vascular cell proliferation — a hypothesis that requires further investigation and confirmation.
In patients with atherosclerosis, serum total cholesterol or LDL cholesterol levels correlated with arterial chymase-dependent Ang-II forming activity [158]. Hamsters consuming a high-cholesterol diet developed lipid deposition in the aortic cusp. Plasma total or LDL cholesterol levels also correlated with adventitial Ang-II immunoreactivity. Oral administration of an orally active, non-peptide chymase inhibitor, SUN-C8257, completely suppressed aortic lipid deposition and Ang-II forming activity, although this inhibitor did not affect systolic blood pressure or serum total and LDL cholesterol levels [158, 159]. Similar observations were made in atherosclerosis-prone Apoe−/− mice [160]. A newly developed chymase inhibitor, RO5066852, reduced chymase activity and possibly tryptase activity; reduced spontaneous atherosclerosis; prevented local mast cell activation-induced acceleration of plaque progression; enhanced lesion collagen content and reduced lesion necrotic core size; and completely normalized the frequency and size of intraplaque hemorrhages in Apoe−/− mice after acute perivascular collar placement-induced carotid plaque [160].
CHYMASE INHIBITORS IN AAA
The best-studied chymase inhibitor in AAA is also NK3201. This inhibitor has been used in at least three experimental AAA models. In aortic elastase perfusion-induced experimental AAA in hamsters, treatment with NK3201 significantly reduced postelastase perfusion (2 weeks) aortic diameters (from 2.18±0.04 mm to 1.66±0.06 mm, P < 0.01), lesion tissue chymase activity (3.44±0.62 mU/mg to 2.03±0.31 mU/mg, P < 0.05), media thinning (media area to total area ratio from 41.3±6.7% to 68.4±7.5%, P < 0.05), and lesion mast cell numbers (14.3±1.3 cells/mm2 to 8.58±0.9 cells/mm2, P < 0.01) [41]. In the same experimental model, dogs receiving NK3201 showed significantly reduced aortic diameters 2–8 weeks post-elastase perfusion. NK3201 treatment decreased luminal area, increased media-to-total area percentage, reduced AAA lesion chymase-positive mast cells and neutrophils, and reduced lesion chymase activity, Ang-II forming activity, and MMP-9 activity [143].
When Apoe−/− mice receive systemic infusion of Ang-II, they develop suprarenal AAAs [161, 162]. Chymase inhibitor NK3201 suppressed lumen areas and arterial wall inflammatory cell (e.g., MOMA-2-positive monocytes and macrophages) infiltration and expansion in these mice. Mechanistically, in addition to inhibiting chymase activities, NK3201 also inhibited chymase-mediated MMP-9 activation in AAA lesions [144]. These animal experiments, as summarized in (Table 1), support an essential role of mast cells and mast cell proteases in AAA pathogenesis, but whether chymase or tryptase can be drug targets for human AAA therapy remains unknown.
CHYMASE AND TRYPTASE INHIBITORS IN METABOLIC DISEASES
MC stabilizers are probably the best-studied anti-allergy drugs in metabolic disorders, and many are used to treat patients with allergies. Drugs in this category include cromolyn (Gastrocrom, or DSCG), ketotifen (Zaditor), and tranilast (Rizaben). Cromolyn and ketotifen are used in pediatric allergic disorders. Tranilast is used in bronchial asthma, atopic dermatitis, and allergic conjunctivitis, and recently has shown anti-angiogenic activity via inhibition of chymase and TGF-β [35]. Cromolyn [163] exerts its effect only on certain populations of mast cells, with limited effect on intestinal mucosal mast cells in rodents [164] and humans [165]. These effects are particularly important because cromolyn may inhibit mast cells in the connective tissues — such as those in the vasculature and WAT — without affecting the innate immunity of the mucosa against pathogens. In addition to its anti-allergy effect, ketotifen can control chronic inflammation processes such as autoimmune encephalomyelitis and arthritis [166], if given sufficient time points. Our most recent observations indicate that cromolyn and ketotifen can prevent diet-induced diabetes in mice. We also demonstrated that these mast cell stabilizers can reverse pre-established diabetes [93]; this result is particularly important for future clinical application. We need more comprehensive human trials to establish the applications of these mast cell drugs in patients; studies of mast cell protease inhibitors have just begun, however, and are still confined within animal models (Table 2).
Table 2.
Chymase Inhibitors in Diabetes
| Inhibitor [Experimental model] (reference) |
Major findings | Mechanisms |
|---|---|---|
| TEI-F00806 TEI-E00548 [Multi-dose streptozotocin- induced diabetes in hamster] [109] |
Reduced proteinuria and systemic oxidative stress Reduced glomerular fibronectin deposition Reduced glomerular mesangial expansion No effect on blood glucose |
Reduced kidney expressions of chymase, Ang-II, NADPH oxidase components, TGF-β1 |
| TY-51469 [Multi-dose streptozotocin- induced diabetes in hamster] [110] |
Attenuated blood glucose level Reduced pancreas oxidative stress Increased pancreatic islets |
Reduced pancreas chymase and Ang-II forming activities Reduced pancreas mast cell number |
TEI-F00806 and TEI-E00548 are chymase-specific inhibitors recently developed by Teijin Pharma of Tokyo, Japan. In a multi-dose streptozotocin (30 mg/kg, one dose every 3 days for 2 weeks) treatment-induced diabetes model, hamsters develop diabetic nephropathy complicated by increased levels of blood glucose, Ang-II, urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG, a marker of oxidative stress), and urine protein (a marker of renal dysfunction). Kidneys from streptozotocin-treated mice show increased expression of chymase, ACE, Ang-II, NADPH oxidase components p22phox and NOX4 (NADPH oxidase 4), TGF-β1, and associated ECM protein fibronectin deposition. These kidneys showed increased oxidative stress and expansion of the glomerular mesangial area. Although TEI-F00806 and TEI-E00548 did not significantly change blood glucose levels or intrarenal ACE expression, both chymase inhibitors ameliorated all other aforementioned renal pathologies [109].
In a similar model of diabetes induced by a single high dose of streptozotocin (60 mg/kg), hamsters showed high blood glucose levels, increased pancreatic chymase, and Ang-II-forming activities. Mice receiving TY-51469, a chymase inhibitor from Toa Eiyo Co., Tokyo, Japan [167], significantly reduced blood glucose, chymase, total Ang II-forming activities, malondialdehyde, and mast cell numbers in pancreatic tissues, compared with those receiving placebo treatment. Furthermore, the TY-51469 group had significantly more pancreatic islets than the placebo group [110]. As summarized in (Table 2), significant improvement in controls of blood glucose and kidney complications after treatment with different chymase inhibitors suggests a direct involvement of this mast cell protease — and possibly tryptase — in the pathogenesis of diabetes. Targeting these mast cell proteases may become a powerful means of managing metabolic diseases and their complications.
FUTURE PERSPECTIVES
Experimental diseases in animals have helped to test the role of mast cell-specific proteases in cardiovascular and metabolic diseases, although many current models do not fully recapitulate human diseases. Protease inhibitors are much more potent and selective than before, but we still lack clinical trials to test the efficacy of these potent and selective inhibitors in humans. Drug companies may be waiting for more mechanistic studies from chymase-deficient or tryptase-deficient animals, as these inhibitors may have off-target effects. For example, chymase inhibitor RO5066852 also targeted cathepsin G, although IC50 is 27-fold higher than chymase [160]. Direct approvals in protease-deficient animals may help to advance the progress of proposing human trials.
ACKNOWLEDGEMENTS
The authors thank Ms. Sara Karwacki for her editorial assistance. This study is supported by grants from the National Natural Science Foundation of China (81001191) (to AH), the Science and Technology Commission of Shanghai (10PJ1408300) (to AH), the National Institutes of Health (HL60942, HL81090, HL88547) (to GPS); and by an Established Investigator Award (0840118N) from the American Heart Association (to GPS).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Di Girolamo N, Indoh I, Jackson N, et al. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J Immunol. 2006;177:2638–2650. doi: 10.4049/jimmunol.177.4.2638. [DOI] [PubMed] [Google Scholar]
- 2.Mallen-St Clair J, Shi GP, Sutherland RE, Chapman HA, Caughey GH, Wolters PJ. Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol Chem. 2006;387:1143–1146. doi: 10.1515/BC.2006.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Sukhova GK, Zhang J, et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–2508. doi: 10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Sukhova GK, Zhang J, et al. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2012;32:15–23. doi: 10.1161/ATVBAHA.111.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang M, Sun J, Zhang T, et al. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. 2008;28:2202–2208. doi: 10.1161/ATVBAHA.108.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Zhang Y, Pan J, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 7.Kitamoto S, Sukhova GK, Sun J, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 8.Sukhova GK, Zhang Y, Pan JH, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutgens E, Lutgens SP, Faber BC, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 10.Pagano MB, Bartoli MA, Ennis TL, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim CS, Shalhoub J, Gohel MS, Shepherd AC, Davies AH. Matrix metalloproteinases in vascular disease--a potential therapeutic target? Curr Vasc Pharmacol. 2010;8:75–85. doi: 10.2174/157016110790226697. [DOI] [PubMed] [Google Scholar]
- 12.Keeling WB, Armstrong PA, Stone PA, Bandyk DF, Shames ML. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39:457–464. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- 13.Hopps E, Caimi G. Matrix metalloproteinases in metabolic syndrome. Eur J Intern Med. 2012;23:99–104. doi: 10.1016/j.ejim.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 14.de Meijer VE, Sverdlov DY, Le HD, Popov Y, Puder M. Tissue-specific differences in inflammatory infiltrate and matrix metalloproteinase expression in adipose tissue and liver of mice with diet-induced obesity. Hepatol Res. 2012;42:601–610. doi: 10.1111/j.1872-034X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsioufis C, Bafakis I, Kasiakogias A, Stefanadis C. The role of matrix metalloproteinases in diabetes mellitus. Curr Top Med Chem. 2012;12:1159–1165. doi: 10.2174/1568026611208011159. [DOI] [PubMed] [Google Scholar]
- 16.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 17.Sanker S, Chandrasekharan UM, Wilk D, Glynias MJ, Karnik SS, Husain A. Distinct multisite synergistic interactions determine substrate specificities of human chymase and rat chymase-1 for angiotensin II formation and degradation. J Biol Chem. 1997;272:2963–2968. doi: 10.1074/jbc.272.5.2963. [DOI] [PubMed] [Google Scholar]
- 18.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 19.Dell’Italia LJ, Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002;17:374–379. doi: 10.1097/00001573-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Lazaar AL, Plotnick MI, Kucich U, et al. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J Immunol. 2002;169:1014–1020. doi: 10.4049/jimmunol.169.2.1014. [DOI] [PubMed] [Google Scholar]
- 21.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 22.Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 23.Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 25.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kido H, Nakano A, Okishima N, et al. Human chymase, an enzyme forming novel bioactive 31-amino acid length endothelins. Biol Chem. 1998;379:885–891. doi: 10.1515/bchm.1998.379.7.885. [DOI] [PubMed] [Google Scholar]
- 27.Omoto Y, Tokime K, Yamanaka K, et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177:8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- 28.Lee-Rueckert M, Kovanen PT. Mast cell proteases: physiological tools to study functional significance of high density lipoproteins in the initiation of reverse cholesterol transport. Atherosclerosis. 2006;189:8–18. doi: 10.1016/j.atherosclerosis.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Kaminska R, Helisalmi P, Harvima RJ, Naukkarinen A, Horsmanheimo M, Harvima IT. Focal dermal-epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J Invest Dermatol. 1999;113:567–573. doi: 10.1046/j.1523-1747.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 30.Pang L, Nie M, Corbett L, Sutcliffe A, Knox AJ. Mast cell beta-tryptase selectively cleaves eotaxin and RANTES and abrogates their eosinophil chemotactic activities. J Immunol. 2006;176:3788–3795. doi: 10.4049/jimmunol.176.6.3788. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Sommerhoff CP, von Eckardstein A, Zettl F, Fritz H, Kovanen PT. Mast cell tryptase degrades HDL and blocks its function as an acceptor of cellular cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:2086–2091. doi: 10.1161/01.atv.0000041405.07367.b5. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz LB, Bradford TR, Littman BH, Wintroub BU. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985;135:2762–2767. [PubMed] [Google Scholar]
- 33.Abe M, Kurosawa M, Ishikawa O, Miyachi Y, Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy. 1998;28:1509–1517. doi: 10.1046/j.1365-2222.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 34.Satomura K, Yin M, Shimizu S, et al. Increased chymase in livers with autoimmune disease: colocalization with fibrosis. J Nihon Med Sch. 2003;70:490–495. doi: 10.1272/jnms.70.490. [DOI] [PubMed] [Google Scholar]
- 35.Jones SE, Gilbert RE, Kelly DJ. Tranilast reduces mesenteric vascular collagen deposition and chymase-positive mast cells in experimental diabetes. J Diabetes Complications. 2004;18:309–315. doi: 10.1016/j.jdiacomp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Blair RJ, Meng H, Marchese MJ, et al. Human mast cells stimulate vascular tube formation Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo A, Russo G, Peticca M, Pietropaolo C, Di Rosa M, Iuvone T. Inhibition of granuloma-associated angiogenesis by controlling mast cell mediator release: role of mast cell protease-5. Br J Pharmacol. 2005;145:24–33. doi: 10.1038/sj.bjp.0706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa T, Imada T, Nakakubo H, Nakamura N, Naito K. Rat mast cell protease-I enhances immunoglobulin E production by mouse B cells stimulated with interleukin-4. Immunology. 2001;104:333–340. doi: 10.1046/j.1365-2567.2001.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamada H, Terai M, Kimura H, Hirano K, Oana S, Niimi H. Increased expression of mast cell chymase in the lungs of patients with congenital heart disease associated with early pulmonary vascular disease. Am J Respir Crit Care Med. 1999;160:1303–1308. doi: 10.1164/ajrccm.160.4.9810058. [DOI] [PubMed] [Google Scholar]
- 40.Ortlepp JR, Janssens U, Bleckmann F, et al. A chymase gene variant is associated with atherosclerosis in venous coronary artery bypass grafts. Coron Artery Dis. 2001;12:493–497. doi: 10.1097/00019501-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Tsunemi K, Takai S, Nishimoto M, et al. A specific chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N- [ [3,4-dioxo-1-pheny l-7-(2-pyridyloxy)]-2-heptyl]acetamide (NK3201), suppresses development of abdominal aortic aneurysm in hamsters. J Pharmacol Exp Ther. 2004;309:879–883. doi: 10.1124/jpet.103.063974. [DOI] [PubMed] [Google Scholar]
- 42.Tsunemi K, Takai S, Nishimoto M, et al. Possible roles of angiotensin II-forming enzymes, angiotensin converting enzyme and chymase-like enzyme, in the human aneurysmal aorta. Hypertens Res. 2002;25:817–822. doi: 10.1291/hypres.25.817. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino F, Urata H, Inoue Y, et al. Chymase inhibitor improves survival in hamsters with myocardial infarction. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S11–S18. [PubMed] [Google Scholar]
- 44.Takai S, Sakonjo H, Fukuda K, et al. A novel chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N- [[,4-dioxo-1-phenyl −7-(2-pyridyloxy)]2-heptyl]acetamide (NK3201), suppressed intimal hyperplasia after balloon injury. J Pharmacol Exp Ther. 2003;304:841–844. doi: 10.1124/jpet.102.042580. [DOI] [PubMed] [Google Scholar]
- 45.Cairns A, Constantinides P. Mast cells in human atherosclerosis. Science. 1954;120:31–32. doi: 10.1126/science.120.3105.31. [DOI] [PubMed] [Google Scholar]
- 46.Constantinides P. Mast cells and susceptibility to experimental atherosclerosis. Science. 1953;117:505–506. doi: 10.1126/science.117.3045.505. [DOI] [PubMed] [Google Scholar]
- 47.Ihara M, Urata H, Kinoshita A, et al. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–1405. doi: 10.1161/01.hyp.33.6.1399. [DOI] [PubMed] [Google Scholar]
- 48.Kokkonen JO, Kovanen PT. Stimulation of mast cells leads to cholesterol accumulation in macrophages in vitro by a mast cell granule-mediated uptake of low density lipoprotein. Proc Natl Acad Sci USA. 1987;84:2287–2291. doi: 10.1073/pnas.84.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kokkonen JO, Kovanen PT. Proteolytic enzymes of mast cell granules degrade low density lipoproteins and promote their granule-mediated uptake by macrophages in vitro . J Biol Chem. 1989;264:10749–10755. [PubMed] [Google Scholar]
- 50.Wang Y, Lindstedt KA, Kovanen PT. Mast cell granule remnants carry LDL into smooth muscle cells of the synthetic phenotype and induce their conversion into foam cells. Arterioscler Thromb Vasc Biol. 1995;15:801–810. doi: 10.1161/01.atv.15.6.801. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Kovanen PT. Heparin proteoglycans released from rat serosal mast cells inhibit proliferation of rat aortic smooth muscle cells in culture. Circ Res. 1999;84:74–83. doi: 10.1161/01.res.84.1.74. [DOI] [PubMed] [Google Scholar]
- 52.Lappalainen H, Laine P, Pentikäinen MO, Sajantila A, Kovanen PT. Mast cells in neovascularized human coronary plaques store and secrete basic fibroblast growth factor, a potent angiogenic mediator. Arterioscler Thromb Vasc Biol. 2004;24:1880–1885. doi: 10.1161/01.ATV.0000140820.51174.8d. [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 54.Sun J, Sukhova GK, Yang M, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 56.Ma H, Kovanen PT. Inhibition of mast cell-dependent conversion of cultured macrophages into foam cells with antiallergic drugs. Arterioscler Thromb Vasc Biol. 2000;20:E134–E142. doi: 10.1161/01.atv.20.12.e134. [DOI] [PubMed] [Google Scholar]
- 57.Lindstedt KA, Mäyränpää MI, Kovanen PT. Mast cells in vulnerable atherosclerotic plaques--a view to a kill. J Cell Mol Med. 2007;11:739–758. doi: 10.1111/j.1582-4934.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swedenborg J, Mäyränpää MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–740. doi: 10.1161/ATVBAHA.110.213157. [DOI] [PubMed] [Google Scholar]
- 59.Xu JM, Shi GP. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev. 2012;33:71–108. doi: 10.1210/er.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M, Lindstedt LK, Kovanen PT. Mast cell-mediated inhibition of reverse cholesterol transport. Arterioscler Thromb. 1992;12:1329–1335. doi: 10.1161/01.atv.12.11.1329. [DOI] [PubMed] [Google Scholar]
- 61.Lindstedt L, Lee M, Castro GR, Fruchart JC, Kovanen PT. Chymase in exocytosed rat mast cell granules effectively proteolyzes apolipoprotein AI-containing lipoproteins, so reducing the cholesterol efflux-inducing ability of serum and aortic intimal fluid. J Clin Invest. 1996;97:2174–2182. doi: 10.1172/JCI118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee M, von Eckardstein A, Lindstedt L, Assmann G, Kovanen PT. Depletion of pre beta 1LpA1 and LpA4 particles by mast cell chymase reduces cholesterol efflux from macrophage foam cells induced by plasma. Arterioscler Thromb Vasc Biol. 1999;19:1066–1074. doi: 10.1161/01.atv.19.4.1066. [DOI] [PubMed] [Google Scholar]
- 63.Lee M, Calabresi L, Chiesa G, Franceschini G, Kovanen PT. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2002;22:1475–1481. doi: 10.1161/01.atv.0000029782.84357.68. [DOI] [PubMed] [Google Scholar]
- 64.Leskinen MJ, Lindstedt KA, Wang Y, Kovanen PT. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol. 2003;23:238–243. doi: 10.1161/01.atv.0000051405.68811.4d. [DOI] [PubMed] [Google Scholar]
- 65.Leskinen MJ, Heikkilä HM, Speer MY, et al. Mast cell chymase induces smooth muscle cell apoptosis by disrupting NF-kappaB-mediated survival signaling. Exp Cell Res. 2006;312:1289–1298. doi: 10.1016/j.yexcr.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 66.Leskinen M, Wang Y, Leszczynski D, Lindstedt KA, Kovanen PT. Mast cell chymase induces apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:516–522. doi: 10.1161/01.atv.21.4.516. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Shiota N, Leskinen MJ, Lindstedt KA, Kovanen PT. Mast cell chymase inhibits smooth muscle cell growth and collagen expression in vitro: transforming growth factor-beta1-dependent and -independent effects. Arterioscler Thromb Vasc Biol. 2001;21:1928–1933. doi: 10.1161/hq1201.100227. [DOI] [PubMed] [Google Scholar]
- 68.Metsärinne KP, Vehmaan-Kreula P, Kovanen PT, et al. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted Peptide. Arterioscler Thromb Vasc Biol. 2002;22:268–273. doi: 10.1161/hq0202.103994. [DOI] [PubMed] [Google Scholar]
- 69.Lätti S, Leskinen M, Shiota N, Wang Y, Kovanen PT, Lindstedt KA. Mast cell-mediated apoptosis of endothelial cells in vitro: a paracrine mechanism involving TNF-alpha-mediated down-regulation of bcl-2 expression. J Cell Physiol. 2003;195:130–138. doi: 10.1002/jcp.10235. [DOI] [PubMed] [Google Scholar]
- 70.Heikkilä HM, Lätti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2008;28:309–314. doi: 10.1161/ATVBAHA.107.151340. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, Adachi R, Libby P, Thompson RW, Shi GP. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 74.Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell alpha-chymase activates progelatinase B by cleaving the Phe88-Gln89 and Phe91-Glu92 bonds of the catalytic domain. J Biol Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 75.Gruber BL, Marchese MJ, Suzuki K, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994;223:171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto K, Kumagai N, Fukuda K, Fujitsu Y, Nishida T. Activation of corneal fibroblast-derived matrix metalloproteinase-2 by tryptase. Curr Eye Res. 2006;31:313–317. doi: 10.1080/02713680600629789. [DOI] [PubMed] [Google Scholar]
- 78.Wågsäter D, Zhu C, Björkegren J, Skogsberg J, Eriksson P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(−/−)Apob(100/100) mouse. Int J Mol Med. 2011;28:247–253. doi: 10.3892/ijmm.2011.693. [DOI] [PubMed] [Google Scholar]
- 79.Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1120–1125. doi: 10.1161/01.ATV.0000218496.60097.e0. [DOI] [PubMed] [Google Scholar]
- 80.Galis ZS, Johnson C, Godin D, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 81.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindstedt KA, Wang Y, Shiota N, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 84.Buday A, Orsy P, Godó M, et al. Elevated systemic TGF-beta impairs aortic vasomotor function through activation of NADPH oxidase-driven superoxide production and leads to hypertension, myocardial remodeling, and increased plaque formation in apoE(−/−) mice. Am J Physiol Heart Circ Physiol. 2010;299:H386–H395. doi: 10.1152/ajpheart.01042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otsuka G, Agah R, Frutkin AD, Wight TN, Dichek DA. Transforming growth factor beta 1 induces neointima formation through plasminogen activator inhibitor-1-dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:737–743. doi: 10.1161/01.ATV.0000201087.23877.e1. [DOI] [PubMed] [Google Scholar]
- 86.Xiang M, Sun J, Lin Y, et al. Usefulness of serum tryptase level as an independent biomarker for coronary plaque instability in a Chinese population. Atherosclerosis. 2011;215:494–499. doi: 10.1016/j.atherosclerosis.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filipiak KJ, Tarchalska-Krynska B, Opolski G, et al. Tryptase levels in patients after acute coronary syndromes: the potential new marker of an unstable plaque? Clin Cardiol. 2003;26:366–372. doi: 10.1002/clc.4950260804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deliargyris EN, Upadhya B, Sane DC, et al. Mast cell tryptase: a new biomarker in patients with stable coronary artery disease. Atherosclerosis. 2005;178:381–386. doi: 10.1016/j.atherosclerosis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Rizas KD, Ippagunta N, Tilson MD3rd. Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 90.Mäyränpää MI, Trosien JA, Fontaine V, et al. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. discussion 395–6. [DOI] [PubMed] [Google Scholar]
- 91.Qin Y, Cao X, Guo J, et al. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs263. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tchougounova E, Forsberg E, Angelborg G, Kjéllen L, Pejler G. Altered processing of fibronectin in mice lacking heparin. a role for heparin-dependent mast cell chymase in fibronectin degradation. J Biol Chem. 2001;276:3772–3777. doi: 10.1074/jbc.M008434200. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka A, Nomura Y, Matsuda A, Ohmori K, Matsuda H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am J Physiol Cell Physiol. 2011;301:C1360–C1367. doi: 10.1152/ajpcell.00514.2010. [DOI] [PubMed] [Google Scholar]
- 95.Szukiewicz D, Szukiewicz A, Maslinska D, Szewczyk G, Watroba M. Mast cell-derived vascular endothelial growth factor (VEGF) and microvascular density in diabetic placentae. Inflamm Res. 2003;52(Suppl 1):S09–S10. doi: 10.1007/s000110300030. [DOI] [PubMed] [Google Scholar]
- 96.Hübner MP, Larson D, Torrero MN, et al. Anti-FceR1 antibody injections activate basophils and mast cells and delay Type 1 diabetes onset in NOD mice. Clin Immunol. 2011;141:205–217. doi: 10.1016/j.clim.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilbert RE, Rumble JR, Cao Z, et al. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ Res. 2000;86:158–165. doi: 10.1161/01.res.86.2.158. [DOI] [PubMed] [Google Scholar]
- 98.Batbayar B, Somogyi J, Zelles T, Fehér E. Immunohistochemical analysis of substance P containing nerve fibres and their contacts with mast cells in the diabetic rat’s tongue. Acta Biol Hung. 2003;54:275–283. doi: 10.1556/ABiol.54.2003.3-4.6. [DOI] [PubMed] [Google Scholar]
- 99.Bonnet F, Cao Z, Cooper ME, Cox AJ, Kelly DJ, Gilbert RE. Tranilast attenuates vascular hypertrophy, matrix accumulation and growth factor overexpression in experimental diabetes. Diabetes Metab. 2003;29:386–392. doi: 10.1016/s1262-3636(07)70049-6. [DOI] [PubMed] [Google Scholar]
- 100.Taguchi S, Ozaki N, Umeda H, Mizutani N, Yamada T, Oiso Y. Tranilast inhibits glucose-induced insulin secretion from pancreatic beta-cells. Horm Metab Res. 2008;40:518–523. doi: 10.1055/s-2008-1073163. [DOI] [PubMed] [Google Scholar]
- 101.Lessmann E, Grochowy G, Weingarten L, et al. Insulin and insulin-like growth factor-1 promote mast cell survival via activation of the phosphatidylinositol-3-kinase pathway. Exp Hematol. 2006;34:1532–1541. doi: 10.1016/j.exphem.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 102.Nagai K, Fukushima T, Oike H, Kobori M. High glucose increases the expression of proinflammatory cytokines and secretion of TNFα and β-hexosaminidase in human mast cells. Eur J Pharmacol. 2012 doi: 10.1016/j.ejphar.2012.04.038. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 103.Oskeritzian CA, Wang Z, Kochan JP, et al. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol. 1999;163:5105–5115. [PubMed] [Google Scholar]
- 104.Shimizu Y, Suga T, Maeno T, et al. Functional expression of high-affinity receptor for immunoglobulin E on mast cells precedes that of tryptase during differentiation from human bone marrow-derived CD34 progenitors cultured in the presence of stem cell factor and interleukin-6. Clin Exp Allergy. 2004;34:917–925. doi: 10.1111/j.1365-2222.2004.01971.x. [DOI] [PubMed] [Google Scholar]
- 105.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J Immunol. 2004;172:593–600. doi: 10.4049/jimmunol.172.1.593. [DOI] [PubMed] [Google Scholar]
- 106.Altintas MM, Rossetti MA, Nayer B, et al. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 2011;10:198. doi: 10.1186/1476-511X-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fenger RV, Linneberg A, Vidal C, et al. Determinants of serum tryptase in a general population: the relationship of serum tryptase to obesity and asthma. Int Arch Allergy Immunol. 2012;157:151–158. doi: 10.1159/000327535. [DOI] [PubMed] [Google Scholar]
- 108.Wang B, Sun J, Kitamoto S, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 109.Maeda Y, Inoguchi T, Takei R, et al. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010;299:F1328–F1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- 110.Takai S, Jin D, Ohzu M, Tanaka K, Miyazaki M. Chymase inhibition provides pancreatic islet protection in hamsters with streptozotocin-induced diabetes. J Pharmacol Sci. 2009;110:459–65. doi: 10.1254/jphs.09115fp. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z, Zhang H, Shen XH, et al. Immunoglobulin E and mast cell proteases are potential risk factors of human pre-diabetes and diabetes mellitus. PLoS One. 2011;6:e28962. doi: 10.1371/journal.pone.0028962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53:858–862. doi: 10.1136/jcp.53.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balakumar P, Singh AP, Ganti SS, Krishan P, Ramasamy S, Singh M. Resident cardiac mast cells: are they the major culprit in the pathogenesis of cardiac hypertrophy? Basic Clin Pharmacol Toxicol. 2008;102:5–9. doi: 10.1111/j.1742-7843.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 114.Singh AP, Singh M, Balakumar P. Effect of mast cell stabilizers in hyperhomocysteinemia-induced cardiac hypertrophy in rats. J Cardiovasc Pharmacol. 2008;51:596–604. doi: 10.1097/FJC.0b013e31817ae60f. [DOI] [PubMed] [Google Scholar]
- 115.Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int. 2003;64:906–913. doi: 10.1046/j.1523-1755.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 116.Stevens RL, Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989;10:381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 117.Raible DG, Schulman ES, DiMuzio J, Cardillo R, Post TJ. Mast cell mediators prostaglandin-D2 and histamine activate human eosinophils. J Immunol. 1992;148:3536–3542. [PubMed] [Google Scholar]
- 118.Silver RB, Reid AC, Mackins CJ, et al. Mast cells: a unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Helske S, Syväranta S, Kupari M, et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 121.Ritz E. Chymase: a potential culprit in diabetic nephropathy? J Am Soc Nephrol. 2003;14:1952–1954. doi: 10.1097/01.asn.0000076125.12092.c6. [DOI] [PubMed] [Google Scholar]
- 122.Koka V, Wang W, Huang XR, Kim-Mitsuyama S, Truong LD, Lan HY. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation. 2006;113:1353–1360. doi: 10.1161/CIRCULATIONAHA.105.575589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ishida K, Takai S, Murano M, et al. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2008;324:422–426. doi: 10.1124/jpet.107.131946. [DOI] [PubMed] [Google Scholar]
- 124.van der Zijl NJ, Hanemaaijer R, Tushuizen ME, et al. Urinary matrix metalloproteinase-8 and −9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin Biochem. 2010;43:635–639. doi: 10.1016/j.clinbiochem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 125.Kowluru RA, Zhong Q, Santos JM. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin Investig Drugs. 2012;21:797–805. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andersson MK, Enoksson M, Gallwitz M, Hellman L. The extended substrate specificity of the human mast cell chymase reveals a serine protease with well-defined substrate recognition profile. Int Immunol. 2009;21:95–104. doi: 10.1093/intimm/dxn128. [DOI] [PubMed] [Google Scholar]
- 127.Masuko K, Murata M, Xiang Y, et al. Tryptase enhances release of vascular endothelial growth factor from human osteoarthritic chondrocytes. Clin Exp Rheumatol. 2007;25:860–865. [PubMed] [Google Scholar]
- 128.Balakumar P, Arora MK, Ganti SS, Reddy J, Singh M. Recent advances in pharmacotherapy for diabetic nephropathy: current perspectives and future directions. Pharmacol Res. 2009;60:24–32. doi: 10.1016/j.phrs.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 129.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 130.Kondo S, Kagami S, Kido H, Strutz F, Müller GA, Kuroda Y. Role of mast cell tryptase in renal interstitial fibrosis. J Am Soc Nephrol. 2001;12:1668–1676. doi: 10.1681/ASN.V1281668. [DOI] [PubMed] [Google Scholar]
- 131.Miyazaki M, Wada T, Shiota N, Takai S. Effect of an angiotensin II receptor antagonist, candesartan cilexetil, on canine intima hyperplasia after balloon injury. J Hum Hypertens. 1999;13(Suppl 1):S21–S25. doi: 10.1038/sj.jhh.1000745. [DOI] [PubMed] [Google Scholar]
- 132.Kunori Y, Muroga Y, Iidaka M, Mitsuhashi H, Kamimura T, Fukamizu A. Species differences in angiotensin II generation and degradation by mast cell chymases. J Recept Signal Transduct Res. 2005;25:35–44. doi: 10.1081/rrs-200054355. [DOI] [PubMed] [Google Scholar]
- 133.Tsunemi K, Takai S, Nishimoto M, et al. Lengthy suppression of vascular proliferation by a chymase inhibitor in dog grafted veins. J Thorac Cardiovasc Surg. 2002;124:621–625. doi: 10.1067/mtc.2002.125164. [DOI] [PubMed] [Google Scholar]
- 134.Fóris G, Dezsö B, Medgyesi GA, Füst G. Effect of angiotensin II on macrophage functions. Immunology. 1983;48:529–535. [PMC free article] [PubMed] [Google Scholar]
- 135.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 136.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 137.Helske S, Lindstedt KA, Laine M, et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–1866. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 138.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 139.Takai S, Jin D, Sakaguchi M, et al. A novel chymase inhibitor, 4-[1-([bis-(4-methyl-phenyl)-methyl]-carbamoyl)3-(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid (BCEAB), suppressed cardiac fibrosis in cardiomyopathic hamsters. J Pharmacol Exp Ther. 2003;305:17–23. doi: 10.1124/jpet.102.045179. [DOI] [PubMed] [Google Scholar]
- 140.Jin D, Takai S, Yamada M, Sakaguchi M, Yao Y, Miyazaki M. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol. 2001;86:203–214. doi: 10.1254/jjp.86.203. [DOI] [PubMed] [Google Scholar]
- 141.Jin D, Takai S, Yamada M, Sakaguchi M, Miyazaki M. Beneficial effects of cardiac chymase inhibition during the acute phase of myocardial infarction. Life Sci. 2002;71:437–446. doi: 10.1016/s0024-3205(02)01689-2. [DOI] [PubMed] [Google Scholar]
- 142.Jin D, Takai S, Yamada M, et al. Impact of chymase inhibitor on cardiac function and survival after myocardial infarction. Cardiovasc Res. 2003;60:413–420. doi: 10.1016/s0008-6363(03)00535-2. [DOI] [PubMed] [Google Scholar]
- 143.Furubayashi K, Takai S, Jin D, et al. The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res. 2007;30:349–357. doi: 10.1291/hypres.30.349. [DOI] [PubMed] [Google Scholar]
- 144.Inoue N, Muramatsu M, Jin D, et al. Effects of chymase inhibitor on angiotensin II-induced abdominal aortic aneurysm development in apolipoprotein E-deficient mice. Atherosclerosis. 2009;204:359–364. doi: 10.1016/j.atherosclerosis.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 145.Weisinger RS, Begg DP, Jois M. Antagonists of the renin-angiotensin system and the prevention of obesity. Curr Opin Investig Drugs. 2009;10:1069–1077. [PubMed] [Google Scholar]
- 146.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J Hypertens. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 147.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhisitkul RB, Robinson GS, Moulton RS, Claffey KP, Gragoudas ES, Miller JW. An antisense oligodeoxynucleotide against vascular endothelial growth factor in a nonhuman primate model of iris neovascularization. Arch Ophthalmol. 2005;123:214–219. doi: 10.1001/archopht.123.2.214. [DOI] [PubMed] [Google Scholar]
- 149.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 150.Ishizaki E, Takai S, Ueki M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129–134. doi: 10.1016/j.ajo.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 151.Sugiyama T, Okuno T, Fukuhara M, et al. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85:406–412. doi: 10.1016/j.exer.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 2004;53:989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 153.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 154.Devereux RB, Dahlöf B, Kjeldsen SE, et al. LIFE Study Group. Effects of losartan or atenolol in hypertensive patients without clinically evident vascular disease: a substudy of the LIFE randomized trial. Ann Intern Med. 2003;139:169–177. doi: 10.7326/0003-4819-139-3-200308050-00006. [DOI] [PubMed] [Google Scholar]
- 155.Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–611. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- 156.Yeong P, Ning Y, Xu Y, Li X, Yin L. Tryptase promotes human monocyte-derived macrophage foam cell formation by suppressing LXRalpha activation. Biochim Biophys Acta. 2010;1801:567–576. doi: 10.1016/j.bbalip.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 157.Takai S, Jin D, Nishimoto M, et al. Oral administration of a specific chymase inhibitor, NK3201, inhibits vascular proliferation in grafted vein. Life Sci. 2001;69:1725–1732. doi: 10.1016/s0024-3205(01)01255-3. [DOI] [PubMed] [Google Scholar]
- 158.Arakawa K, Urata H. Hypothesis regarding the pathophysiological role of alternative pathways of angiotensin II formation in atherosclerosis. Hypertension. 2000;36:638–641. doi: 10.1161/01.hyp.36.4.638. [DOI] [PubMed] [Google Scholar]
- 159.Uehara Y, Urata H, Ideishi M, Arakawa K, Saku K. Chymase inhibition suppresses high-cholesterol diet-induced lipid accumulation in the hamster aorta. Cardiovasc Res. 2002;55:870–876. doi: 10.1016/s0008-6363(02)00458-3. [DOI] [PubMed] [Google Scholar]
- 160.Bot I, Bot M, van Heiningen SH, et al. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/− mice. Cardiovasc Res. 2011;89:244–252. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- 161.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang YX, Martin-McNulty B, Freay AD, et al. Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol. 2001;159:1455–1464. doi: 10.1016/S0002-9440(10)62532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patalano F, Ruggieri F. Sodium cromoglycate: a review. Eur Respir J Suppl. 1989;6:556s–560s. [PubMed] [Google Scholar]
- 164.Pearce FL, Befus AD, Gauldie J, Bienenstock J. Mucosal mast cells II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982;128:2481–2486. [PubMed] [Google Scholar]
- 165.Befus AD, Dyck N, Goodacre R, Bienenstock J. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol. 1987;138:2604–2610. [PubMed] [Google Scholar]
- 166.De Filippis D, D’Amico A, Iuvone T. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol. 2008;20(Suppl 1):20–25. doi: 10.1111/j.1365-2826.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 167.Palaniyandi SS, Nagai Y, Watanabe K, et al. Chymase inhibition reduces the progression to heart failure after autoimmune myo-carditis in rats. Exp Biol Med (Maywood) 2007;232:1213–1221. doi: 10.3181/0703-RM-85. [DOI] [PubMed] [Google Scholar]