Abstract
Bombyx mori (silkworm) silk proteins have been utilized as unique biomaterials for various medical applications. To develop a novel affinity silk material, we generated a transgenic silkworm that spins silk protein containing the fibroin L-chain linked with the single-chain variable fragment (scFv) as a fusion protein. Previously, the scFv-conjugated “affinity” silk powder specifically immunoprecipitated its target protein, Wiskott-Aldrich syndrome protein. To expand the applicability of affinity silk materials, we processed the scFv-conjugated silk protein into a thin film by dissolving it in lithium bromide, then drying it in the wells of 96-well plates. Enzyme-linked immunosorbent assay demonstrated specific detection of Wiskott-Aldrich syndrome protein, both as a recombinant protein and in its native form extracted from mouse macrophages. These findings suggest that this scFv-conjugated silk film serves as the basis for an alternative immunodetection system.
Bombyx mori (silkworm) silk proteins are widely utilized as unique natural biopolymers for biomaterial applications. The silk fibers in cocoons are composed of fibroin and sericin; fibroin is a silk-fiber core protein, and sericin is a group of soluble glycoproteins that covers the surface of fibroin fiber. Fibroin fibers have been directly utilized as threads for surgical suture, as this protein has low toxicity and high biocompatibility with human tissues. Silk fibroin fibers are first dissolved in aqueous solution, then processed into various formats such as powders, fibers, gels, sponges or films1,2,3,4. Additionally, fibroin can be chemically modified5,6,7 or post-conjugated with bioactive peptides and/or proteins8,9,10 to alter its physical or biological properties. For example, fibroin films covalently coupled with arginine-glycine-aspartic acid peptides or bone morphogenic protein 2 showed enhancement of cell adhesion and osteogenic differentiation of human bone marrow stromal cells, respectively8,9,10. However, the modification procedure is often encumbered by technical difficulties, such as the loss of bioactivity; high manufacturing costs are inevitable.
By virtue of recent technological developments, bioactive proteins can be produced in the silk glands of transgenic silkworms, either independently from the silk protein11,12 or fused with fibroin proteins13,14,15,16. The fibroin L-chain (FibL) fused with basic fibroblast growth factor previously led to enhancement of cell growth15, suggesting that the recombinant protein retains its biological activity even when fused to silk fibroin proteins. Such bioactive ligand-conjugated transgenic silk fibroin can be used as scaffolding for tissue engineering.
To expand the applicability of transgenic silk fibroins toward a novel affinity reagent, we previously generated a transgenic silkworm strain that produces silk fibroin fused to the single-chain variable fragment (scFv), which is composed of the VH and VL domains from the original antibody17,18. The scFv construct was derived from a monoclonal antibody (mAb) against Wiskott-Aldrich syndrome protein (WASP), an important immune adaptor molecule in mammals19,20,21,22. After dissolving the cocoons in lithium bromide (LiBr), the silk solution was dialyzed, concentrated, freeze-dried, and crushed into powder. Immunoprecipitation analyses demonstrated that anti-WASP-scFv conjugated to FibL retains its specific binding activity to the target molecule after multiple processing steps23. These results strongly suggest that scFv-conjugated silk powder may open new avenues for the development of affinity purification systems.
In this investigation, cocoons expressing scFv-conjugated fibroin protein were processed into a thin film, and the specific affinity of this film to the target protein was evaluated via enzyme-linked immunosorbent assay (ELISA). The present work reveals that scFv-conjugated silk film is a potentially useful material for alternative immunodetection systems.
Results
Solubilization of silk cocoons from wild-type and transgenic silkworms
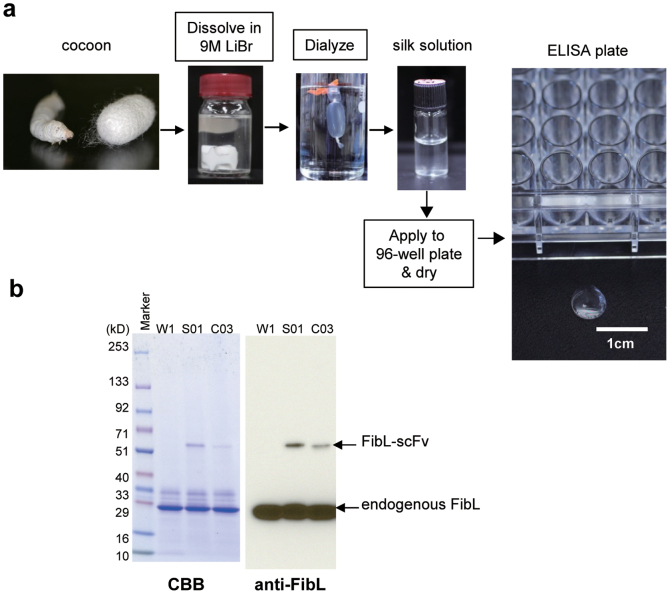
Cocoon shells produced by wild-type w1-pnd (W1) silkworms and silkworms harboring a transgenic construct of FibL fused with anti-WASP-scFv (S01) or a control scFv construct (C03) were chopped, dissolved in LiBr solution, and dialyzed in 1 mM Tris-HCl (pH 9.0), as described in Methods. The resulting silk solutions derived from each strain were clear (Fig. 1a). Expression of the transgenes encoding the S01 construct or the control C03 construct was confirmed in each silk solution by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by staining with Coomassie brilliant blue and immunoblotting with anti-FibL antibody (Fig. 1b). The expression levels of scFv-conjugated FibL were only 5–10% of endogenous FibL, which is in accordance with previous transgenic silkworm experiments; the average expression level of transgenes in the fused form with FibL is approximately 5–20%13,14,15.
Figure 1. Production of scFv-conjugated silk film via transgenic silkworm technology.
(a) Schematic of the procedure for preparing silk film. Cocoons produced by silkworms were dissolved in LiBr, then processed into the film. (b) SDS-PAGE and immunoblot analysis showing expression of the transgenes FibL-anti-WASP-scFv (S01) and FibL-control-scFv (C03) in the silk solution. Silk solutions derived from wild-type (W1), S01, and C03 strains were separated by SDS-PAGE and stained with Coomassie brilliant blue. Immunoblots were probed with anti-FibL polyclonal antibody.
Comparison of binding activity of scFv-conjugated silk solution and its parental mAb in ELISA
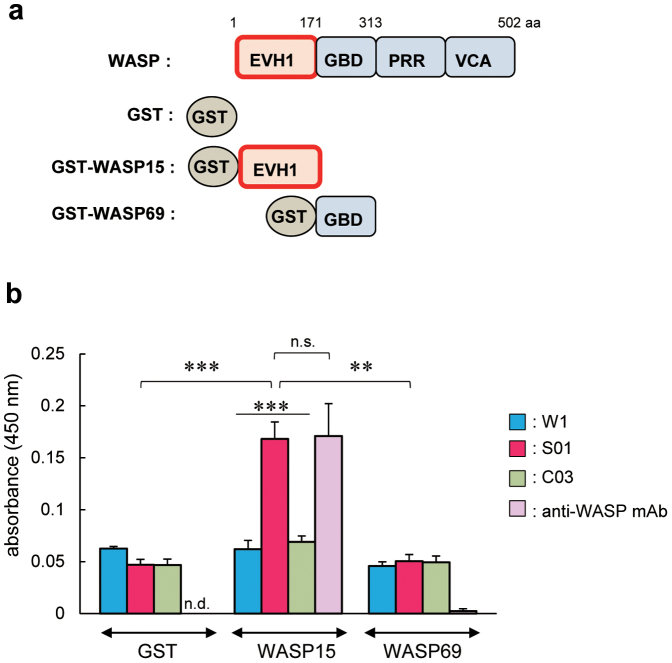
The affinity of scFv-conjugated silk solution to the target protein was confirmed by ELISA using recombinant probe proteins (glutathione S-transferase (GST), GST-WASP15, and GST-WASP69 fusion proteins; Fig. 2a) that were produced and affinity-purified from Escherichia coli cells23. GST-WASP15 is the specific antigen against which the parental anti-WASP mAb19,22 was raised. In this assay, S01 but not control C03 silk solution-coated wells detected the target protein as efficiently as wells coated with parental anti-WASP mAb (Fig. 2b). Anti-WASP mAb-coated wells displayed significant reactivity only to the target protein, whereas all of the silk solution-coated wells (W1, S01, and C03) showed similar amounts of non-specific binding regardless of the probe proteins (Fig. 2b). These results suggest that it is possible to detect the target molecule by ELISA using silk solution, but the inherent binding activity of the silk solution to unrelated proteins, anti-GST polyclonal antibody (pAb), or horseradish peroxidase (HRP)-conjugated anti-rabbit Igs should be reduced.
Figure 2. Specific binding to the target molecule using transgenic silk solution.
(a) Schematic representation of WASP and WASP truncations (WASP15 and WASP69). The major functional domains of WASP are shown, as are the EVH1 domain (EVH1), GTPase-binding domain (GBD), proline rich region (PRR), and verproline/cofiline/acidic domain (VCA). aa, amino acids. GST was fused with the N-terminus of each WASP deletion mutant. (b) ELISA using silk solution from W1, S01, or C03 strains, or parental anti-WASP mAb coating the 96-well assay plate. The recombinant probe proteins (GST, GST-WASP15, and GST-WASP69) were added to each silk solution or anti-WASP mAb-coated well. Values are mean ± standard error of the mean from three independent experiments. **P < 0.01; ***P < 0.001; n.s., not significant; n.d., not detectable.
ScFv-conjugated silk films efficiently detect the target protein in ELISA
In an attempt to reduce non-specific binding in the ELISA system, the prepared silk solutions were applied to the wells of 96-well plates and dried until they formed thin silk films on the wells (Fig. 1a). We previously demonstrated that W1 and S01 silk powders, which were made by concentrating and freeze-drying silk solution and then crushing it into powder, efficiently blocked non-specific interactions23. Silk films derived from each strain exhibited similar thickness, morphology, and permeability (data not shown).
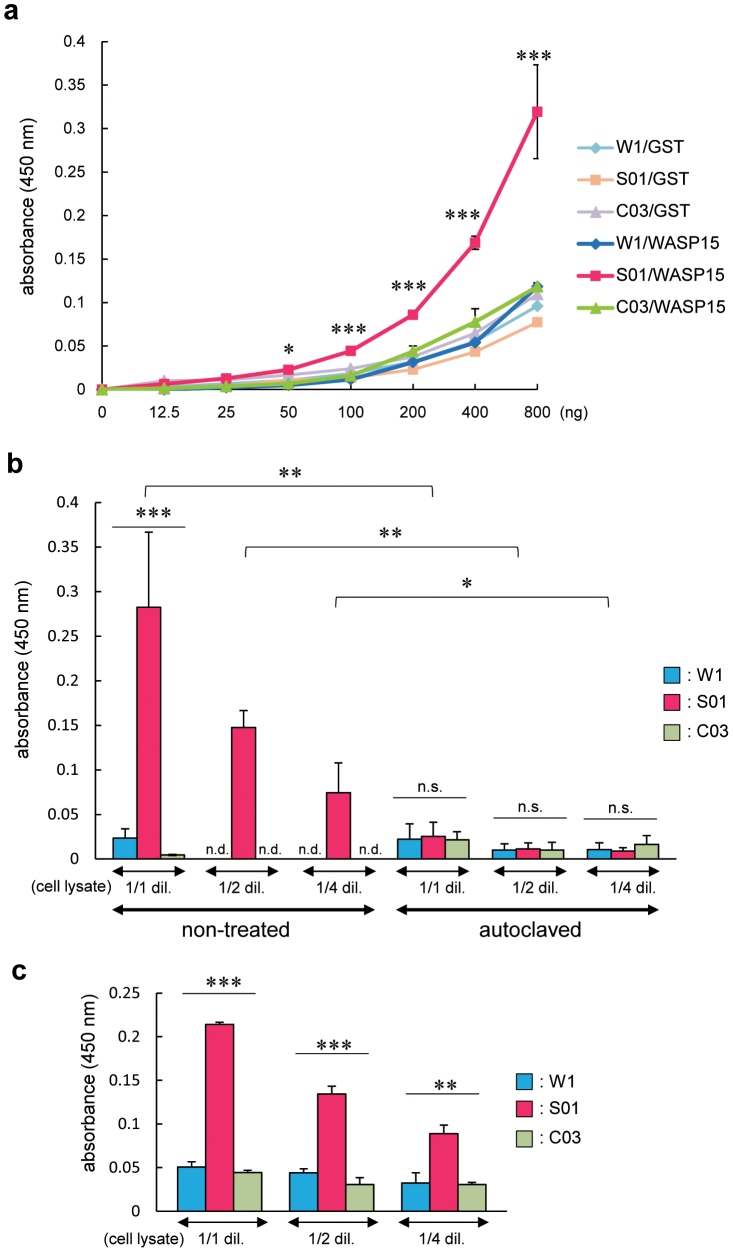
To test the specific affinity of silk film derived from anti-WASP-scFv-conjugated S01 silk solution to the target protein, we performed ELISA using wild-type or transgenic silk film-coated 96-well plates. The intensity of absorbance of the S01 silk-film coated wells exponentially increased as the concentration of the target protein (GST-WASP15) increased, whereas W1 and C03 silk film-coated wells did not (Fig. 3a). These results suggest that anti-WASP-scFv fused with FibL preserves its specific affinity to the target protein in the silk-film format.
Figure 3. ScFv-conjugated silk film efficiently detects the target protein.
Specific binding was quantified by ELISA using 96-well plates coated with silk film derived from W1, S01, or C03 strains. (a) The indicated amount of GST or GST-WASP15 was added to each silk film-coated well. (b) A mouse macrophage RAW264.7 cell line was lysed. The lysate was diluted by half (1/2 dil.), one-quarter (1/4 dil.), or not diluted (1/1 dil.) and added to each silk film-coated well or control well coated with silk film made from silk solution after autoclaving. (c) The diluted or non-diluted cell lysate was added to each silk film-coated well which 3 months have passed since it was made. Values are mean ± standard error of the mean from three independent experiments. *P < 0.05; ** P < 0.01; ***P < 0.001; n.s., not significant; n.d., not detectable.
To examine whether S01 silk film specifically detects native WASP extracted from mammalian immune cells, a RAW264.7 cell lysate was incubated in W1, S01, or C03 silk film-coated 96-well plates and analyzed by ELISA. In this assay, the S01 silk film efficiently detected native WASP, and the levels of non-specific interactions of the W1 and C03 silk films were lower than those observed when the recombinant proteins were incubated (Fig. 3b). The intensity of absorbance was reduced as the cell extract was diluted, suggesting that scFv-conjugated silk-film ELISA can be used in quantitative analyses (Fig. 3b).
As a control, we performed ELISA using 96-well plates which were coated with wild-type or transgenic silk film made from autoclaved silk solution. Although silk film made from autoclaved silk solution could not be distinguished in appearance compared with silk film made from non-treated silk solution (data not shown), the binding activity of anti-WASP-scFv-conjugated S01 silk film was completely diminished by autoclaving of its silk solution (Fig. 3b). These results suggest that some or most of anti-WASP-scFv fused with FibL may be not denatured by dissolving with 9 M LiBr and preserve their proper folding required for binding activity during the processing into film.
Another advantage of the affinity silk film is its longer shelf life. We performed ELISA using the silk-film coated plates stored at room temperature (RT) for three months. Although the intensity of absorbance of S01 silk film-coated wells was reduced by 10–20% and non-specific binding of W1 and C03 silk film-coated wells was slightly increased, the specific affinity to the target protein was still remained (Fig. 3c). Given these observations, silk film made from transgenic silk cocoon shells expressing scFv fused to fibroin protein may be potentially useful for developing an alternative immunodetection system that has comparable sensitivity to that of conventional systems but is less expensive to manufacture and has a longer shelf life at RT.
Discussion
In the present study, we demonstrate that thin silk films derived from cocoon shells containing FibL fused with anti-WASP-scFv specifically bind to purified recombinant GST-WASP15 and native WASP in a mouse RAW264.7 macrophage extract. These observations strongly suggest that scFv-conjugated silk fibroin proteins can be fabricated into films as well as into powders; the films retain their binding activity and can be used to construct alternative ELISA systems.
Traditional ELISA systems need several steps for their manufacture, such as the preparation of antigen-capturing antibodies, which are mostly produced in mammalian cells, as well as isolation, purification, and immobilization of these antibodies on the surface of assay plates. In contrast, affinity silk film is a “ready-made” reagent produced by transgenic silkworm technology that requires only a few processing steps, such as dissolving the cocoons and dialyzing and drying the silk solution in assay plates (Fig. 1a). Therefore, the costs of manufacturing a silk-film ELISA system may be substantially lower than those required for a traditional ELISA system. Furthermore, once affinity silk is processed into thin film, it can be stored at RT for a long time until use, as was previously demonstrated with affinity silk powder23. These findings indicate that affinity silk film may provide a novel immunodetection system at lower manufacturing cost, but also with convenient storage and quality-control of products.
The amount of scFv-conjugated FibL is only 10% of the total FibL expression (Fig. 1b). Nonetheless, S01 transgenic silk film specifically bound the target protein, WASP (Fig. 3). Although we do not know precisely how the fibroin and sericin are combined and reconstituted during the processing of silk film, at least some of the anti-WASP scFv moieties conjugated to the C-terminus of FibL are exposed on the surface of the silk film and specifically capture the target protein. As the background ELISA levels were lower with the silk film (Fig. 3b) than with the silk solution (Fig. 2b), non-specific binding of silk proteins to unrelated proteins could be reduced after processing into film.
We previously demonstrated that silk powder made from cocoon shells expressing anti-WASP-scFv fused to fibroin protein retains its specific binding activity to the target molecule after dissolution in 9 M LiBr, dialysis, concentration, freeze-drying, and mechanical crushing23. Also, silk film, derived from anti-WASP-scFv-conjugated S01 silk solution after dissolution with 9 M LiBr and dialysis, exhibited its specific affinity to the target protein in ELISA. However, the binding activity of S01 silk film was almost diminished by the treatment of autoclaving of its silk solution (Fig. 3b). These results suggest that some or most of anti-WASP-scFv fused with FibL may preserve its proper structure for specific binding during the processing into powder or film. Furthermore, the anti-WASP-scFv construct that we used to generate affinity silk materials can bind to its target molecule in the cytosol when scFv is expressed in mouse immune cells as an intracellular antibody (intrabody)19,22. These results suggest that this anti-WASP-scFv construct autonomously folds into the conformation required to specifically bind the WASP molecule, even when the construct was expressed and secreted into the lumen of the silk gland as a fusion protein with FibL. Given these observations, the selection of scFv constructs that fold autonomously into the correct conformation under any conditions is a key factor for the successful production of affinity silk materials. Further investigations using silk proteins conjugated via scFv to other target molecules will be necessary to improve affinity silk technology.
In addition to the film format, affinity silk protein could also be converted into microspheres or magnetic beads for purification and detection of the target protein. Furthermore, the combination of nanoparticle technology for biomedical applications24,25, silk fibroin's biocompatibility, and the controlled degradation of silk fibroin makes it an ideal candidate for a nanoscale drug-delivery vehicle26,27,28. Conjugation of scFv, which specifically recognizes a marker expressed on cancer cells and other disease-related cells, may lead to the production of affinity silk fibroin nanoparticles that exhibit specific recognition of and therapeutic effects on these cells.
Methods
Solubilization of silk cocoons and preparation of silk solution and silk film
The experimental transgenic silkworm strain S01carries a transgene encoding FibL fused with anti-WASP-scFv23. Another transgenic strain, C03, carries a transgene encoding FibL fused with a scFv construct against E. coli cells (manuscript in preparation) and was used as a control. One hundred and fifty milligrams of cocoon shells obtained from wild-type w1-pnd (W1) and transgenic S01 and C03 silkworms were chopped into 2–3 mm squares and immersed in 1.5 mL of 70% ethanol, followed by addition of 13.5 mL of 9 M LiBr and 100 mM Tris-HCl (pH 9.0). The cocoon squares were suspended for 4 h at 37°C until the silk proteins had completely dissolved. The resulting silk solutions were placed in a cellulose dialysis membrane bag (Cellulose tube UC20-32-100, MWCO = 14,000; Eidia Co., Ltd, Tokyo, Japan) and dialyzed five times against 8 L of 1 mM Tris-HCL (pH 9.0) at RT (the 1 mM Tris-HCl (pH 9.0) solution was changed every 12 h) and one time against 8 L of 1 mM Tris-HCl (pH 8.0) for 12 h at RT. The dialyzed silk solutions were centrifuged at 10,000 × g for 30 min at RT and the supernatants were adjusted to a concentration of 1 mg/mL. Fifty microliters of these prepared silk solutions were applied to individual wells of 96-well polystyrene plates and dried at 50°C for 3.5 h until a thin film formed. The silk film-coated plates were kept at RT, and then used for the binding assay. The control silk film plates were made from the each strain's silk solution which was autoclaved (120°C, 20 min) after dissolving with 9 M LiBr solution. The processing of silk film on the wells was similar to the non-treated silk solution.
Immunoblotting
Silk solutions from W1, S01, and C03 strains were treated with 2 × SDS sample buffer, separated by 12% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The blots were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) for 1 h at RT and incubated with anti-FibL pAb (raised against a synthetic peptide representing FibL residues 67–80), followed by incubation with HRP-conjugated anti-rabbit Igs (Dako, Glostrup, Denmark). Immunoreactive proteins were detected using Chemi-Lumi One L (Nacalai Tesque).
ELISA
One hundred microliters of the dialyzed silk solution or anti-WASP mAb (a parental antibody for anti-WASP scFv, 1/2000 dilution in Blocking One) were applied to the wells of 96-well plates and incubated overnight at 4°C. After three washes with phosphate-buffered saline (PBS), each well was blocked with Blocking One at RT for 1 h. After five washes with PBS with Tween 20, 100 ng of GST, GST-WASP15 (aa 1–171), or GST-WASP69 (aa 172–313) fusion protein22 were applied to the wells and incubated at RT for 2 h. Binding was detected by sequential incubation of plates with anti-GST pAb (MBL, Nagoya, Japan) and HRP-conjugated anti-rabbit Igs (Dako), followed by incubation with SureBlue™ TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD, USA). After color development, the reaction was stopped with 1 N HCl and the absorbance of the solution was read at a wavelength of 450 nm using a microplate reader (iMark™ Microplate Reader; Bio-Rad). The silk film-coated plates were directly reacted with the indicated amounts of GST fusion proteins.
A murine macrophage RAW264.7 cell line29,30 (American Type Culture Collection No. TIB-71) was lysed with RIPA buffer (50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and protease inhibitor cocktail (Nacalai Tesque)) on ice for 60 min. Cell lysates were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were incubated with silk film-coated plates for 2 h at RT. Binding was detected via sequential incubation of plates with anti-WASP pAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and HRP-conjugated anti-rabbit Igs (Dako), followed by incubation with SureBlue™ TMB Microwell Peroxidase Substrate (KPL).
Statistical analysis
Statistical significance was assessed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Sample pairs were analyzed with the Student's t-test. Multiple samples were evaluated by one-way ANOVA with Tukey's test. Differences were considered significant when P < 0.05.
Author Contributions
M.S., K.K., Y.T. and H.K. conceived and designed the experiments. M.S. and K.K. performed the experiments. C.S., M.M. and Y.T. assisted with experiments and commented on the manuscript. M.S., K.K. and H.K. wrote the manuscript.
Acknowledgments
This work was supported by the Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency, and the Strategic Research Fund of National Institute of Agrobiological Sciences.
References
- Rockwood D. N. et al. Materials fabrication from Bombyx mori silk fibroin. Nat. Protocols 6, 1612–1631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U. J. et al. Structure and properties of silk hydrogels. Biomacromolecules 5, 786–792 (2004). [DOI] [PubMed] [Google Scholar]
- Nazarov R., Jin H. J. & Kaplan D. L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 5, 718–726 (2004). [DOI] [PubMed] [Google Scholar]
- Tamada Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules 6, 3100–3106 (2005). [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Tsukada M., Minoura N. & Imai Y. Synthesis of poly(ethylene glycol)-silk fibroin conjugates and surface interaction between L929 cells and the conjugates. Biomaterials 18, 267–271 (1997). [DOI] [PubMed] [Google Scholar]
- Tamada Y. Sulfation of silk fibroin by chlorosulfonic acid and the anticoagulant activity. Biomaterials 25, 377–383 (2004). [DOI] [PubMed] [Google Scholar]
- Murphy A. R., John P. S. & Kaplan D. L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMCS proliferation and differentiation. Biomaterials 29, 2829–2838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia S., McCarthy M. B., Gronowicz G. & Kaplan D. L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 54, 139–148 (2001). [DOI] [PubMed] [Google Scholar]
- Meinel L. et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J. Biomed. Mater. Res. A. 71, 25–34 (2004). [DOI] [PubMed] [Google Scholar]
- Karageorgiou V. et al. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A. 71, 528–537 (2004). [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tomita M., Shimizu K. & Yoshizato K. Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: production of recombinant human serum albumin. J. Biotechnol. 128, 531–544 (2007). [DOI] [PubMed] [Google Scholar]
- Iizuka M. et al. Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J. 276, 5806–5820 (2009). [DOI] [PubMed] [Google Scholar]
- Tomita M. et al. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 21, 52–56 (2003). [DOI] [PubMed] [Google Scholar]
- Inoue S. et al. A fibroin secretion-deficient silkworm mutant, Nd-sD, provides an efficient system for producing recombinant proteins. Insect Biochem. Mol. Biol. 35, 51–59 (2005). [DOI] [PubMed] [Google Scholar]
- Hino R., Tomita M. & Yoshizato K. The generation of germline transgenic silkworms for the production of biologically active recombinant fusion proteins of fibroin and human basic fibroblast growth factor. Biomaterials 27, 5715–5724 (2006). [DOI] [PubMed] [Google Scholar]
- Kojima K. et al. A new method for the modification of fibroin heavy chain protein in the transgenic silkworm. Biosci. Biotechnol. Biochem. 71, 2943–2951 (2007). [DOI] [PubMed] [Google Scholar]
- Bird R. E. et al. Single-chain antigen-binding proteins. Science 242, 423–426 (1988). [DOI] [PubMed] [Google Scholar]
- Huston J. S. et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 85, 5879–5883 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. et al. Intrabodies against the EVH1 domain of Wiskott-Aldrich syndrome protein inhibit T cell receptor signaling in transgenic mice T cells. FEBS J. 272, 6131–6144 (2005). [DOI] [PubMed] [Google Scholar]
- Derry J. M., Ochs H. D. & Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 78, 635–644 (1994). [DOI] [PubMed] [Google Scholar]
- Thrasher A. J. WASp in immune-system organization and function. Nat. Rev. Immunol. 2, 635–646 (2002). [DOI] [PubMed] [Google Scholar]
- Sato M., Sawahata R., Takenouchi T. & Kitani H. Identification of Fyn as the binding partner for the WASP N-terminal domain in T cells. Int. Immunol. 23, 493–502 (2011). [DOI] [PubMed] [Google Scholar]
- Sato M. et al. Production of scFv-conjugated affinity silk powder by transgenic silkworm technology. PLoS ONE 7, e34632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Aseh A., Rios C. N., Aggarwal B. B. & Mathur A. B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 4, 115–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A. B. & Gupta V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 5, 807–820 (2010). [DOI] [PubMed] [Google Scholar]
- Cheema S. K. et al. Silk fibroin mediated delivery of liposomal emodin to breast cancer cells. Int. J. Pharm. 341, 221–229 (2007). [DOI] [PubMed] [Google Scholar]
- Gobin A. S., Rhea R., Newman R. A. & Mathur A. B. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int. J. Nanomed. 1, 81–87 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu J., Chung Y., Kim Y. H., Tae G. & Kundu S. C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 388, 242–250 (2010). [DOI] [PubMed] [Google Scholar]
- Ralph P. & Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J. Immunol. 119, 950–954 (1977). [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P. & Nakoinz I. Functional macrophage cell lines transformed by abelson leukemia virus. Cell 15, 261–267 (1978). [DOI] [PubMed] [Google Scholar]