Abstract
Background
CD4-specific rates of mortality in sub-Saharan African adults with high CD4 counts have rarely been estimated. This estimation is useful to the when to start antiretroviral treatment (ART) debate.
Methods
We pooled data from ANRS-funded research cohorts in West Africa. All HIV-infected adults (≥18 years) with available follow-up time off ART were eligible. We used a joint model to estimate CD4 count evolution. We estimated CD4-specific rates of mortality, loss-to-follow-up (LTFU) and ART initiation by dividing the number of first event by the follow-up time off ART within each CD4 category.
Results
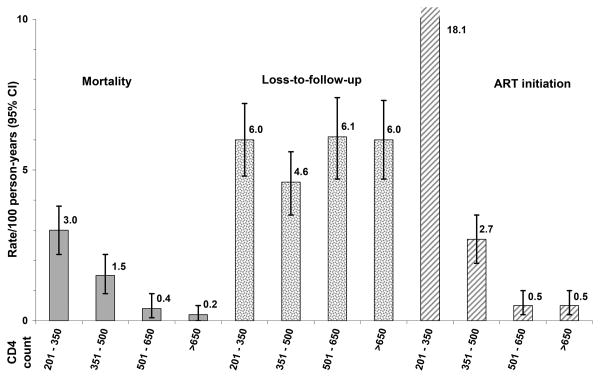
Between 1996 and 2009, 2,588 adults (80% women) from five cohorts in Cote d'Ivoire and Burkina Faso were followed off ART during 6,862 person-years (PY). In the 201-350, 351-500, 501-650 and >650/mm3 CD4 categories, mortality rates were: 3.0, 1.5, 0.4, 0.2 per 100 PY; LTFU rates: 6.0, 4.6, 6.1, 6.0 per 100 PY; and ART initiation rates: 18.1, 2.7, 0.5, 0.5 per 100 PY, respectively. All estimates varied across cohorts; mortality rates were higher when rates of LFTU and ART initiation were lower; LTFU rates were two to 40 times higher than mortality rates.
Conclusions
Among untreated West African adults with high CD4 counts, mortality and LTFU rates were substantial. Even when data is collected under research conditions, informative censoring due to ART initiation and LTFU could lead to significantly underestimate mortality figures.
Keywords: HIV infection, CD4 lymphocyte count, mortality, lost to follow-up, informative censoring, sub-Saharan Africa, adults
Introduction
The absolute number of CD4+ cells /mm3 (“CD4 count”) is the most common marker of disease progression in HIV-infected adults (1) with a lower CD4 count predicting a higher frequency of severe morbidity and mortality (2). It is recommended to start patients on antiretroviral treatment (ART) before they reach a CD4 threshold under which their risk of mortality is significantly increased. It is now admitted that a CD4 count of 350 /mm3 is the minimal threshold to recommend ART initiation worldwide (3,4). However, recent data from in Africa and Thailand strengthen the case in favour of earlier initiation of ART (5), some national guidelines recommend starting ART at 500 CD4/mm3 (6), and randomized trials are currently assessing the benefits and risks of initiating ART in patients with more than 500 CD4/mm3 (7,8). It is still unclear whether there is a universal CD4 threshold to be applied in all settings and all patients, or whether ‘when to start ART?’ is a context-driven question (9-11).
The rationale for raising the question of “should we start ART at higher CD4 counts than currently recommended?” is based on three hypotheses. First, that the short-term risk of mortality or severe morbidity is higher in HIV-infected patients with high CD4 counts than in HIV-negative patients; second, that the short-term risk of mortality or severe morbidity increases with decreasing CD4 counts, even at high CD4 counts; and third, that controlling viral load earlier could prevent some HIV-related diseases in the long term. The two former hypotheses can be explored by estimating CD4-specific rates of mortality in cohort studies of patients with high CD4 counts. The latter hypothesis can only be addressed through randomized trials with long term follow-up. In Europe and in North America, mortality rates in patients with high CD4 counts have been repeatedly estimated in large cohort collaborations (10,12-15). In sub-Saharan Africa, cohort studies providing estimates in individuals without ART and with high CD4 counts are scarce (16,17).
In this study, we pooled data from longitudinal studies funded by the National Agency for Research on AIDS and Viral Hepatitis (ANRS, France) in West Africa. Our aim was to estimate CD4-specific rates of mortality in untreated adults with high CD4 counts.
Methods
Study population
Research studies sponsored by the ANRS or associated partners in resource-limited countries were eligible if the study procedures included (i) repeated CD4 counts; (ii) a follow-up period without ART and (iii) an active strategy to retain patients.
Within eligible studies, patients were included in this analysis if: (i) they were aged ≥18 years at enrolment; (ii) they had at least one CD4 measurement available and (iii) they were followed at least one day off ART.
Procedures and definitions
In all participating studies, transport to the clinic, visits, drugs, hospitalizations and biological or radiological tests were free of charge for patients. All studies included the following procedures: scheduled visits, ranging from every 3 months to every 6 months; CD4 counts at inclusion and every 6 months thereafter; active strategies to contact patients who did not show up for a scheduled visit (including phone calls, home visits and hospital records); standardized definitions and procedures to document follow-up events and ART initiation, and standardized data collection. All study protocols had been approved by national ethics committees or institutional review boards.
Patients were defined as LTFU if their last contact was more than six months before the database cut-off for this study, if they had not started ART before their last contact and if they were not known to be dead.
Statistical analysis
For the present analysis, the date of inclusion of patients was the date of first contact reported in the database. Data were censored at the date of the first event among the following: last contact with the study team, death, ART initiation, or database cut-off date for this study.
We considered the following CD4 strata: 201 to 350, 351 to 500, 501 to 650 and >650/mm3. The rationale for breaking down the >650/mm3 stratum into two strata, 501-650 and >650/mm3, was based on the assumption that the short-term risk of mortality could decrease with increasing CD4 counts, even at very high CD4 counts, and that this could be especially true in low resource settings were major causes of HIV-related severe morbidity are common community infections (eg: tuberculosis) (17).
In order to determine the time spent in a given CD4 stratum, we estimated the CD4 evolution at individual level by jointly modelling the correlation between repeated measures in each subject through a linear mixed model and the time to drop out through a survival model. We were thus able to adjust inferences on longitudinal measurements in the presence of non-ignorable missing values (18,19). The linear mixed model had two random effects (intercept and slope), adjusted on participating study, gender and baseline CD4 count (<200, 200-350, 351-500, >500/mm3). The underlying assumptions were verified by graphically studying model residuals. The time-to-drop-out analyses were based on an exponential model, adjusted on participating study and baseline CD4 count (<200, 200-350, 351-500, >500/mm3). CD4 counts evolution and time to drop out were linked by two random effects parameters (random intercept and random slope). The joint model was performed using the NLMIXED procedure of the SAS® software, version 9.1 (SAS institute Inc.Cary, NC,USA).
We estimated CD4-specific rates of mortality, LTFU and ART initiation per 100 person-years (PY) by dividing the number of first events that occurred in each CD4 stratum by the time spent in the corresponding stratum (for patients who did not have the event) or the time between entry in the stratum and first event (for patients who experienced the event). Confidence intervals (95% CI) were calculated assuming a Poisson distribution if the number of events was lower than 50 and normal approximation otherwise.
Results
Studies sites characteristics and populations
Among 17 longitudinal studies of HIV-infected adults sponsored by the ANRS in low resource settings from 1996 to 2009, five included the follow-up of HIV-infected adults without ART. These five studies were conducted in Cote d'Ivoire and in Burkina Faso (Table 1). Their main objective was to study: HIV disease natural history (n=2) (2,20), sexually transmitted infections in vulnerable women (n=1) (21), tolerance and efficacy of interventions to prevent of mother-to-child transmission of HIV (n=1) (22) and feasibility of HIV care and treatment in a pilot program including pregnant and post-partum women with a family-focused approach (n=1) (23). In these five studies, between April 1996 and March 2009, 2,699 adults were followed at least one day without ART, of whom 2,588 (96%) had at least one measurement of CD4 count. Their main characteristics are shown in Table 1.
Table 1. Characteristics of studies, HIV-infected adults and follow-up without antiretroviral treatment (ART) in West Africa, ANRS 12222 Morbidity/Mortality Collaboration.
| 1 | 2 | 3 | 4 | 5 | Overall | |
|---|---|---|---|---|---|---|
| Country | Côte d'Ivoire | Côte d'Ivoire | Burkina Faso | Côte d'Ivoire | Côte d'Ivoire | - |
| Study Period | 1996-2003 | 1997-2009 | 1998-2008 | 2000-2005 | 2003-2008 | - |
| Main inclusion criteria | Adults, WHO stage 2-3 |
Adults recent seroconversion | Women ≥ 15 years, sex workers | Pregnant women | Pregnant and post-partum women and their family | - |
| Main outcome | Natural history | Natural history | Sexual transmitted infections | Tolerance, efficacy of pMTCT | Pilot ART program | - |
| Number of participants | 719 | 275 | 256 | 709 | 974 | 2588* |
| Baseline characteristics | ||||||
| Women, % | 69 | 40 | 100 | 100 | 88 | 80§ |
| Median age, years (IQR) |
31 | 29 | 31 | 27 | 29 | 29 |
| (26-37) | (25-34) | (25-38) | (23-30) | (25-33) | (25-34) | |
| Median CD4 count/mm3 (IQR) |
297 | 470 | 364 | 544 | 363 | 395 |
| (156-511) | (331-645) | (218-568) | (348-756) | (231-539) | (234-612) | |
| WHO stage 3 or 4, % | 56 | 0 | 24 | 25 | 22 | 30 |
|
| ||||||
| Follow-up characteristics | ||||||
|
| ||||||
| Median follow-up, years, (IQR) | 3.1 | 4.2 | 1.8 | 1.8 | 1.5 | 1.9§ |
| (1.5-5.2) | (2.4-6.1) | (0.8-4.1) | (1.7-1.8) | (0.1-3.0) | (1.1-4.1) | |
| Number of CD4 count per patient, median (IQR) |
5 | 8 | 3 | 3 | 2 | 4#,§ |
| (2-8) | (4-12) | (2-6) | (2-4) | (1-6) | (2-7) | |
|
| ||||||
| Person-years of follow-up | ||||||
| Overall | 2336 | 1217 | 565 | 1022 | 1722 | 6862§ |
| Per CD4 stratum /mm3** | ||||||
| 0-50 | 99 | 1 | 0 | 0 | 0 | 100 |
| 51-100 | 175 | 3 | 5 | 6 | 3 | 194 |
| 101-200 | 514 | 69 | 30 | 58 | 23 | 693 |
| 201-350 | 623 | 355 | 172 | 212 | 320 | 1684 |
| 351-500 | 377 | 365 | 135 | 205 | 499 | 1580 |
| 501-650 | 250 | 243 | 111 | 241 | 409 | 1254 |
| >650 | 297 | 181 | 111 | 300 | 467 | 1357 |
| Status at study termination, % | ||||||
| Alive without ART | 29 | 43 | 41 | 89 | 37 | 40 |
| ART initiation | 19 | 38 | 40 | 0 | 43 | 31 |
| Lost to follow-up | 8 | 12 | 12 | 9 | 19 | 14 |
| Dead | 43 | 7 | 6 | 2 | 2 | 15 |
345 participated both to studies 4 and 5;
modelized
During follow-up, 312 patients (12%) had at least one CD4 count missing, including 107 with only one missing CD4 count and 205 with >1 missing CD4 count
1307 women were pregnant at study entry or became pregnant during follow-up. The median time of follow-up during pregnancy was 0.4 year (IQR : 0.3-0.5), and the median number of CD4 measurements during pregnancy was 1 (IQR: 0-1). Overall follow-up during pregnancy was 605 person-years, representing 9% of the total follow-up in the study.
- ART: antiretroviral treatment; pMTCT: Prevention of Mother to Child Transmission; IQR: interquartile range
Mortality, LTFU and ART initiation
The 2,588 patients were followed during 6,862 PY (Table 1). In pooled analysis, CD4-specific mortality rates decreased with increasing CD4 counts, from 3.0 per 100 PY in the 201-350 / mm3 CD4 strata to 0.2 per 100 PY above 650 CD4 / mm3 (Figure 1). ART initiation rates also decreased with increasing CD4 counts, ranging from 18.1 per 100 PY in the 201-350 /mm3 CD4 strata to 0.5 per 100 PY above 650 CD4 /mm3. LTFU rates ranged from 4.6 to 6.0 per 100 PY, with no significant difference between CD4 strata.
Figure 1. CD4-specific rates of death, loss-to-follow-up and antiretroviral treatment initiation in untreated HIV-infected adults with CD4 ≥200 /mm3 in West Africa: pooled estimates, ANRS 12222 Morbidity/Mortality Collaboration.
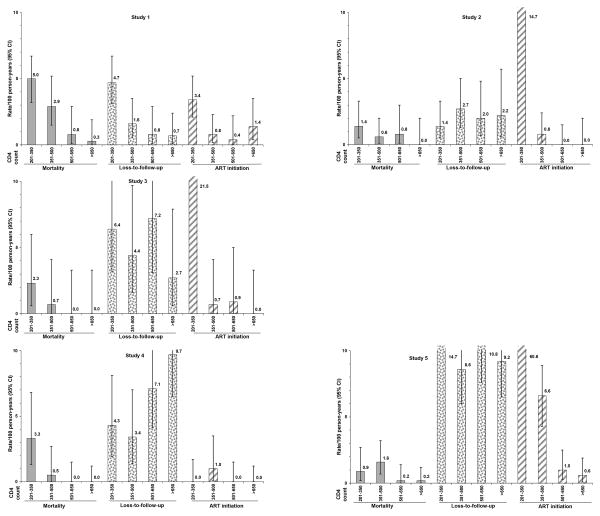
Estimates varied across cohorts (Table 2, Figure 2). Within the 201-350/mm3 CD4 stratum, the highest mortality rate was 5.0 per 100 PY in a cohort with a low rate of ART initiation (3.4 per 100 PY). In this cohort, the LTFU rate was 4.7 per 100 PY. Conversely, the lowest mortality rate was 0.9 per 100 PY in this CD4 stratum in a cohort with high rates of both LTFU and ART initiation. Within all CD4 strata above 350 /mm3, rates of ART initiation were low and mortality rates tended to be lower when the rate of LFTU was higher. The range of variability across cohorts of mortality rates decreased when CD4 count increased.
Table 2. CD4-specific mortality rates in untreated HIV-infected adults with CD4 ≥200 /mm3 in West Africa: pooled estimates and estimates of mortality, LTFU and ART initiation rates in studies with the highest and lowest mortality rates, ANRS 12222 Morbidity/Mortality Collaboration.
| Pooled estimates | Mortality Highest and lowest estimates | LTFU in corresponding studies | ART initiation in corresponding studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Mortality/100 PY | Mortality/100 PY | LTFU/100 PY | ART initiation/100 PY | ||||||||||
|
|
|
|
|
||||||||||
| CD4 strata/mm3 | N | PY | n | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | ||
| 201 to 350 | 1092 | 1684 | 50 | 3.0 | 2.2-3.8 | Study 5 | 0.9 | 0.2-2.7 | 14.7 | 10.5-18.9 | 60.6 | 52.1-69.2 | |
| Study 1 | 5.0 | 3.2-6.7 | 4.7 | 3.1-6.7 | 3.4 | 2.1-5.2 | |||||||
|
| |||||||||||||
| 351 to 500 | 944 | 1580 | 23 | 1.5 | 0.9-2.2 | Study 4 | 0.5 | 0.0-2.7 | 3.4 | 1.4-7.0 | 1.0 | 0.1-3.5 | |
| Study 1 | 2.9 | 1.5-5.2 | 1.6 | 0.6-3.5 | 0.8 | 0.2-2.3 | |||||||
|
| |||||||||||||
| 501 to 650 | 760 | 1254 | 5 | 0.4 | 0.1-0.9 | Study 4 | 0.0 | 0.0-1.5 | 7.1 | 4.1-11.3 | 0.0 | 0.0-1.5 | |
| Study 2 | 0.8 | 0.1-3.0 | 2.0 | 0.7-4.8 | 0.0 | 0.0-1.5 | |||||||
|
| |||||||||||||
| >650 | 670 | 1357 | 2 | 0.2 | 0.0-0.5 | Study 4 | 0.0 | 0.0-1.2 | 9.7 | 6.5-13.9 | 0.0 | 0.0-1.2 | |
| Study 1 | 0.3 | 0.0-1.9 | 0.7 | 0.1-2.4 | 1.4 | 0.4-3.5 | |||||||
LTFU: loss-to-follow-up; ART: antiretroviral treatment; N: number of patients; n: number of deaths; PY: person-years of follow-up; 95% CI: 95% confidence interval
Figure 2. CD4-specific rates of death, loss-to-follow-up and antiretroviral treatment initiation in untreated HIV-infected adults with CD4 ≥200/mm3 in West Africa, by participating study, ANRS 12222 Morbidity/Mortality Collaboration.
Discussion
To our knowledge, this is the first report of CD4-specific mortality rates in HIV-infected adults with high CD4 count and no ART in West Africa.
Estimating CD4-specific rates requires longitudinal observational databases with repeated CD4 counts and standardized procedures. In sub-Saharan Africa, most of the large databases with longitudinal follow-up enrol HIV-infected patients at ART initiation (24). In untreated individuals, CD4 counts are often low at first contact (25) and data on follow-up with high CD4 counts are scarce. Finally, most databases are rather program-based than research-oriented, meaning that data is recorded in real life conditions, with rare CD4 count measurements and high rates of loss to follow-up (26).We pooled data from ANRS-funded cohort studies in West Africa. In these studies, procedures, definitions and data collection were standardized. Patients were followed free of charge, had systematic CD4 measurements every six months, and were systematically traced when they missed scheduled appointments.
CD4-specific mortality rates largely varied across cohorts. We censored follow-up at last contact in patients LTFU, and at ART initiation in those who started ART. By doing so, we found the highest estimates of mortality within cohorts with the lowest rates of LTFU, and in cohorts who were implemented during the pre-ART era and had the lowest rates of ART initiation. This strongly suggests that data censoring due to LTFU and ART initiation was informative, and that mortality estimates are more accurate when rates of LTFU and ART initiation are low. As a consequence, in our study, true mortality figures are probably more accurately estimated in cohorts with the highest mortality rates and the lowest rates of LTFU and ART initiation; furthermore, in these cohorts with even low rates of LTFU and ART, mortality rates might be still underestimated, because of informative censoring.
In our study, in the cohort with the lowest LTFU and ART initiation rates, mortality estimates were 5.0/100 PY, 2.9 and 0.8 /100 PY in the 200-350, 350-500 and 500-650 CD4/mm3 strata, respectively. These rates are consistent with previous reports from Zimbabwe and South Africa (16,17). They are much higher than those reported by cohort collaborations from high-income countries (12,13). No formal comparison between settings is allowed, especially because our sample size is small and our confidence interval wide compared to those of large cohort collaborations from high-income countries. However, our findings strengthen the rationale for the 2010 WHO guidelines to increase the CD4 threshold for ART initiation from 200/mm3 to 350 /mm3 in low resource settings (3), and suggest that the rationale for starting ART earlier than currently recommended might be even stronger in sub-Saharan Africa than in Europe or in North America. This also suggests that randomised trials assessing the benefits and risks of starting ART at high CD4 counts should carefully take into account the fact that mortality rates at high CD4 count might be different in high-resource as compared to low-resource settings.
Finally, our study confirms previous reports that many people are LTFU while waiting for ART, and suggestions by some authors that earlier initiation of ART may contribute to a better retention of patient in care (27).
Our study has several limitations. The first limitation is its sample size. Yet our estimates are probably the best possible in the current context of obviously scarce data. Thus, there is clearly a need for large cohorts studies to be implemented in Africa, which would include patients with high CD4 counts and provide standardized follow-up before they initiate ART. These cohorts should ideally be multi-country, and their funding should provision for a high level of data collection standardization, LTFU prevention and morbidity documentation, conditions which are not always fulfilled in routine program databases (28,29).
The second limitation is the extent of mortality underestimation. Our data suggest that LTFU and ART initiation probably led to underestimate true mortality rates in patients off ART through informative censoring, but the extent of this underestimation cannot be measured. LTFU may be a source of mortality misclassification, while ART initiation may be a source of both mortality and LTFU misclassification, as patients who start ART may be better retained into care than those who do not. The proportion of deaths among LTFU patients is unknown and may vary across CD4 strata. Available data on the outcomes of patients LTFU while on ART is increasing (30). Unfortunately, such data are scarce for individuals LTFU without ART with high CD4 counts (31,32). Even if recorded in the context of cohort studies, LTFU rates were similar or even higher than mortality rates, suggesting that even a low proportion of deaths among LTFU patients may have led to significantly underestimating mortality. We took into account informative censoring in the model estimating the CD4 count evolution (33), and by doing so we were able to accurately estimate the time spent within each CD4 strata. However, this did not allow us to adjust mortality rates with non-observed events due to LTFU or initiation of ART (34).
The third limitation is that we describe here crude mortality rates and not standardized mortality ratios. Therefore, we don't know how the mortality rates in patients with CD4 in the two strata >500/mm3 relate to the background population mortality.
The fourth limitation is that we did not address morbidity rates and causes of death in the present study. In fact standardized morbidity data were recorded in only 2 out of the 5 participating studies. Morbidity data from these two specific studies will be analyzed further.
Finally, our study included 80% of women. This percentage is higher than in the HIV-infected adult population in West Africa, thus limiting generalisability. Furthermore, 9% of the overall follow-up time was during pregnancy, which might influence data through both maternal mortality and the natural lowering of CD4 during pregnancy (35). The former might lead to overestimating mortality rates across the entire CD4 spectrum, while the latter might lead to misallocate some deaths to wrong CD4 strata.
In conclusion, mortality rates were substantial in our collaborative study conducted in West Africa in HIV-infected adults with more than 200 CD4/mm3; all the more so as these mortality rates were probably underestimated because of informative censoring due to LTFU and ART initiation. We suggest that large multi-country cohorts including patients without ART with high CD4 counts should be implemented, in order to better estimate the risk of early mortality in HIV-infected adults in sub-Saharan Africa. In such cohorts, underestimation of mortality due to informative censoring should be systematically discussed and the incidence of LTFU and ART initiation should be systematically provided when reporting CD4-specific rates of mortality.
Acknowledgments
The ANRS 12222 Morbidity / Mortality Study Group
Steering Comittee: Xavier Anglaret, Robert Colebunders, François Dabis, Joseph Drabo, Serge Eholié, Delphine Gabillard, Pierre-Marie Girard, Karine Lacombe, Christian Laurent, Vincent Le Moing, Charlotte Lewden
Other representants of participating studies: Gérard Allou, Clarisse Amani-Bossé, Divine Avit, Aida Benalycherif, Pierre de Beaudrap, Charlotte Boullé, Patrick Coffie, Ali Coulibaly, Eric Delaporte, Lise Denoeud, Serge Diagbouga, Didier Koumavi Ekouevi, Jean-François Etard, Sabrina Eymard-Duvernay, Patricia Fassinou, Isabelle Fournier-Nicolle, Hervé Hien, Charlotte Huet, Issouf Konate, Sinata Koulla-Shiro, Valériane Leroy, Olivier Marcy, Pierre Régis Martin, Nicolas Meda, Eugène Messou, Albert Minga, Eitel Mpoudi-Ngolé, Philippe Msellati, Boubacar Nacro, Nicolas Nagot, Ibra Ndoye, Thérèse N'Dri-Yoman, Abdoulaye Ouédraogo, Men Pagnaroat, Roger Salamon, Vonthanak Saphonn, Olivier Segeral, Catherine Seyler, Besigin Tonwe-Gold, Moussa Traore, Philippe Van de Perre, Ida Viho, Marcel Zannou
Funding Source: French National Agency for Research on Aids and Viral Hepatitis (ANRS) (Grants ANRS 12222)
Financial support: The ANRS 12222 Morbidity/Mortality Study Group is funded by the French National Agency for Research on Aids and Viral Hepatitis (ANRS).
Footnotes
This data was presented at the 5th AFRAVIH, Maroc, 28-31 March 2010, the 14th Workshop on HIV Observational Databases, Sitges, Spain, 25-27 March 2010 and the 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Rome, Italy, 17-20 July 2011 (abstract # 2509).
Conflict of interest: We declare no conflict of interest
Contributions: Xavier Anglaret, Delphine Gabillard, Christian Laurent and Charlotte Lewden developed the analysis plan, to which all authors then contributed. Delphine Gabillard performed statistical analyses. All authors contributed to interpretation of the data. Charlotte Lewden and Xavier Anglaret wrote the report, to which all authors then contributed. Delphine Gabillard, Charlotte Lewden and Xavier Anglaret had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Anglaret X, Messou E, Oaussa T, et al. Pattern of bacterial disease in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis, Abidjan, Côte d'Ivoire. AIDS. 2003;17:575–84. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral Therapy for HIV infection in adults and adolescents. Recommendations for a public health approach 2010 revision. 2010 [cited 2010/25/10]. Available from: http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 4.European AIDS Clinical Society E. Guidelines Clinical Management and Treatment of HIV-infected Adults in Europe. Version 5-4. 2011 [cited 07/08/2011]. Available from: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/1_treatment_of_hiv_infected_adults.pdf.
- 5.Grinsztejn B, Ribaudo H, Cohen MS, et al. Effects of early versus delayed initiation of antiretroviral therapy (ART) on HIV clinical outcomes: results from the HPTN 05 randomized clinical trial. Proceedings of the: 6th International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention; 2011 17-20 July; Roma, Italy. 2011.[MOAX0105] [Google Scholar]
- 6.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 7.Strategic Timing of Antiretroviral Treatment (START) [cited 2011/05/05]. Available from: http://clinicaltrials.gov/ct2/show/NCT00867048?term=start&rank=1.
- 8.Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-infected Adults (ANRS 12136 TEMPRANO). [cited 2011/05/05]. Available from: http://clinicaltrials.gov/ct2/show/NCT00495651?term=temprano&rank=1.
- 9.When to start consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd BE, Jenkins CA, Rebeiro PF, et al. Estimating the optimal CD4 count for HIV-infected persons to start antiretroviral therapy. Epidemiology. 2010;21:698–705. doi: 10.1097/EDE.0b013e3181e97737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The UK Collaborative HIV Cohort (CHIC) Study Steering Committee. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 13.Dunn D, Woodburn P, Duong T, et al. Current CD4 Cell Count and the Short-Term Risk of AIDS and Death before the Availability of Effective Antiretroviral Therapy in HIV-Infected Children and Adults. J Infect Dis. 2008;197:398–404. doi: 10.1086/524686. [DOI] [PubMed] [Google Scholar]
- 14.Study Group on Death Rates at High CD4 Count in Antiretroviral Naive Patients. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per μL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–5. doi: 10.1016/S0140-6736(10)60932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CASCADE Collaboration. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18:51–8. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- 16.Badri M, L SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 17.Hargrove JW, Humphrey JH. Mortality among HIV-positive postpartum women with high CD4 cell counts in Zimbabwe. AIDS. 2010;24:F11–4. doi: 10.1097/qad.0b013e328335749d. [DOI] [PubMed] [Google Scholar]
- 18.Thiebaut R, Jacqmin-Gadda H, Babiker A, et al. Joint modelling of bivariate longitudinal data with informative dropout and left-censoring, with application to the evolution of CD4+ cell count and HIV RNA viral load in response to treatment of HIV infection. Stat Med. 2005;24:65–82. doi: 10.1002/sim.1923. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Carlin B. Separate and joint modeling for longitudinal and event time data using standard computer packages. Am Stat. 2004;58:1–9. [Google Scholar]
- 20.Minga A, Danel C, Abo Y, et al. Progression to WHO criteria for antiretroviral therapy in a 7-year cohort of adult HIV-1 seroconverters in Abidjan, Cote d'Ivoire. Bull World Health Organ. 2007;85:116–123. doi: 10.2471/BLT.06.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagot N, Ouangre A, Ouedraogo A, et al. Spectrum of commercial sex activity in Burkina Faso: classification model and risk of exposure to HIV. J Acquir Immune Defic Syndr. 2002;29:517–21. doi: 10.1097/00126334-200204150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–18. [PMC free article] [PubMed] [Google Scholar]
- 23.Tonwe-Gold B, Ekouevi DK, Bosse CA, et al. Implementing family-focused HIV care and treatment: the first 2 years' experience of the mother-to-child transmission-plus program in Abidjan, Cote d'Ivoire. Trop Med Int Health. 2009;14:204–12. doi: 10.1111/j.1365-3156.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett JA, Shao JF. Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. Lancet Infect Dis. 2009;9:637–49. doi: 10.1016/S1473-3099(09)70227-0. [DOI] [PubMed] [Google Scholar]
- 25.Wools-Kaloustian K, Kimaiyo S, Musick B, et al. The impact of the President's Emergency Plan for AIDS Relief on expansion of HIV care services for adult patients in western Kenya. AIDS. 2009;23:195–201. doi: 10.1097/QAD.0b013e32831cc0e6. [DOI] [PubMed] [Google Scholar]
- 26.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen S, Fox MP. Retention in HIV Care between Testing and Treatment in Sub-Saharan Africa: A Systematic Review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tayler-Smith K, Zachariah R, Massaquoi M, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104:313–9. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Ekouevi DK, Balestre E, Ba-Gomis FO, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15(Suppl 1):34–42. doi: 10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MP, Brennan A, Maskew M, et al. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lessells RJ, Mutevedzi PC, Cooke GS, et al. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anglaret X, Toure S, Gourvellec G, et al. Impact of Vital Status Investigation Procedures on Estimates of Survival in Cohorts of HIV-Infected Patients From Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–3. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 33.Duvignac J, Anglaret X, Kpozehouen A, et al. CD4+ T-lymphocytes natural decrease in HAART-naive HIV-infected adults in Abidjan. HIV Clin Trials. 2008;9:26–35. doi: 10.1310/hct0901-26. [DOI] [PubMed] [Google Scholar]
- 34.Brinkhof MW, Spycher BD, Yiannoutsos C, et al. Adjusting Mortality for Loss to Follow-Up: Analysis of Five ART Programmes in Sub-Saharan Africa. PLoS ONE. 2010;5:e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekouevi DK, Inwoley A, Tonwe-Gold B, et al. Variation of CD4 count and percentage during pregnancy and after delivery: implications for HAART initiation in resource-limited settings. AIDS Res Hum Retroviruses. 2007;23:1469–74. doi: 10.1089/aid.2007.0059. [DOI] [PubMed] [Google Scholar]