Abstract
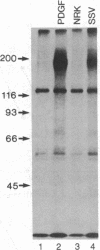
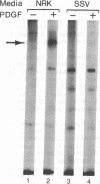
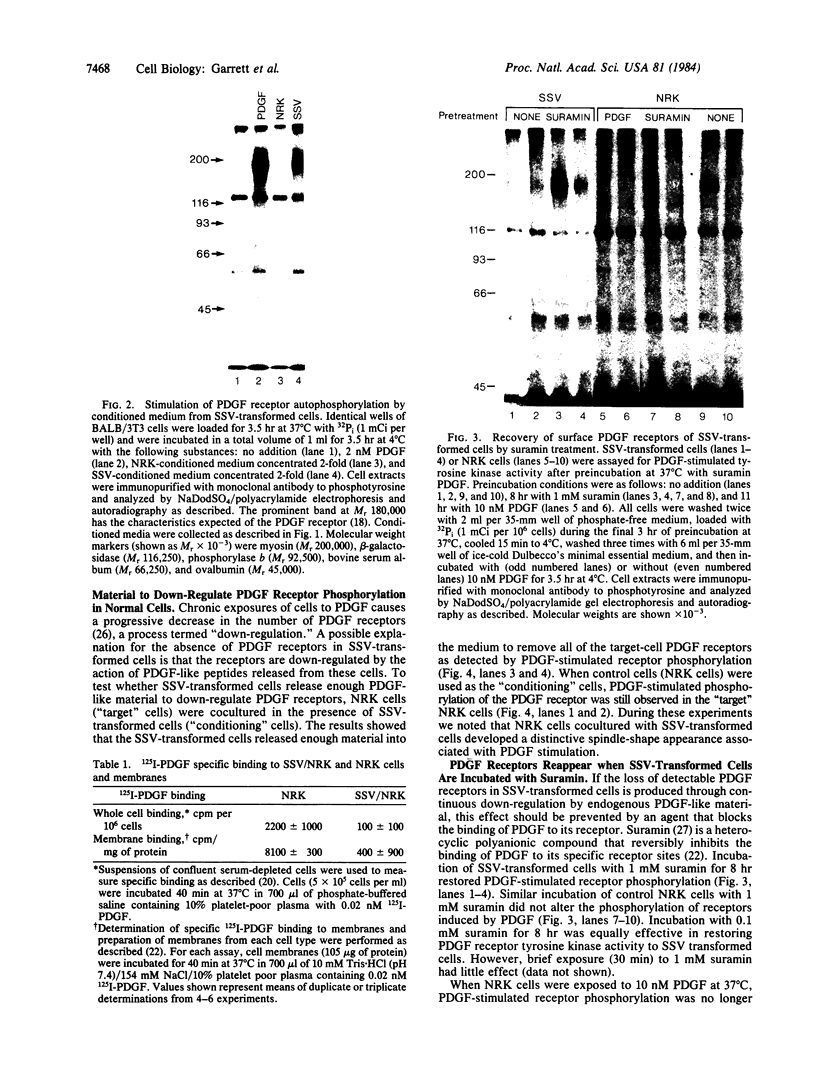
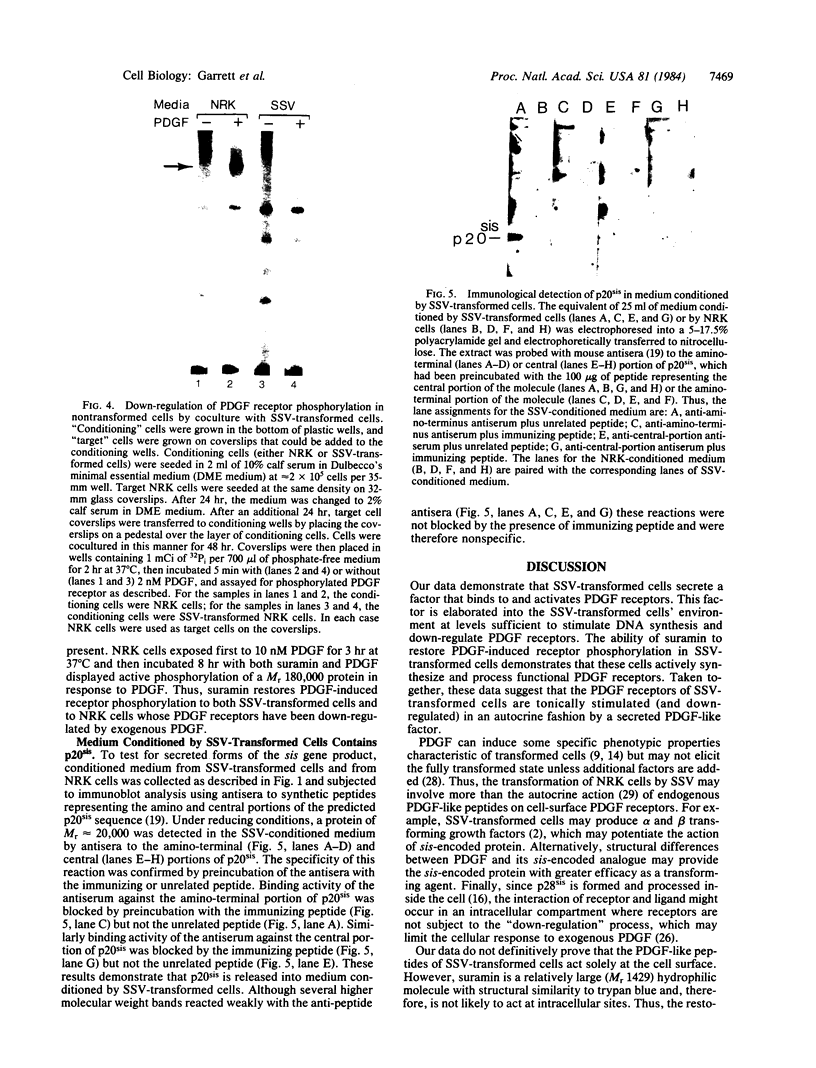
The viral (v)-sis oncogene encodes a protein (p28sis) that is structurally homologous to platelet-derived growth factor (PDGF). We have shown that simian sarcoma virus (SSV)-transformed cells containing the v-sis oncogene release a Mr 20,000 substance that is recognized by antisera to synthetic peptide sequences contained in p28sis. Medium conditioned by SSV-transformed cells competes with 125I-labeled PDGF for specific PDGF receptor sites, initiates DNA synthesis, and stimulates tyrosine phosphorylation of the PDGF receptor when added to normal cells. When normal cells are co-cultured with SSV-transformed cells, the PDGF receptors of the normal cells are down-regulated by factors released from the transformed cells. Thus, SSV-transformed cells release material that is functionally similar to PDGF. We have used anti-phosphotyrosine antibodies to purify PDGF receptors and to detect PDGF-stimulated receptors in normal cells. SSV-transformed cells have no PDGF receptors detectable by these antibodies or by 125I-labeled PDGF binding studies. However, when SSV-transformed cells are exposed to suramin, a compound that blocks binding of PDGF to its receptors, the receptors reappear on the cell surface and within 8 hr are present at the same levels as in control cells. These "new" receptor sites can be phosphorylated in response to PDGF. Thus, the absence of PDGF receptors in SSV-transformed cells is due to down-regulation of the receptors by an autocrine mechanism that can be blocked by suramin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Hawking F. Suramin: with special reference to onchocerciasis. Adv Pharmacol Chemother. 1978;15:289–322. doi: 10.1016/s1054-3589(08)60486-x. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Cellular responsiveness to growth factors correlates with a cell's ability to express the transformed phenotype. Cell. 1983 Jul;33(3):931–938. doi: 10.1016/0092-8674(83)90036-3. [DOI] [PubMed] [Google Scholar]
- Niman H. L. Antisera to a synthetic peptide of the sis viral oncogene product recognize human platelet-derived growth factor. Nature. 1984 Jan 12;307(5947):180–183. doi: 10.1038/307180a0. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Heldin C. H., Wasteson A., Westermark B. A glioma-derived analog to platelet-derived growth factor: demonstration of receptor competing activity and immunological crossreactivity. Proc Natl Acad Sci U S A. 1984 Feb;81(3):926–930. doi: 10.1073/pnas.81.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Wheeler T., Kaplan P. L. Cells transformed by RNA and DNA tumor viruses produce transforming factors. Fed Proc. 1982 Nov;41(13):3004–3007. [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Frolik C. A., Anzano M. A., Sporn M. B. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983 Jun;42(9):2621–2626. [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Williams L. T. A v-sis oncogene protein produced in bacteria competes for platelet-derived growth factor binding to its receptor. J Biol Chem. 1984 Sep 10;259(17):10645–10648. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Antoniades H. N., Goetzl E. J. Platelet-derived growth factor stimulates mouse 3T3 cell mitogenesis and leukocyte chemotaxis through different structural determinants. J Clin Invest. 1983 Nov;72(5):1759–1763. doi: 10.1172/JCI111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Williams L. T., Tremble P., Antoniades H. N. Platelet-derived growth factor binds specifically to receptors on vascular smooth muscle cells and the binding becomes nondissociable. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5867–5870. doi: 10.1073/pnas.79.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]