ABSTRACT
Background
Aortic stiffness and left ventricular (LV) diastolic dysfunction are common and associated with increased morbidity and mortality in systemic lupus erythematosus (SLE).
Hypothesis
In SLE, aortic stiffness and LV diastolic dysfunction may be associated.
Methods
This 6‐year‐duration, cross‐sectional, and controlled study was conducted in 76 SLE patients (69 women; mean age, 37 ± 12 years) and 26 age‐ and sex‐matched healthy controls. All subjects underwent clinical and laboratory evaluations and transesophageal echocardiography (TEE) to assess LV diastolic function and stiffness of the descending thoracic aorta using the pressure‐strain elastic modulus (PSEM). To validate results using PSEM, aortic strain, stiffness, and distensibility were assessed.
Results
Patients as compared with controls had higher PSEM (8.14 ± 4.25 vs 5.97 ± 2.31 U, P < 0.001) and had lower mitral inflow E/A and septal and lateral mitral annulus tissue Doppler E′/A′ velocity ratios, longer isovolumic relaxation time, lower septal and lateral mitral annulus E′ velocities, and higher mitral E/septal E′ and mitral E/lateral E′ velocity ratios (all P ≤ 0.03), all indicative of LV diastolic dysfunction. In patients, PSEM was correlated with parameters of LV diastolic dysfunction (all P < 0.05), was independently negatively associated with E/A and E′/A′ ratios and E′ velocities, and was positively associated with E/E′ ratios (P ≤ 0.02 for each parameter and P < 0.001 for all parameters as a profile). Aortic strain, stiffness, and distensibility were also worse in patients than in controls (all P < 0.05) and were correlated with parameters of LV diastolic dysfunction (all P ≤ 0.03).
Conclusions
Aortic stiffness is independently associated with LV diastolic dysfunction in young adult patients with SLE.
Introduction
Aortic stiffness and left ventricular (LV) diastolic dysfunction are common and associated with increased morbidity and mortality in systemic lupus erythematosus (SLE).1, 2, 3, 4, 5, 6, 7 Aortic stiffness may cause earlier return of the pulse wave reflection to the heart and lead to increased aortic systolic but decreased diastolic pressure, increased LV afterload and LV mass, decreased coronary perfusion, and consequently to LV diastolic dysfunction.8 To our knowledge, an association of aortic stiffness with LV diastolic dysfunction in autoimmune inflammatory diseases including SLE has not been reported, but may be etiologically and clinically important. Therefore, this study was designed to determine whether aortic stiffness and LV diastolic dysfunction simultaneously assessed by transesophageal echocardiography (TEE) are independently associated.
Methods
Study Populations
This 6‐year (December 2006–December 2012) cross‐sectional and controlled study is part of a protocol approved by the National Institutes of Health and our institutional review board for the study of cardiovascular disease using TEE and cerebrovascular disease in SLE. The study was conducted according to the Declaration of Helsinki and all participants provided signed informed consent.
Seventy‐six consecutive patients with SLE (69 women; mean age, 37 ± 12 years [range, 18–60 years]) participated in the study. Patients were recruited from 266 well‐characterized patients actively followed at the Rheumatology Clinics of the University of New Mexico Health Sciences Center. Patients were excluded due to age <18 or >60 years, pregnancy, atrial fibrillation or flutter, cardiomyopathy, drug abuse, renal dysfunction, difficult venous access, self‐withdrawal or noncompliance with study protocol, or contraindications to TEE or magnetic resonance imaging.
To provide a normal reference and validate blinded interpretation and diagnostic accuracy of tests, 26 age‐ and sex‐matched healthy controls (22 women; mean age, 34 ± 11 years [range, 18–57 years]) were studied.
Clinical and Laboratory Evaluations
Patients and controls underwent clinical and laboratory evaluations including parameters of inflammation, platelet activity, coagulation, and fibrinolysis (Supplemental Table 1). Patients were also characterized with regard to SLE duration, activity, injury, therapy, and standard serologies, including antiphospholipid antibodies (Supplemental Table 2).
Transesophageal Echocardiography
All subjects underwent multiplane TEE with the I‐E33 imaging system (Philips; Andover, MA) using a 7‐MHz transducer. Studies of patients and controls were codified, digitally stored, and randomly intermixed for blinded analysis.
Aortic Diameters and Intima‐Media Thickness
At a low depth (3–4 cm) and using a narrow sector scan to improve image resolution, 2‐dimensional guided M‐mode images were used to assess systolic and diastolic diameters of proximal aorta (25–30 cm from incisors), mid aorta (30–35 cm), and distal descending thoracic aorta (35–40 cm3; Supplemental Figure 1). At each location, 3 end‐systolic and end‐diastolic aortic diameters were measured from short‐ or long‐axis views. At each aortic location, 3 measurements of aortic intima‐media thickness (IMT) were performed at end‐diastole using M‐mode imaging.9 To assess intraobserver variability, 26 randomly selected studies had repeat aortic measurements. The mean percent coefficient of variation in systolic and diastolic diameters of the proximal, mid, and distal aorta were 1.51% and 1.55%, 0.69% and 0.70%, and 1.21% and 1.76%, respectively.
Blood Pressures
During assessment of the aorta, 3 to 6 automatic brachial blood pressures were obtained and matched in time with measurement of aortic diameters.
Aortic Stiffness
Stiffness of the proximal, mid, and distal thoracic aorta was assessed with the pressure‐strain elastic modulus (PSEM), a well‐validated parameter of static arterial stiffness, as = [k(sBP − dBP)/(sD − dD/dD)]/10 000, where k = 133.3 is the conversion factor from mm Hg to Nm−2 (Pascal units), sBP is brachial systolic blood pressure, dBP is brachial diastolic blood pressure, sD is systolic diameter, and dD is diastolic diameter.3, 10 To validate the results derived from using PSEM, we also measured (1) strain (%) = (sD − dD)/dD, which assesses percent change of vessel deformation independent of blood pressure; (2) stiffness (units) = (sBP/dBP)/(sD − dD/dD), which, as PSEM, assesses the amount of pressure required to distend a vessel; and (3) distensibility (units) = (dD/IMT)/(sBP/dBP)/(sD − dD/dD), which assesses changes in vessel diameter as a function of blood pressure and wall thickness.10
Left Ventricular Structure and Function and Left Atrial Volume
Left ventricular end‐diastolic diameter and inferior and anterior end‐diastolic wall thicknesses were measured at the papillary muscles level from transgastric TEE short‐ or long‐axis views using 2‐dimensional guided M‐mode images. Left ventricular mass was calculated using the following formula: 0.80 × 1.05 [(inferior wall thickness + anterior wall thickness + LV internal diameter)3 − (LV internal diameter)3].11 Left ventricular wall motion and systolic function were visually assessed. Because TEE visualization of the entire left atrium (LA) is uncommon, LA volumes were measured from transthoracic echocardiography (TTE) obtained immediately after TEE.
Left Ventricular Diastolic Function
From basal 4‐chamber TEE view and using pulsed‐wave Doppler, LV diastolic function was assessed according to American Society of Echocardiography guidelines12: (1) mitral inflow early filling (E wave) and atrial contraction (A wave) peak velocities at the leaflets tip; (2) E‐wave deceleration time; (3) early (E′) and late (A′) peak velocities of septal and lateral mitral annulus; (4) isovolumic relaxation time (IVRT) using septal mitral annulus tissue Doppler recordings; and (5) left or right upper pulmonary vein systolic and diastolic peak velocities (Supplemental Figure 1). Measurements of LV diastolic function were performed using electronic calipers and averaged over 3 cardiac cycles. To assess intraobserver variability, 14 randomly selected TEE studies had repeat measurements. The mean percent coefficient of variation of mitral E and A, septal E′ and A′, IVRT, and pulmonary vein systolic and diastolic velocities were 2.67% and 2.88%, 2.59% and 1.74%, 4.44%, and 3.06% and 4.21%, respectively.
To avoid interpretation bias, one observer measured aortic diameters, a second observer measured parameters of LV diastolic function, a third observer measured LV size and wall thickness and aortic IMT, and a fourth observer measured LA volumes.
Statistical Analysis
The Student t test or Wilcoxon rank‐sum test (for non‐normally distributed data) and Fisher exact test were used for comparison of continuous and categorical variables among groups, respectively. Pressure‐strain elastic modulus, strain, stiffness, and distensibility at each aortic location and across 3 locations were compared between patients and controls. To adjust for confounding effects of heart rate, and especially of blood pressure, on the association of PSEM and LV diastolic dysfunction with disease state (SLE vs controls), we standardized these outcomes for each individual to the same pooled (patients and controls) mean heart rate (73.7 beats per minute) and mean arterial blood pressure (MAP; 85.6 mm Hg) using multiple linear regression. Also, PSEM in patients who were normotensive during TEE, normotensive on no vasodilators, ambulatory normotensive (blood pressure during enrollment clinical evaluation), and normotensive without prehypertension (blood pressure ≤135/85 mm Hg) and without aortic atherosclerosis was compared with that of controls. Pressure‐strain elastic modulus, strain, stiffness, and distensibility were correlated with LV mass, LA volume, and LV diastolic dysfunction using Pearson correlations. Because a single, specific definition of LV diastolic dysfunction applicable to young women is not feasible based on current guidelines, the 5 most accepted parameters of LV diastolic dysfunction were analyzed as a profile by repeated measures analysis of variance (rANOVA). Because the profile of LV diastolic dysfunction differed between patients and controls, we analyzed each variable separately as post hoc tests to our overall analysis. These analyses determined the independent effect of PSEM (after adjusting for blood pressure and left ventricular mass index [LVMI]) and clinical, laboratory, and therapy variables (described in Supplemental Tables 1 and 2) on these 5 parameters of LV diastolic dysfunction. The effect sizes of predictor variables are reported as standardized β (number of SD change in the outcome for a 1‐SD change in the predictor variable). A 2‐tailed P value <0.05 was considered significant.
Results
Clinical and Laboratory Characteristics
Patients and controls were similar in age, sex, body mass index, and atherogenic risk factors. Patients as compared with controls had higher nonhypertensive systolic and diastolic blood pressures (122.5 ± 15.02 mm Hg and 76.18 ± 10.29 mm Hg vs 115.5 ± 8.02 mm Hg and 71.12 ± 8.68 mm Hg, respectively, both P ≤ 0.02) but had no significantly higher frequency of hypertension (10 [13%] vs 0%, P = 0.06). Patients as compared with controls also had lower hemoglobin, platelets, albumin, and peak thrombin generation; and higher creatinine, quantitative D‐dimer, and tissue plasminogen activator antigen (tPA; all P ≤ 0.04; Supplemental Table 1). Clinical, therapy, and laboratory data were typical of young adult SLE patients (Supplemental Table 2).
Aortic Stiffness in Patients and Controls
As shown in Supplemental Table 3, during TEE patients as compared with controls had similar pulse pressures, and yet the differential aortic diameters were smaller in patients, suggesting that aortic stiffness may occur independent of blood pressure. Aortic PSEM was higher in patients as compared with controls even after standardizing both groups to the same mean heart rate and MAP (P < 0.001; Table 1). Furthermore, PSEM was higher in 59 patients vs 23 controls who were normotensive during TEE (7.55 ± 3.71 vs 5.58 ± 1.99 units, P = 0.003), in 47 patients vs 23 controls who were normotensive during TEE and on no antihypertensive therapy (7.22 ± 3.58 vs 5.58 ± 1.99 units, P = 0.02), in 66 patients vs 26 controls who were normotensive during initial enrollment clinical evaluation (8.03 ± 4.34 vs 5.97 ± 2.31 units, P = 0.004), in 54 patients vs 25 controls who were neither hypertensive nor prehypertensive during initial enrollment clinical evaluation (8.07 ± 4.29 vs 5.95 ± 2.35 units, P = 0.006), and in 59 patients vs 26 controls who had no aortic plaques (7.74 ± 4.20 vs 5.97 ± 2.31 units, P = 0.01). Aortic strain, stiffness, and distensibility were also worse in patients than in controls (all P < 0.05; Table 1).
Table 1.
Aortic Pressure‐Strain Elastic Modulus in SLE Patients and Controls
| Aortic Location | Patients, n = 76 | Controls, n = 26 | P Valuea |
|---|---|---|---|
| Aortic PSEM, U | |||
| Proximal | 7.77 ± 4.09 | 5.40 ± 2.28 | <0.001/<0.001 |
| Mid | 7.87 ± 4.65 | 6.15 ± 2.93 | 0.03/0.006 |
| Distal | 9.08 ± 6.62 | 6.37 ± 3.17 | 0.01/0.003 |
| Overallb | 8.14 ± 4.25 | 5.97 ± 2.31 | 0.002/<0.001 |
| Aortic strain, stiffness, and distensibility in SLE patients and controls | |||
| Overall strain, %b | 10.7 ± 4 | 12.3 ± 3 | 0.049 |
| Overall stiffness, Ub | 6.55 ± 3.20 | 5.26 ± 1.88 | 0.02 |
| Overall distensibility, Ub | 0.446 ± 0.25 | 0.568 ± 0.18 | 0.01 |
Abbreviations: PSEM, pressure‐strain elastic modulus; SD, standard deviation; SLE, systemic lupus erythematosus.
Data are presented as mean ± SD.
Second P value after standardizing both groups to the same pooled mean heart rate and blood pressure using regression coefficients.
Overall refers to the average of proximal, mid, and distal thoracic aortic PSEM, strain, stiffness, and distensibility.
Left Ventricular Wall Thickness, Mass, and Diastolic Function
Unadjusted and after standardizing each subject to the same pooled mean heart rate (73.7 bpm) and blood pressure (85.6 mm Hg), patients as compared with controls had (1) greater inferior and anterior wall thickness and higher LV mass and index; (2) lower E/A and E′/A′ ratios, longer IVRT, and lower septal and lateral mitral annulus E′ velocities consistent with impaired LV relaxation; and (3) higher mitral E/septal E′, mitral E/lateral E′, and average mitral E/E′ velocities ratios suggestive of decreased LV compliance (all P ≤ 0.03; Table 2). Left ventricular size, wall motion, and systolic function were similar among groups.
Table 2.
TEE Doppler Echocardiographic Findings in Systemic Lupus Erythematosus Patients and Controls
| Variable | Patients (n = 76) | Controls (n = 26) | P Valuea |
|---|---|---|---|
| Heart rate, bpm | 76.14 ± 13.24 | 66.60 ± 11.82 | 0.001 |
| Mean arterial BP, mm Hg | 87.70 ± 16.20 | 79.56 ± 10.55 | 0.005 |
| LVEDD, cm | 4.65 ± 0.64 (74) | 4.66 ± 0.48 | 0.94 |
| LV wall‐motion abnormalities, n (%) | 2 (3) | 0 | 1.00 |
| LVEF <50%, n (%) | 2 (3) | 0 | 1.00 |
| LV inferior wall thickness, cm | 0.81 ± 0.20 (73) | 0.71 ± 0.10 | <0.001/0.001 |
| LV anterior wall thickness, cm | 0.78 ± 0.18 (73) | 0.65 ± 0.10 | <0.001/<0.001 |
| LV mass, g | 123 ± 50 (73) | 99 ± 23 | 0.002/0.007 |
| LVMI, g/m2 | 67 ± 26 (73) | 54 ± 11 | <0.001/0.003 |
| LA volume, mL | 44.43 ± 19.07 (71) | 37.76 ± 11.03 | 0.04/0.88 |
| LAVI | 24.63 ± 9.99 (71) | 20.76 ± 6.03 (25) | 0.02/0.82 |
| Mitral E velocity, cm/sec | 80 ± 23 (75) | 79 ± 16 | 0.90/0.77 |
| Mitral A velocity, cm/sec | 64 ± 25 (75) | 45 ± 9 | <0.001/<0.0001 |
| Mitral E/A ratio | 1.39 ± 0.61 (75) | 1.81 ± 0.43 | <0.001/<0.001 |
| Mitral E deceleration time, msec | 177 ± 38 (73) | 166 ± 35 (24) | 0.19/0.15 |
| Septal IVRT, msec | 115 ± 16 (74) | 72 ± 14 | 0.02/0.03 |
| Septal E′ velocity, cm/sec | 9 ± 3 (75) | 12 ± 3 | <0.001/0.006 |
| Septal A′ velocity, cm/sec | 8.47 ± 2.05 (75) | 8.35 ±1.69 | 0.78/0.16 |
| Septal E′/A′ ratio | 1.14 ± 0.48 (75) | 1.44 ± 0.43 | 0.004/0.001 |
| Lateral E′ velocity, cm/sec | 12 ± 4 | 15 ± 2 | 0.001/0.002 |
| Lateral A′ velocity, cm/sec | 8.91 ± 2.28 (74) | 8.40 ± 1.66 (25) | 0.24/0.07 |
| Lateral E′/A′ ratio | 1.55 ± 0.76 (73) | 1.92 ± 0.62 (23) | 0.008/0.003 |
| Septal E/E′ ratio | 9.99 ± 4.66 (75) | 7.27 ± 1.96 (25) | <0.001/<0.001 |
| Lateral E/E′ ratio | 7.68 ± 4.64 (75) | 5.59 ± 1.35 (25) | <0.001/0.004 |
| Average, septal/lateral E/E′ ratio | 8.46 ± 4.29 (75) | 6.27 ± 1.52 (25) | <0.001/0.002 |
| PV systolic velocity, cm/sec | 21.55 ± 15.16 (73) | 15.63 ± 4.04 | 0.003/0.006 |
| PV diastolic velocity, cm/sec | 13.96 ± 13.90 (73) | 10.56 ± 3.48 | 0.06/0.37 |
| PV systolic/diastolic ratio | 1.84 ± 0.66 (73) | 1.56 ± 0.44 | 0.02/0.002 |
Abbreviations: BP, blood pressure; IVRT, isovolumic relaxation time; LA, left atrium; LAVI, left atrial volume index; LV, left ventricle; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PV, pulmonary vein; SD, standard deviation; TEE, transesophageal echocardiography.
Cell formats are mean ± standard deviation (SD) or mean ± SD (n) for subsets.
The first P value is unadjusted, and the second P value is after standardizing patients and controls to same mean heart rate and blood pressure using regression coefficients.
Correlation of Aortic Stiffness With Left Ventricular Mass, Left Atrial Volume, and Left Ventricular Diastolic Dysfunction
In SLE, PSEM was correlated with LV mass and LA volume indexes and with parameters of LV diastolic dysfunction (all P < 0.05; Table 3). Aortic strain, stiffness, and distensibility were also correlated with parameters of LV diastolic dysfunction in SLE (all P ≤ 0.03; Supplemental Table 4).
Table 3.
Correlation of Aortic Stiffness With LV Mass, LA Volume, and Parameters of LV Diastolic Function in SLE Patients
| Variable | PSEM | |
|---|---|---|
| Pearson Correlations (r) | P Value | |
| SLE patients | ||
| LVMI, g/m2 (n = 73) | 0.23 | 0.05 |
| LAVI (n = 71) | 0.27 | 0.02 |
| Mitral A peak velocity, cm/sec (n = 75) | 0.40 | <0.001 |
| Mitral E′/A′ ratio (n = 75) | −0.37 | <0.001 |
| Septal E′ peak velocity, cm/sec (n = 75) | −0.24 | 0.03 |
| Septal A′ peak velocity, cm/sec (n = 75) | 0.33 | 0.004 |
| Septal E′/A′ ratio (n = 75) | −0.42 | <0.001 |
| Septal IVRT, msec (n = 74) | 0.34 | 0.004 |
| Lateral E′ peak velocity, cm/sec (n = 76) | −0.45 | <0.001 |
| Lateral A′ peak velocity, cm/sec (n = 74) | 0.20 | 0.08 |
| Lateral E′/A′ ratio (n = 74) | −0.46 | <0.001 |
| Average of septal and lateral E′/A′ ratio (n = 75) | −0.38 | <0.001 |
| Septal E/E′ ratio (n = 75) | 0.23 | 0.045 |
| Lateral E/E′ ratio (n = 75) | 0.40 | <0.001 |
| Average E/E′ ratio (n =75) | 0.32 | 0.006 |
Abbreviations: IVRT, isovolumic relaxation time; LA, left atrial; LAVI, left atrial volume index; LV, left ventricular; LVMI, left ventricular mass index; PSEM, pressure‐strain elastic modulus; SLE, systemic lupus erythematosus.
Independent Association of Aortic Stiffness and Other Variables With Left Ventricular Diastolic Dysfunction
By multivariate analysis including all clinical, laboratory, and therapy variables listed in Supplemental Tables 1 and 2, PSEM was independently negatively associated with mitral E/A and E′/A′ ratios and E′ velocity and positively associated with E/E′ ratios after adjustment including MAP and LVMI (P ≤ 0.02 for each parameter and P < 0.001 for all parameters as a profile by rANOVA; Table 4 and Figure 1). These data support that LV diastolic dysfunction in SLE patients can occur independently of hypertension and LV hypertrophy. Mean arterial blood pressure, complement C3 levels, non‐neuro–Systemic Lupus International Collaborating Clinics score (SLE damage score), tPA levels, antithrombotic therapy, and SLE duration were also independently associated with LV diastolic dysfunction (all P ≤ 0.04).
Table 4.
Independent Association of Aortic Stiffness (PSEM) and Other Variables with LV Diastolic Function in SLE Patients by Multivariate Analysisa
| Best Predictors | Standardized βb | P Value |
|---|---|---|
| Mitral E/A ratio | ||
| PSEM | −0.32/−0.34c | 0.004/0.003c |
| tPA | −0.28 | 0.009 |
| Septal mitral annulus tissue Doppler E′/A′ ratio | ||
| PSEM | −0.30/−26c | 0.009/0.03c |
| Mean blood pressure | −0.29 | 0.01 |
| Complement C3 levels | −0.28 | 0.009 |
| Mitral annulus tissue Doppler E′ velocity | ||
| PSEM | −0.35/−0.33c | <0.001/0.002c |
| tPA | −0.29 | 0.005 |
| Mean blood pressure | −0.25 | 0.02 |
| ASA/warfarin | −0.20 | 0.04 |
| Lateral mitral annulus tissue Doppler E/E′ ratio | ||
| PSEM | 0.34/0.34c | 0.002/0.003c |
| Non‐neuro‐SLICC | 0.32 | 0.004 |
| Average mitral annulus tissue Doppler E/E′ ratio | ||
| PSEM | 0.26/0.27c | 0.02/0.02c |
| SLE duration | 0.31 | 0.007 |
Abbreviations: ASA, aspirin; LV, left ventricular; LVMI, left ventricular mass index; PSEM, pressure‐strain elastic modulus; SD, standard deviation; SLE, systemic lupus erythematosus; SLICC, Systemic Lupus International Collaborating Clinics; tPA, tissue plasminogen antigen.
Multivariate models were determined by stepwise variable selection and verified by backward regression. Candidate predictor variables include all variables statistically significant and clinically meaningful in univariate analyses.
Standardized β represent the number of SD change in the outcome for 1 SD change in the predictor variable (ie, a 1‐SD increase in PSEM is associated with a 0.32‐SD decrease in mitral E/A ratio).
Standardized β and P value when LVMI was added in the model.
Figure 1.
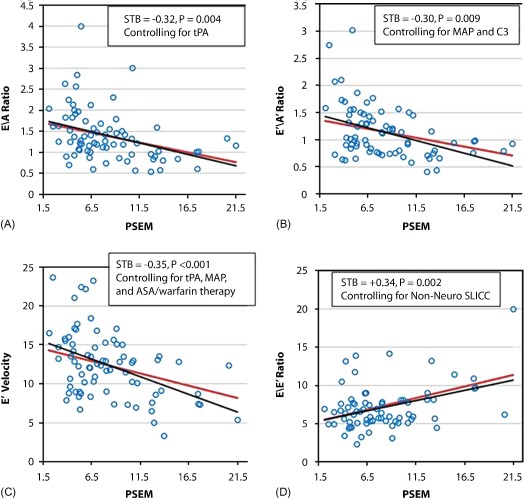
Association of aortic stiffness with LV diastolic dysfunction. Independent association of PSEM (units) with (A) mitral E/A ratio, (B) E′/A′ ratio, (C) E′ peak velocity, and (D) E/E′ ratio (P ≤ 0.009 for each parameter and P < 0.001 by rANOVA as a profile). The association of PSEM with LV diastolic dysfunction was computed as least squares means over the range of PSEM values, controlling for relevant covariates set to their mean values (red lines). The unadjusted linear regression fits (black lines) to the scatter plots of raw data (open blue circles) are shown for comparison purposes. Abbreviations: ASA, aspirin; C3, complement C3; LV, left ventricular; MAP, mean arterial blood pressure; PSEM, pressure‐strain elastic modulus; rANOVA, repeated measures analysis of variance; SLICC, Systemic Lupus International Collaborating Clinics; STB, standardized β; tPA, tissue plasminogen antigen.
Discussion
Major Findings
There are 3 major findings in this study: (1) SLE patients as compared with controls have a higher degree of aortic stiffness independently of atherogenic risk factors including hypertension and aortic atherosclerosis, have higher LV mass, and have a greater degree of LV diastolic dysfunction; (2) in SLE patients, aortic stiffness is correlated with increased LV mass, LA volume, and LV diastolic dysfunction; and (3) in SLE patients, aortic stiffness (even after adjusting for LVMI), MAP, inflammation (complement C3 levels and SLE damage score), thrombogenesis (tPA levels and antithrombotic therapy), and SLE duration are independently associated with LV diastolic dysfunction.
To our knowledge, this is the first study to demonstrate that aortic stiffness is independently associated with LV diastolic dysfunction in SLE even after adjusting for blood pressure and LVMI. These findings suggest that SLE and other autoimmune diseases–associated chronic or recurrent systemic inflammation may cause endothelial dysfunction and apoptosis, increased thrombogenesis, smooth‐muscle‐cell proliferation, intima‐media thickening, vessel stiffness and increased impedance, and consequently prehypertension or hypertension—which, in combination and a self‐perpetuating vicious cycle, may cause further aortic stiffness, increased blood pressure, and LV afterload; may or not increase LV mass; and ultimately may cause LV diastolic dysfunction.8, 13, 14, 15 Arterial hypertension is highly prevalent (29% to 36%) in SLE patients.3, 13, 16 In this study, SLE patients had higher nonhypertensive‐range blood pressures than controls, 10 patients (13%) were hypertensive on enrollment, 24 patients (32%) were either ambulatory hypertensive or on vasodilator therapy, and aortic stiffness was highly correlated with hypertension or vasodilator therapy (standardized β: 0.37, P = 0.001). The rates of hypertension in this study are similar to those reported in studies of premenopausal women with SLE16, 17 but higher than those reported in similar general populations (∼5%).18
Aortic stiffness in SLE may be associated with reduced coronary flow reserve or microvascular coronary artery disease, leading to LV diastolic dysfunction.8, 14, 15, 19 Previous series demonstrated decreased in coronary flow reserve during dipyridamole or adenosine Doppler TTE and vasodilatory stress‐induced reversible, fixed, or mixed myocardial perfusion defects in young SLE patients with normal or minimally diseased epicardial coronary arteries on angiography.19, 20 In this study, 17 patients (22%) had atherosclerosis of the descending thoracic aorta, and other studies have reported an association of carotid arterial stiffness with similar rates of subclinical carotid and coronary atherosclerosis.21, 22
Comparison With Previous Studies
To our knowledge, there are no previous studies correlating aortic stiffness with LV diastolic dysfunction in autoimmune inflammatory diseases, including SLE. Previous studies using TTE for assessment of LV diastolic dysfunction and arterial tonometry for assessment of carotid‐to‐femoral‐artery pulse wave velocity as an indicator of arterial stiffness have shown that SLE patients do develop premature LV diastolic dysfunction and arterial stiffness independently of age and hypertension, but an association of arterial stiffness with LV diastolic dysfunction was not reported in those studies.2, 3, 4, 5, 6, 23, 24
Study Limitations
The strength of association of aortic stiffness with LV diastolic dysfunction may have been underestimated by (1) study of a low proportion (8%) of patients with renal dysfunction and therefore of less severe SLE, (2) exclusion of patients age >60 years with expected longer SLE duration and greater vascular disease, (3) no application of myocardial strain and strain rate for detection of LV diastolic dysfunction, and (4) assessment of aortic stiffness and LV diastolic dysfunction during conscious sedation. Although the study lacks a gold standard of invasively measured LV end‐diastolic pressure, studies in non‐SLE populations have shown a high correlation of LV diastolic dysfunction by Doppler echocardiography with that by cardiac catheterization.25, 26 Finally, the use of 24‐hour ambulatory blood‐pressure monitoring and blood pressure assessed in response to stressors may have reflected better the prevalence and impact of systemic hypertension on aortic stiffness and LV diastolic function in both study groups (SLE patients and controls). In this study, systemic hypertension was more common during a stressor such as TEE than during enrollment clinical evaluation (22% vs 13% in SLE patients and 12% vs 0% in controls, respectively), and therefore the prevalence and effect of systemic hypertension may have been overestimated. However, after adjustment to blood pressure and exclusion of subjects with hypertension during TEE and initial clinical evaluation, aortic stiffness and LV diastolic dysfunction were still higher in patients than in controls. Thus, these tests should be considered in future studies of the association of systemic hypertension, aortic stiffness, and LV diastolic dysfunction.
Conclusion
Systemic lupus erythematosus‐associated aortic stiffness may not only be associated with, but also may be a marker or risk factor for, hypertension, LV hypertrophy, and LV diastolic dysfunction.17, 21, 22, 27 This study suggests that subsets of SLE patients are at higher risk for LV diastolic dysfunction and include those with (1) longer SLE duration, (2) severe SLE (high damage score), (3) elevated acute‐phase reactants (C3 levels), (4) hypercoagulability (requiring antithrombotic therapy or with elevated tPA), and (5) inadequately treated hypertension. Therefore, an earlier diagnosis of SLE; effective immunosuppressive, anti‐inflammatory, and antithrombotic therapy; and treatment of commonly associated traditional atherogenic risk factors may prevent the development or progression of aortic stiffness, hypertension, atherosclerosis, LV diastolic dysfunction, and clinical diastolic heart failure. In fact, immunosuppressive therapy, statins, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers or calcium channel blockers, and antiplatelet therapy have shown to have a beneficial effect on aortic stiffness in SLE and non‐SLE populations.28, 29, 30 The findings of this study may also apply to other autoimmune‐mediated inflammatory diseases such as rheumatoid arthritis, spondyloarthropathies, scleroderma, and polymyositis/dermatomyositis. Furthermore, in SLE patients undergoing TEE for appropriate indications, assessment of aortic stiffness and LV diastolic dysfunction is feasible and clinically relevant. Finally, a larger controlled cross‐sectional and longitudinal study is needed to confirm our findings and determine the short‐ and long‐term prognosis and effect of therapy on the incidence and progression of aortic stiffness and LV diastolic dysfunction in young adult SLE patients.
Supporting information
Figure S1 A‐C: Normal aortic stiffness and normal LV diastolic function in a 27 year old female with SLE. A. Short axis M‐mode of the mid descending thoracic aorta showing systolic (SD) and diastolic (DD) diameters of 17.3 and 16 mm, respectively. The calculated PSEM was 7.9 Pascal units. B. Pulse wave Doppler of the mitral inflow showing an E/A ratio of 1.42. C. Septal mitral annulus tissue Doppler showing an E'/A' ratio of 1.5, isovolumic relaxation time (IVRT) of 60 msec, and a mitral E/septal E' ratio of 7.3. D‐F. Abnormal aortic stiffness and LV diastolic dysfunction in a 38 year old female with SLE. D. Short axis Mmode of the mid descending thoracic aorta showing systolic and diastolic diameters of 19 and 18 mm, respectively. The calculated PSEM was 11.04 Pascal units. E. Mitral inflow showing an E/A ratio of 0.57. F. Septal mitral annulus tissue Doppler showing low E' peak velocities of 4.0 cm/sec, E'/A' ratio of 0.5, IVRT of 87 msec, and a mitral E/septal E' ratio of 10.5.
Table S1 Clinical and Laboratory Data in SLE Patients and Controls
Table S2 Clinical, Therapy, and Laboratory Data in SLE Patients
Table S3 Pulse Pressures, Differential Aortic Diameters, and Aortic Intima‐Media Thickness in SLE Patients and Controls
Table S4 Correlation of Aortic Strain, Stiffness, and Distensibility with Left Ventricular Diastolic Dysfunction in SLE Patients
Acknowledgments
The authors thank Dr. Warren Laskey for his critical review of the manuscript; Julia Middendorf, RN, for her outstanding job in the coordination of this study; and Diana Maynard, RCDS, for performing measurements of left atrial volumes.
Carlos A. Roldan, MD, designed and conducted the study, performed and interpreted transesophageal echocardiograms and measured left ventricular end‐diastolic diameters and aortic wall intima‐media thicknesses, analyzed and interpreted the data, and wrote the manuscript. Ihab B. Alomari, MD, participated in the conduction of the study, performed measurements of left ventricular diastolic function, participated in the analysis and interpretation of the data, and reviewed and edited the manuscript. Khaled Awad, MD, participated in the conduction of the study, performed measurements of left ventricular diastolic function, participated in the analysis and interpretation of the data, and reviewed and edited the manuscript. Nathan M. Boyer, MD, participated in the conduction of the study, performed measurements of aortic diameters, participated in the analysis and interpretation of the data, and reviewed and edited the manuscript. Clifford R. Qualls, PhD, participated in the design of the study, managed the databases, performed all statistical analyses, and critically reviewed and edited the manuscript. Ernest R. Greene, PhD, participated in the design and conduction of the study, participated in the analysis and interpretation of the data, and critically reviewed and edited the manuscript. Wilmer L. Sibbitt Jr, MD, participated in the design and conduction of the study, recruited and evaluated SLE patients, participated in the analysis and interpretation of the data, and critically reviewed and edited the manuscript.
This research was funded by the grant RO1‐HL04722‐01‐A6 by the National Institutes of Health, National Heart, Lung, and Blood Institute, and in part by the National Center for Research Resources and National Center for Advancing Translational Sciences through grant number 8UL1‐TR00004‐1. The funding institution was not involved in the analyses of the data or writing of the manuscript.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Becker‐Merok A, Nossent J. Prevalence, predictors and outcome of vascular damage in systemic lupus erythematosus. Lupus. 2009;18:508–515. [DOI] [PubMed] [Google Scholar]
- 2. Bjarnegråd N, Bengtsson C, Brodszki J, et al. Increased aortic pulse wave velocity in middle aged women with systemic lupus erythematosus. Lupus. 2006;15:644–650. [DOI] [PubMed] [Google Scholar]
- 3. Roldan CA, Joson J, Qualls CR, et al. Premature aortic stiffness in systemic lupus erythematosus by transesophageal echocardiography. Lupus. 2010;19:1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SW, Park MC, Park YB, et al. E/E′ ratio is more sensitive than E/A ratio for detection of left ventricular diastolic dysfunction in systemic lupus erythematosus. Lupus. 2008;17:195–201. [DOI] [PubMed] [Google Scholar]
- 5. Buss SJ, Wolf D, Korosoglou G, et al. Myocardial left ventricular dysfunction in patients with systemic lupus erythematosus: new insights from tissue Doppler and strain imaging. J Rheumatol. 2010;37:79–86. [DOI] [PubMed] [Google Scholar]
- 6. Blasco Mata LM, Acha Salazar O, González‐Fernández CR, et al. Systemic lupus erythematosus and systemic autoimmune connective tissue disorders behind recurrent diastolic heart failure. Clin Dev Immunol. 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nossent J, Cikes N, Kiss E, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus. 2007;16:309–317. [DOI] [PubMed] [Google Scholar]
- 8. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging. J Am Coll Cardiol. 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 9. Roldan CA, Joson J, Sharrar J, et al. Premature aortic atherosclerosis in systemic lupus erythematosus a controlled transesophageal echocardiographic study. J Rheumatol. 2010;37:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godia EC, Madhok R, Pittman J, et al. Carotid artery distensibility a reliability study. J Ultrasound Med. 2007;26:9:1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Appleton CP, Gilbert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 13. Barnes JN, Nualnim N, Sugawara J, et al. Arterial stiffening, wave reflection, and inflammation in habitually exercising systemic lupus erythematosus patients. Am J Hypertens. 2011;24:1194–1200. [DOI] [PubMed] [Google Scholar]
- 14. Zardi EM, Afeltra A. Endothelial dysfunction and vascular stiffness in systemic lupus erythematosus: are they early markers of subclinical atherosclerosis? Autoimmun Rev. 2010;9:684–686. [DOI] [PubMed] [Google Scholar]
- 15. El‐Magadmi M, Bodill H, Ahmad Y, et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404. [DOI] [PubMed] [Google Scholar]
- 16. Sabio JM, Vargas‐Hitos JA, Navarrete‐Navarrete N, et al. Grupo Lupus Virgen de las Nieves. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026–1032. [DOI] [PubMed] [Google Scholar]
- 17. Urowitz MB, Gladman D, Ibañez D, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus. 2007;16:731–735. [DOI] [PubMed] [Google Scholar]
- 18. Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 19. Hirata K, Kadirvelu A, Kinjo M, et al. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56:1904–1909. [DOI] [PubMed] [Google Scholar]
- 20. Sella EM, Sato EI, Barbieri A. Coronary artery angiography in systemic lupus erythematosus patients with abnormal myocardial perfusion scintigraphy. Arthritis Rheum. 2003;48:3168–3175. [DOI] [PubMed] [Google Scholar]
- 21. Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003:349:2407–2415. [DOI] [PubMed] [Google Scholar]
- 22. Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. [DOI] [PubMed] [Google Scholar]
- 23. Selzer F, Sutton‐Tyrrell K, Fitzgerald SG, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50:151–159. [DOI] [PubMed] [Google Scholar]
- 24. Yildiz M, Yildiz BS, Soy M, et al. Impairment of arterial distensibility in premenopausal women with systemic lupus erythematosus. Kardiol Pol. 2008;66:1194–1201. [PubMed] [Google Scholar]
- 25. Hsiao SH, Chiou KR, Lin KL, et al. Left atrial distensibility and E/e′ for estimating left ventricular filling pressure in patients with stable angina a comparative echocardiography and catheterization study. Circ J. 2011;75:1942–1950. [DOI] [PubMed] [Google Scholar]
- 26. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures a comparative simultaneous Doppler‐catheterization study. Circulation. 2000;102:1788–1794. [DOI] [PubMed] [Google Scholar]
- 27. Ryan MJ. The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1258–R1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soltész P, Kerekes G, Dér H, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. 2011;10:416–425. [DOI] [PubMed] [Google Scholar]
- 29. Avalos I, Chung CP, Oeser A, et al. Aspirin therapy and thromboxane biosynthesis in systemic lupus erythematosus. Lupus. 2007;16:981–986. [DOI] [PubMed] [Google Scholar]
- 30. Ferreira GA, Navarro TP, Telles RW, et al. Atorvastatin therapy improves endothelial‐dependent vasodilation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology (Oxford). 2007;46:1560–1565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 A‐C: Normal aortic stiffness and normal LV diastolic function in a 27 year old female with SLE. A. Short axis M‐mode of the mid descending thoracic aorta showing systolic (SD) and diastolic (DD) diameters of 17.3 and 16 mm, respectively. The calculated PSEM was 7.9 Pascal units. B. Pulse wave Doppler of the mitral inflow showing an E/A ratio of 1.42. C. Septal mitral annulus tissue Doppler showing an E'/A' ratio of 1.5, isovolumic relaxation time (IVRT) of 60 msec, and a mitral E/septal E' ratio of 7.3. D‐F. Abnormal aortic stiffness and LV diastolic dysfunction in a 38 year old female with SLE. D. Short axis Mmode of the mid descending thoracic aorta showing systolic and diastolic diameters of 19 and 18 mm, respectively. The calculated PSEM was 11.04 Pascal units. E. Mitral inflow showing an E/A ratio of 0.57. F. Septal mitral annulus tissue Doppler showing low E' peak velocities of 4.0 cm/sec, E'/A' ratio of 0.5, IVRT of 87 msec, and a mitral E/septal E' ratio of 10.5.
Table S1 Clinical and Laboratory Data in SLE Patients and Controls
Table S2 Clinical, Therapy, and Laboratory Data in SLE Patients
Table S3 Pulse Pressures, Differential Aortic Diameters, and Aortic Intima‐Media Thickness in SLE Patients and Controls
Table S4 Correlation of Aortic Strain, Stiffness, and Distensibility with Left Ventricular Diastolic Dysfunction in SLE Patients


