Abstract
Background
Glioblastoma is a fatal brain tumor in dire need of effective therapy. Treatments with both oncolytic viruses and immunotherapy have shown preclinical efficacy and clinical promise. We sought to exploit possible synergies between oncolytic herpes simplex virus – type I (oHSV-1) infection of intracranial gliomas and delivery of immune stimulating fms-like tyrosine kinase 3 ligand (Flt3-L) by engineering a herpes vector to express the cytokine.
Objective
To construct an oHSV-1 vector that expresses high levels of Flt3-L and examine its antiglioma efficacy in an immunocompetent murine model.
Methods
G47Δ and a bacterial artificial chromosome (BAC) system were used to generate a novel oHSV-1, termed G47Δ-Flt3L, expressing Flt3-L. Cytokine expression was confirmed, and G47Δ-Flt3L was injected intratumorally into established intracranial CT-2A gliomas in syngeneic C57/Bl6 mice. Animals were followed for survival and assessed by the Kaplan-Meier method.
Results
G47Δ-Flt3L expressed high levels of Flt3-L in culture. Expression of Flt3-L impacted neither viral replication nor had a cytotoxic effect against CT2A glioma cells. Direct inoculation into intracerebral CT2A glioma cells resulted in high levels of detectable Flt3-L in mouse blood and was superior to parental G47Δ at prolonging survival in glioma-bearing animals.
Conclusion
Treatment with G47Δ-Flt3L improves survival of glioma-bearing mice.
Keywords: brain tumor, Flt3-ligand, glioma, herpes, immunotherapy, oncolytic virus, vaccine
Recently, there has been increased interest in immunotherapy approaches for treatment of malignant glioma. Better understanding of the cellular entities that drive or suppress antitumor immunity, enhanced ex vivo cellular engineering techniques, and recognition of a growing number of glioma-associated antigens have lead to successful preclinical models of vaccination, and early-phase clinical trials have demonstrated safety, systemic biological effect, and suggestions of disease stabilization and extended survival. Currently, phase II multicenter dendritic cell vaccination1 and epidermal growth factor variant III (EGFRvIII) peptide vaccination2 protocols are being conducted for patients with newly diagnosed glioblastoma.
Although the immune system is able to develop antibody and T-lymphocte responses against growing glioblastomas, tolerance wins out over antitumor immunity, and the tumor effectively shields itself from immune effectors. Therefore, the key to clinical efficacy is the successful breaking of tolerance. In some fashion, tumor-associated antigens require unveiling so that they can be presented to effector lymphocytes which can be activated and positioned to infiltrate and target the tumor. Given the lack of draining lymphatics in the central nervous system and the lack of potent antigen presenting cells in the immunosuppressed brain tumor microenvironment, driving an effective anti-glioma response presents particular challenges.
Treatment of malignant tumors with oncolytic herpes simplex virus 1 (oHSV) vectors is promising because of the opportunity to target cancerous cells while sparing neighboring normal tissues. Cancer clinical trials examining direct intratumoral or intravascular injection of oHSV in patients with solid tumors inside and outside of the brain have been completed without evidence of treatment-associated toxicity and with some objective clinical and radiographic responses3–6. The dynamic interplay between oHSV with the immune system is a critical factor in understanding how to optimize the vigor and the durability of the antitumor effect7. As expected, antiviral immunity develops or re-emerges after infection and can limit the viral replicative cycle and abrogate the direct cytocidal impact of the therapy8. In fact, pre-infection suppression of innate immunity with cyclophosphamide or inhibitors of complement is associated with enhanced oHSV replication and tumor killing in rodent models. Our group and others have demonstrated that oHSV infection of flank tumors initiates an inflammatory cascade that results in the development of systemic and specific adaptive antitumor immunity9. In an effort to take advantage of this anticancer vaccine effect, investigators have armed oHSV with genes for immunostimulatory cytokines such as GM-CSF6 and IL-1210, which have variably yielded improved tumor control in several models.
Dendritic cells (DCs) are professional antigen-presenting cells that have the capacity to migrate to sites of inflammation, to ingest and process antigenic material, and, then, to traffic to draining lymph nodes where cross-presentation of tumor antigens to lymphocyte receptors occurs. DCs may represent the link between the initial innate immune response to viral infection and subsequent adaptive antiviral or, antitumor immunity. This is underscored by the fact that combining oHSV infection of flank tumors with intratumoral injection of ex vivo generated immature DC’s generates a powerful antitumor immune response that is nearly 100% curative 11. oHSV infection appears to break tolerance to tumors by exposing tumor-associated antigens and by elaboration of inflammatory danger signals, but the subsequent enhancement of antigen presentation by DCs appears to be requisite 12, 13. We, therefore, set out to engineer an oHSV that expresses soluble Flt3L, a cytokine and growth factor associated with the development of hematopoietic precursors into both plasmacytoid (pDCs) and conventional (cDCs) dendritic cells, as well as their mobilization out of bone marrow 14. We hypothesized that infection of an intracranial glioma by an oHSV armed with Flt3-L transgene would exert an antitumor immune effect by creating an inflammatory environment in situ, while systemically mobilizing DC precursors.
Materials and Methods
Engineering and production of virus
Flt3 Ligand shuttle vector construction
The open reading frame of human fms-like tyrosine kinase 3 ligand (hFlt3-L), as determined by Immunex was obtained in a pBluescript II SK plasmid from Epoch Biolabs 15. Human Flt3L has high homology with the murine product, and has activity in mice. All experiments herein utilize the human sequence and protein. The sequences containing a 5′ BamH1 restriction site followed by a kozak sequence, the hFlt3-L ORF and a 3′ Not1 restriction site were inserted into an EcoRV-digested pBluscript II SK derivative from Stratagene. The pFLS-Express #4 shuttle vector containing the CMV promoter followed by multiple cloning sites (provided by Dr. Toshihiko Kuroda, Massachusetts General Hospital) was used for inserting the Flt3L transgene into the G47ΔBAC, a bacterial artificial chromosome (BAC) containing the entire genome of oncolytic HSV-1 G47Δ 16, 17. The BamH1/Not1 fragment of the hFlt3L cDNA from the hFlt3L-containing pBluescript II SK was subcloned to the BamH1/ Not1 sites of the pFLS-Express #4 shuttle vector. Insertion of the hFlt3L transgene was confirmed by sequencing.
Confirmation of shuttle vector expression of Flt3L
To determine expression and secretion of hFlt3L from the shuttle vectors containing the hFLT3-L inserts, the plasmids were transfected into 293T cells Lipofectamine™ 2000. Supernatants were collected 48 and 72 hours later and cytokine levels quantified using human Flt-3 Ligand Quantikine ELISA Kit (cat# DFK00) from R&D Systems.
Insertion of Flt3-Ligand into G47ΔBAC backbone
The process of inserting a transgene into the G47ΔBAC viral vector has previously been described 18, 19. Briefly, the G47ΔBAC plasmid was incubated with each of the shuttle plasmids containing the Flt3L inserts in the setting of Cre recombinase. The recombined DNA was then electroporated into DH10B E. coli and selected for kanamycin and chloramphenicol resistance. The resulting bacterial colonies were harvested and grown for DNA mini-prep. The purified DNA was then subjected to HindIII restriction digest and electrophoresis. As a control, the shuttle plasmid without Flt3L cDNA sequences was used to generate “G47ΔBAC-empty.” Restriction analysis confirmed the expected recombination events in all bacterial clones for both recombined DNAs, i.e., G47ΔBAC-Flt3L, and G47ΔBAC-empty (data not shown).
Confirmation of Cytokine Expression by Recombinant G47Δ-Flt3L
After confirmation of Cre-recombination, G47ΔBAC-Flt3L or G47ΔBAC-empty were co-transfected into Vero cells with an FLPe-expressing plasmid using lipofectamine plus (Invitrogen). FLPe removes the BAC-derived sequence, allowing viral particle production. Crude virus was obtained by collecting the supernatant, and clones were obtained by plaque purification. Virus was then titered by infection of Vero cells. After titration, Vero cells were infected at an MOI of 0.1, and supernatant was collected after 24 hours for hFlt3L quantification by ELISA. All Flt3L ELISA assays were performed using the protocol from the Human Flt3 Ligand Quantikine ELISA Kit (cat# DFK00) from R&D Systems.
Viral Replication Assay
Viral replication of clones of G47Δ-Empty and G47Δ-Flt3L was assessed by plaque assay on Vero cells. Vero cells were plated at a concentration of 4x105 per well in 6-well plates. After adherence, they were infected with 4.0x103 PFUs of multiple clones of each virus (MOI=0.01) and incubated for 12, 36, and 60 hours. At each time point, cells and supernatant were harvested and subjected to three freeze/thaw cycles. Released virus was used to infect Vero cells, and 72 hours later cells were stained with X-gal and neutral red. X-gal positive plaques were counted and used for calculation of viral yield.
Oncolytic Virus Cytotoxicity Assay
G47Δ-Flt3L and G47Δ-Empty in MOIs ranging from 0.001 to 10 were used to infect 1x104 CT-2A cells plated in each well of 96-well plates. The 96-well plates were incubated for 72 hours and subjected to counting via a MTS cell proliferation assay, following the manufacturer’s protocol (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS), cat# G5421, Promega).
Plasma analysis of hFlt3L
To confirm in vivo secretion of cytokine by the G47Δ-Flt3L virus, plasma from treated animals was harvested 36, 72, and 168 hours after intratumoral viral injection and subjected to ELISA [Human Flt3 Ligand Quantikine ELISA Kit (cat# DFK00), R&D Systems]. Becton Dickinson yellow top Vacutainer® collection tubes (Cat#364606) were used to collect plasma from mice treated with PBS, 2x106 PFU G47, or 2x106 PFU G47Δ-Flt3L at the indicated time points.
Cell lines
The 20-methylcholanthrene induced, murine anaplastic astrocytoma model CT-2A syngeneic to C57Bl/6 (kindly provided by Dr. Thomas Seyfried, Boston College) was used for all animal experiments 20, 21. Cells were maintained in RMPI-1640 (Cellgro, Cat#10-040-CV) containing 10% FBS (Cellgro, Cat#35-010-CV) and 1% penicillin/streptomycin (Cellgro, Cat#, 30-002-Cl) in a 37°C humidified incubator supplemented with 5% CO2. CT-2A cells stably expressing firefly luciferase (fluc) and mCherry were constructed by transducing cells with the lentiviral vector CSCW2-Fluc-ImC (kindly provided by Dr. Miguel Sena-Esteves, University of Massachusetts, Worcester) that contains the fluc-IRES-mCherry cassette under the immediate early cytomegalovirus promoter 22. These cells were termed CT-2A-fluc and were used for all animal experiments to track intracranial growth by bioluminescence. CT-2A-fluc cells were sorted on a custom BD Biosciences LSRII with a 593nm laser for the highest expressing mCherry cells.
Treatment and efficacy study of syngeneic mouse model of CT-2A-fluc glioma
The procedure for intracerebral tumor implantation has already been described 23. Briefly, cells were loaded into a 250μL Hamilton syringe attached to a 25-gauge needle (Hamilton, Reno, NV) in a 1:1 volumetric mixture with 3% methylcellulose (Sigma, Cat# 9004-67-5). With the aid of a model 900 small animal stereotactic instrument (Kopf Instruments) 5000 CT-2A-fluc cells were injected in the right frontal lobe of anesthesized 6-8-week-old female C57/BL6 mice obtained from the National Cancer Institute (NCI) Frederick, MD. On day 6 post-tumor implantation, mice were divided into three groups of ten and treated with PBS, 2x106 PFU G47Δ-empty, or 2x106 PFU G47Δ-hFLT3-L injected intra-tumorally using a 10μL Hamilton syringe (Hamilton, Reno, NV). Animals were followed for survival and similarly treated animals were used for subsequent ancillary studies.
Bioluminescence Imaging
Animals with intracranial tumors were monitored for tumor growth by bioluminescence using a custom Xenogen IVIS Imaging System 100 built at the Center for Molecular Imaging Research, Massachusetts General Hospital (by Dr. Ralph Weissleder). 300μL of 15mg/mL D-Luciferin potassium salt (Caliper life sciences Cat# 122796) was injected into each animal. Every five minutes photons emitted per second was measured and the peak values determined. Animals were monitored daily after tumor implantation in order to establish the first day of statistically significant tumor growth compared to normal mice. All animal procedures were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital and were performed at least twice.
Biostatistical Analysis
Survival curves were compared by the Kaplan-Meier method using the Log-rank test. Means were compared by either 2-tailed Students’ t-test, analysis of variance (ANOVA), or Two-way ANOVA with Bonferroni post-hoc comparisons. Statistical differences were considered significant if P-values were less than or equal to 0.05. Graphpad Prism software was used for all analyses.
RESULTS
Recombinant virus construction, Flt3L expression, and in vitro replication and cytotoxicity
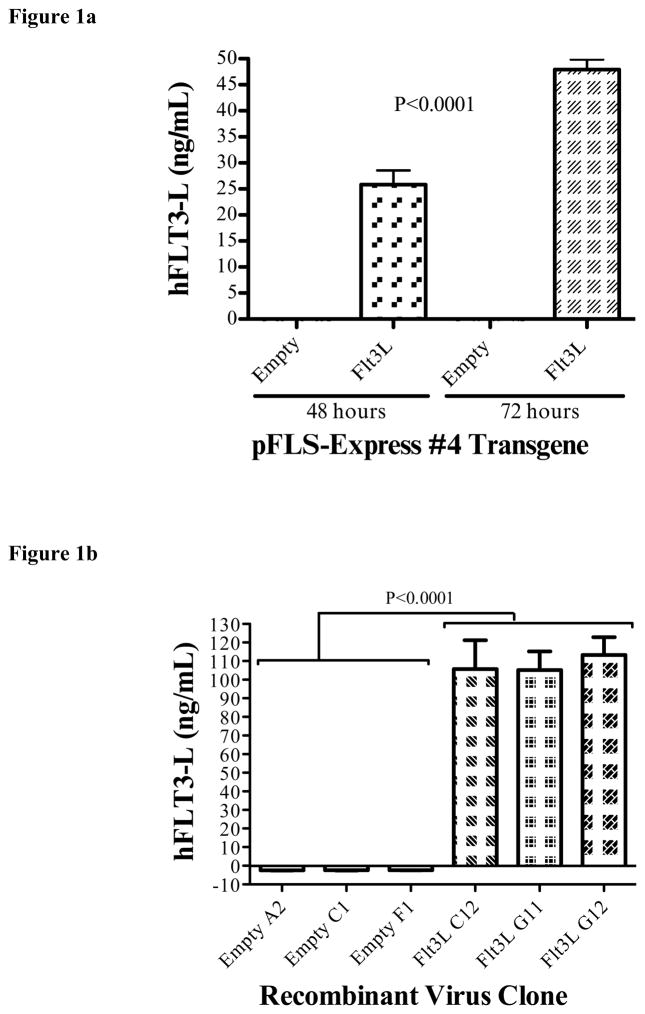
Throughout the process of recombinant virus engineering, we performed validation of sequence, transgene expression, and viral cytotoxicity. Correct ligation of hFlt3L fragments into the pFLS-Express #4 shuttle vector was determined by DNA sequencing. Subsequently, shuttle vectors were transfected into 293T cells to validate proper protein expression and secretion in mammalian cells. ELISA of the transfected 293T supernatants demonstrated a statistically significant increase in the secretion of soluble hFlt3L in pFLS-Express #4-hFlt3L transfected cells compared to pFLS-Express #4 Empty transfected cells. Appropriately, Flt3L-expressing shuttle vectors yielded high levels of cytokine into the supernatant. In addition, after transfection with pFLS-Express#4-hFlt3L, Flt3L levels were significantly higher at 72 hours after transfection of 293 T cells than at 48 hours.(two-way ANOVA, P<0.0001, Fig. 1A).
Figure 1.
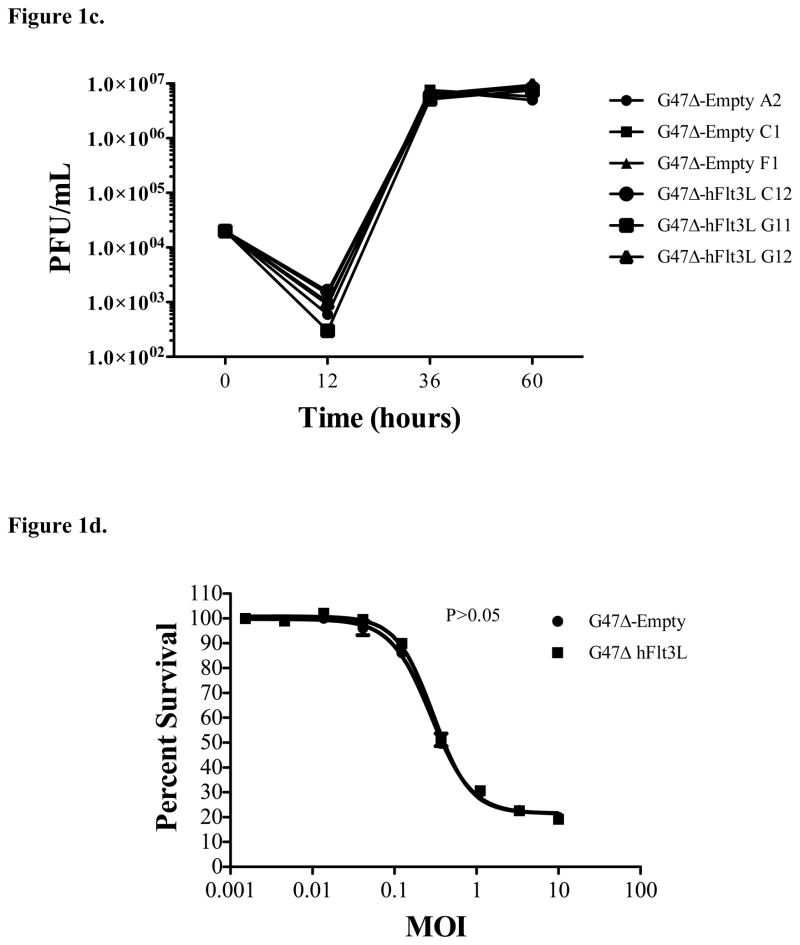
Construction of G47Δ-Flt3L. a) 293T cells were transfected with the shuttle vector containing the insert with the sequence for Flt3L. ELISA assay of the supernatants demonstrates high levels of Flt3-L at 48 hours, increased further at 72 hours. Supernatant at all time points from empty plasmid-transfected 293T cells did not contain detectable Flt3L. b) Quantification of Flt3-L expression by virus-infected Vero cells. Infection of Vero cells with plaque-purified G47Δ-Flt3L resulted in elaboration of high levels of Flt3-L into the supernatant. No Flt3L was detected after infection with G47Δ without transgene expression. c) Single burst assay for viral replication. Viral replication appears to be unaffected by expression of Flt3-L. D) MTS colorimetric assay examining in vitro cytotoxicity of oncolytic virus clones. CT-2A glioma cells were infected at varying MOI’s with G47Δ-empty and G47Δ-Flt3L and assessed for viability 72 hours later. Viral expression of Flt3L did not affect the number of viable cells.
After complete construction and sequence validation of the recombinant viruses, hFlt3-L ELISA was used to determine the ability of viral clones to infect mammalian cells and express soluble hFlt3-L protein. Twenty-four hours after Vero cell infection, the quantity of hFlt3L protein secreted into cell culture supernatant was compared between G47Δ-empty and G47Δ-Flt3L cultures. Results confirmed the absence of hFlt3-L expression in G47Δ-empty and high amounts of hFlt3-L in the G47Δ-Flt3Lsupernatant (Fig. 1B).
We sought to demonstrate that insertion and expression of the flt3L transgene did not impact virus replication and cytotoxic capacity. We, therefore, compared viral yields after infection of Vero cells by various clones at 12, 36, and 60 hours post infection. Viral yields for all tested clones, both with and without the flt3L insertion, were equivalent. (Fig. 1C). Cytotoxicity was determined by infecting CT-2A-fluc cells at MOIs between 0.001 and 10, then measuring the percentages of live cells by MTS assay. Both G47Δ-empty and G47Δ-Flt3L killed CT-2A-fluc cells in an MOI-dependent manner, and, as was the case with viral yield, there was no significant difference in cytocidal/oncolytic activity between the two viruses (Fig. 1D).
In summary, G47Δ-Flt3L expressed high levels of hFlt3L, replicated efficiently in mammalian cells, and killed CT-2A murine glioma cells with equal efficacy to G47Δ-empty.
Flt3L is detected in mouse plasma after G47Δ-Flt3L infection of intracranial syngeneic glioma
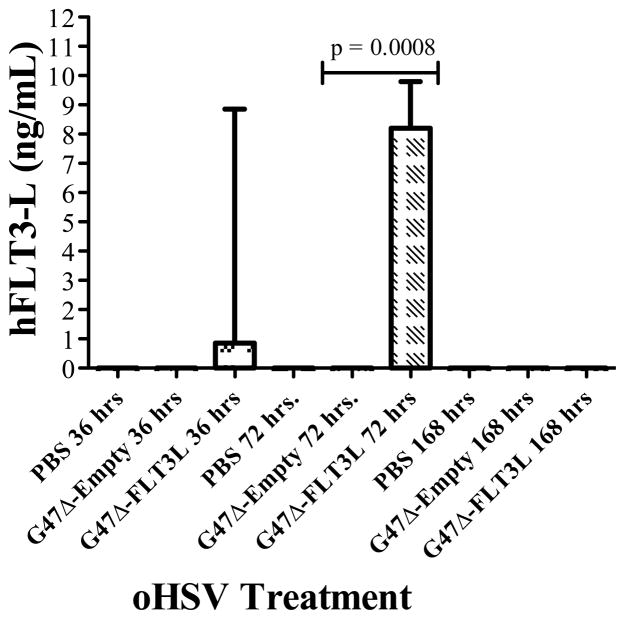
By bioluminescence, implanted CT-2A tumors were clearly viable and detectable against background by day 6 (data not shown). Therefore, we chose to treat tumors with stereotactic injection of virus and controls at this time point. Given that Flt3L can have both local and systemic effects, we sought to determine whether G47Δ-Flt3L infection of intracranial tumors resulted in an increase in levels of circulating cytokine by performing ELISA of plasma harvested via cardiac blood draw of glioma-bearing mice treated with vehicle control, G47Δ-empty, and G47Δ-Flt3L. Flt3L was not identified in the plasma of PBS and G47Δ-empty treated mice, but was detectable 36 hours after treatment with G47Δ-Flt3L. Peak levels were detected at 72 hours after infection. By 168 hours after treatment, Flt3L was no longer detectable in the plasma (Figure 2).
Figure 2.
ELISA assay demonstrating detectable levels of Flt3L in the plasma of G47Δ-Flt3L-treated C57/Bl6 mice. Flt3L was not detectable at any point in the plasma of mice treated with saline injection or G47Δ-Empty. In G47Δ-Flt3L-treated mice, Flt3L levels were higher at 72 hours than at 36 hours and were undetectable by 168 hours after injection. At 72 hours, the level of hFlt3L expression in the plasma of treated tumor-bearing mice was significantly higher in the G47Δ-Flt3L treated group compared to both saline and G47Δ-Empty treated group (p=0.0008, ANOVA and Bonferroni’s post-hoc analysis).
Intratumoral injection of G47Δ-Flt3L enhances survival in mice bearing syngeneic intracranial glioma tumors
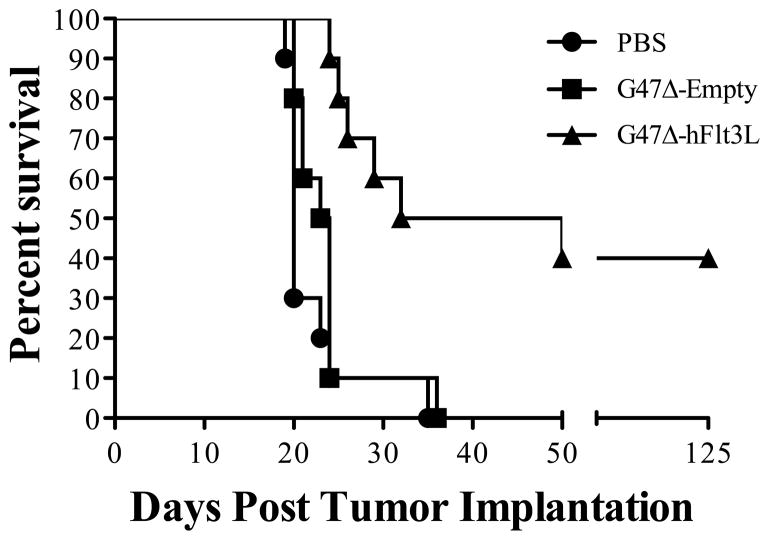
G47Δ effectively kills multiple cancer cell lines in vitro and has greater in vivo efficacy than does its parental virus, G207 16, 24. G47Δ is also effective against intracerebrally implanted gliomas derived from human glioblastoma stem cells 25. We sought to determine whether engineering G47Δ to express Flt3L impacted survival of immunocompetent mice bearing syngeneic intracranial gliomas. C57/Bl6 mice were stereotactically injected with luciferase-expressing CT-2A glioma cells into the right frontal lobe. On day 6 after implantation of CT-2A cells, in accordance with bioluminescence demonstration of viable tumor, 2 x 106 pfu’s of G47Δ-empty, G47Δ-Flt3L, or vehicle control were stereotactically injected into the tumor. Mice treated with G47Δ-Flt3L lived significantly longer than those treated with G47Δ-empty, and long-term survival was observed in 40% of animals (Figure 3). Parental G47Δ-empty, at this dose and schedule, had no impact when compared to treatment with control.
Figure 3.
Kaplan-Meier curves demonstrating that C57/Bl6 mice bearing intracranial syngeneic CT-2A gliomas survived significantly longer after treatment with one injection of 2 x 106 pfu’s of G47Δ-Flt3L than with parental G47Δ-empty (p<0.005, Log-rank test), itself, which was equivalent to PBS controls.
Discussion
In this study, we used a BAC system to generate a recombinant oncolytic virus expressing Flt3L. This is the first example using an oncolytic virus expressing Flt3L in a syngeneic glioma model. We demonstrated that infection of an intracranial tumor with a Flt3L -expressing oHSV generates high systemic levels of cytokine and improves survival.
The ability of oncolytic viruses to kill tumor cells directly provides several advantages over replication-deficient vectors. Cell death is followed by release of multiple viral progeny which infect surrounding cells, and the cycle is repeated. There is, therefore, the potential for spread throughout a tumor mass. Oncolytic cell death is itself a stimulus for the development of antitumor immunity, turning oncolytic therapy into a strategy with systemic possibilities, rather than limiting tumor killing to local cytotoxicity. We have shown that increasing the number of immature cDCs in this inflammatory microenvironment by direct injection enhances the immune effect in the treatment of subcutaneous tumors in mice 11, but this cellular approach may be cumbersome and expensive to clinically translate, particularly for intracranial tumors. Therefore, we set out to duplicate this effect by engineering a third-generation oHSV, G47Δ 16, to express the cytokine Flt3L. oHSV are particularly well-suited for insertion of transgenes because large segments of the genome can be deleted without incapacitating the replication and lytic machinery and thus leave space for large gene insertions 10, 26. Flt3L was chosen because of its function in dendritic cell precursor mobilization and development 27, 28.
With a single intratumoral injection, G47Δ-Flt3L more effectively treated mice bearing a syngeneic intracerebral glioma than did G47Δ without an inserted transgene. In vitro cytotoxicity was identical between the viruses, suggesting that the in vivo effects are related to the impact of the cytokine. Furthermore, viral infection in the intracerebral tumors had systemic effects, demonstrated by high levels of detectable Flt3L in plasma at 36 and 72 hours after infection in treated mice. While G47Δ has been shown to exert a potent antitumor effect in subcutaneous and intracranial tumor models, a single injection has not been shown to be effective against syngeneic intracranial tumors.
Flt3L is a growth factor and differentiation agent for numerous types of immune cells, and it can lead to increased numbers of pre-B cells, as well as enhanced maturation of T, B, and NK cells 29. Perhaps most relevant for cancer immunity is its capacity to expand both cDC and pDC subsets, including increasing numbers of immature and mature cells in bone marrow, spleen, lymph nodes, and peripheral blood. Flt3L, therefore, has putative advantages as an adjuvant to cancer vaccination as it immediately activates a diverse array of immune stimulator and effector cells.
Accordingly, a number of cancer therapies utilizing Flt3L have been examined. Early studies in animals demonstrated that systemic delivery of recombinant Flt3L was associated with both increased numbers of intratumoral DC’s and CTL’s, as well as with improved tumor control 30, 31. In phase I clinical trials, systemic administration of Flt3L has been safe and is associated with increased numbers of both circulating and peritumoral DCs 32, 33.
Most experimental approaches with Flt3L deliver cytokine via a gene or cell-based therapy approach. For instance, Curran and Allison recently demonstrated in mice that administering B16 melanoma cells engineered by gene transfer to express Flt3L may be associated with more effective immunity when combined with CTL-associated antigen 4 (CTLA-4) blockade than is delivery of GM-CSF-expressing cells 34. Furthermore, both the vaccination and tumor sites were more briskly infiltrated with CD8+ T lymphocytes, cDCs, and pDCs when Flt3L-expressing cells were used. Other approaches have seen enhanced effects by combining delivery of Flt3L, either intratumorally or systemically, with complementary cytokine-driven vaccination, such as RANTES 35, which is chemotactic for immature DCs 36, or interleukin 18 37, which induces Th1 immunity. Ramakrishna, et al. 38 have shown that codelivery of oncolytic adenoviruses expressing MIP-1α and Flt3L, respectively, is synergistic and triggers enhanced DC and T cell infiltration into inoculated tumors. In this study, infiltrating DCs were defined by CDllc positivity, not differentiating between plasmacytoid and conventional subsets.
As the resident cell population and barriers to immune cell trafficking may differ in the central nervous system, it is important to examine the impact of Flt3L in the brain and in brain tumors. In early work, Ali et al. demonstrated that injection of intracranial CNS-1 tumors in syngeneic Lewis rats with recombinant adenovirus engineered to express Flt3L, resulted in improved survival and increased infiltration of cells with DC markers, such as OX62 and MHCII 39. Later, the same group 40 demonstrated that infection of rat brain parenchyma with Ad-Flt3L led to an increased recruitment and activation of interferon-α-expressing pDCs, preferentially over cDCs or other inflammatory cells. From a therapeutic standpoint, this paradigm is maximized when in situ Flt3L delivery is coupled with local cell death, i.e., with oncolyis. In established rodent tumors, when neither approach as monotherapy was efficacious, combining Ad-Flt3L injection with HSV-TK delivery in the context of systemic ganciclovir, significantly improved survival 41. Tumor regression was associated with both humoral and cellular immunity 42, appeared to be dependent on activation of toll-like receptor (TLR) 2 on bone-marrow-derived DCs, and was mediated by high-mobility-group-box 1 (HMGB1)43. HMGB1 is a danger signal or “alarmin” released by dying/necrotic tumor cells, and is a TLR ligand associated with cytotoxic therapies such as radiation and chemotherapy, and, in this case, TK- mediated oncolysis. HMGB1 may be required for the maturation of human plasmacytoid DCs 44, as well as the migration of maturing DCs 45.
The efficacy of G47Δ-Flt3L theoretically depends on viral-mediated killing of tumor cells, release of tumor-associated antigens and danger signals, and increased numbers of circulating DCs, both plasmacytoid and conventional. It is likely that release of these oncolysis-associated factors, both locally and into the circulation, impacts differentiation, chemotaxis, and the activation/maturation status of antigen presenting cells resulting in antitumor immunity. In this paradigm, a single agent, G47Δ-Flt3L, is responsible for local and systemic immune effects that drive an enhanced antitumor immune response that is more potent that when treating with parental G47Δ. However, engineering the microenvironment for generation of more vigorous antitumor immunity may be also associated with intensified antiviral immunity and further studies of this are warranted.. The impressive in vivo effect of G47Δ-Flt3L against established syngeneic intracranial glioma merits further investigation of safety and efficacy in human trials and consideration of combination therapy with other immunomodulatory agents and with existing treatment regimens.
Acknowledgments
Funding
WTC – Amos Medical Faculty Development Plan of the Robert Wood Johnson Foundation.; Mass General Hospital Physician Scientist Development Award
RLM - Supported in part by NIH Grant NS-032677
Footnotes
Disclosures - none
References
- 1.Kim W, Liau LM. Dendritic cell vaccines for brain tumors. Neurosurg Clin N Am. Jan;21(1):139–157. doi: 10.1016/j.nec.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanaly CW, Ding D, Heimberger AB, Sampson JH. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg Clin N Am. Jan;21(1):95–109. doi: 10.1016/j.nec.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000 May;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 4.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009 Jan;17(1):199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong Y, Kim T, Bhargava A, et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol Ther. 2009 Feb;17(2):389–394. doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006 Nov 15;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 7.Agarwalla PK, Barnard ZR, Curry WT., Jr Virally mediated immunotherapy for brain tumors. Neurosurg Clin N Am. Jan;21(1):167–179. doi: 10.1016/j.nec.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Rainov NG, Zimmer C, Chase M, et al. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum Gene Ther. 1995 Dec;6(12):1543–1552. doi: 10.1089/hum.1995.6.12-1543. [DOI] [PubMed] [Google Scholar]
- 9.Todo T, Rabkin SD, Sundaresan P, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999 Nov 20;10(17):2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 10.Varghese S, Rabkin SD, Nielsen PG, Wang W, Martuza RL. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin Cancer Res. 2006 May 1;12(9):2919–2927. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- 11.Farrell CJ, Zaupa C, Barnard Z, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008 Dec 1;14(23):7711–7716. doi: 10.1158/1078-0432.CCR-08-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapteva N, Aldrich M, Rollins L, et al. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol Ther. 2009 Sep;17(9):1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapteva N, Aldrich M, Weksberg D, et al. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J Immunother. 2009 Feb-Mar;32(2):145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drexler HG, Quentmeier H. FLT3: receptor and ligand. Growth Factors. 2004 Jun;22(2):71–73. doi: 10.1080/08977190410001700989. [DOI] [PubMed] [Google Scholar]
- 15.Lyman SD, James L, Escobar S, et al. Identification of soluble and membrane-bound isoforms of the murine flt3 ligand generated by alternative splicing of mRNAs. Oncogene. 1995 Jan 5;10(1):149–157. [PubMed] [Google Scholar]
- 16.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Research. 2005 Dec 1;65(23):10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005 Dec 1;65(23):10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda T, Martuza RL, Todo T, Rabkin SD. Flip-Flop HSV-BAC: bacterial artificial chromosome based system for rapid generation of recombinant herpes simplex virus vectors using two independent site-specific recombinases. BMC Biotechnol. 2006;6:40. doi: 10.1186/1472-6750-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Murillo R, Martinez A. Standardization of an orthotopic mouse brain tumor model following transplantation of CT-2A astrocytoma cells. Histol Histopathol. 2007 Dec;22(12):1309–1326. doi: 10.14670/HH-22.1309. [DOI] [PubMed] [Google Scholar]
- 21.Seyfried TN, el-Abbadi M, Roy ML. Ganglioside distribution in murine neural tumors. Mol Chem Neuropathol. 1992 Oct;17(2):147–167. doi: 10.1007/BF03159989. [DOI] [PubMed] [Google Scholar]
- 22.Maguire CA, Meijer DH, LeRoy SG, et al. Preventing growth of brain tumors by creating a zone of resistance. Mol Ther. 2008 Oct;16(10):1695–1702. doi: 10.1038/mt.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson JH, Archer GE, Ashley DM, et al. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10399–10404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res. 2005 Feb 15;65(4):1532–1540. doi: 10.1158/0008-5472.CAN-04-3353. [DOI] [PubMed] [Google Scholar]
- 25.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009 Apr 15;69(8):3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ino Y, Saeki Y, Fukuhara H, Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res. 2006 Jan 15;12(2):643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 27.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996 Nov 1;184(5):1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik SH, Sathe P, Park HY, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007 Nov;8(11):1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 29.Dong J, McPherson CM, Stambrook PJ. Flt-3 ligand: a potent dendritic cell stimulator and novel antitumor agent. Cancer Biol Ther. 2002 Sep-Oct;1(5):486–489. doi: 10.4161/cbt.1.5.161. [DOI] [PubMed] [Google Scholar]
- 30.Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997 Jun;3(6):625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999 Dec 15;59(24):6028–6032. [PubMed] [Google Scholar]
- 32.Morse MA, Nair S, Fernandez-Casal M, et al. Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol. 2000 Dec 1;18(23):3883–3893. doi: 10.1200/JCO.2000.18.23.3883. [DOI] [PubMed] [Google Scholar]
- 33.Marroquin CE, Westwood JA, Lapointe R, et al. Mobilization of dendritic cell precursors in patients with cancer by flt3 ligand allows the generation of higher yields of cultured dendritic cells. J Immunother. 2002 May-Jun;25(3):278–288. doi: 10.1097/01.CJI.0000016307.48397.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009 Oct 1;69(19):7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song S, Liu C, Wang J, et al. Vaccination with combination of Fit3L and RANTES in a DNA prime-protein boost regimen elicits strong cell-mediated immunity and antitumor effect. Vaccine. 2009 Feb 11;27(7):1111–1118. doi: 10.1016/j.vaccine.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 36.Crittenden M, Gough M, Harrington K, Olivier K, Thompson J, Vile RG. Expression of inflammatory chemokines combined with local tumor destruction enhances tumor regression and long-term immunity. Cancer Res. 2003 Sep 1;63(17):5505–5512. [PubMed] [Google Scholar]
- 37.Saito T, Takayama T, Osaki T, et al. Combined mobilization and stimulation of tumor-infiltrating dendritic cells and natural killer cells with Flt3 ligand and IL-18 in vivo induces systemic antitumor immunity. Cancer Sci. 2008 Oct;99(10):2028–2036. doi: 10.1111/j.1349-7006.2008.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramakrishna E, Woller N, Mundt B, et al. Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses. Cancer Res. 2009 Feb 15;69(4):1448–1458. doi: 10.1158/0008-5472.CAN-08-1160. [DOI] [PubMed] [Google Scholar]
- 39.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004 Dec;10(6):1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtin JF, King GD, Barcia C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006 Mar 15;176(6):3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005 Aug 15;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009 Oct 1;15(19):6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009 Jan 13;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005 Jul;26(7):381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007 Jan;81(1):84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]