Abstract
Introduction
There are not any effective method to induce the innervation of urinary bladder wall graft after augmentation. Neurons from urinary bladder wall and omentium can not elongate and branch in graft because of lack of neurotrophic factors. The best source of these neurotrophic factors are Schwann cells which can be transplanted into urinary bladder wall graft. To transplant Schwann cells the proper amount of cells is needed which can be only obtain during in vitro Schwann cell cultivation. We introduce the results of Schwann cell isolation and in vitro cultivation.
Materials and methods
33 Wistar rats, males (350 gr.) were used in this study. Animal were divited into two groups (n = 15). Cell cultures were established in both groups on 5, 6, 7, 8 nad 9 day after nerve injury. In first group the digestion time with colagenase and trypsyne was 2.5 h and in second one 3.5 h.
Results
A larger number of cells were isolated from the degenerated sciatic nerve. Colonies of cells that morphologically resembled Schwann cells were visible by light microscopy on the second day of in vitro cultivation. Homogeneity of the primary cultures increased in the last day of cultivation to 60%.
Conclusions
Schwann cells isolation from predegenerated peripheral nerve is effective and can delivered require amount of cells for transplantation to urinary bladder graft.
Keywords: tissue engineering, urinary bladder, innervation, cell isolation, Schwann cells
INTRODUCTION
Regenerative medicine brings a promise to introduce new therapies based on tissue engineering methods for reconstructive urology. Such an approach has been already reported in a clinical trial by Atala et al. in 2006. Six patients underwent replacement of the urinary bladder with an in vitro constructed graft [1]. Nevertheless, there are still many problems to be solved in order to popularize regenerative medicine therapies in clinical practice. One of the most challenging issues is to achieve the integration of in vitro constructed tissue with host organism. To meet this objective the tissue engineered urinary bladder must be vascularized and innervated after replacement [2].
Despite the advances that have been made in the field of regenerative medicine, there is no method to induce the innervation of tissue-engineered urinary bladder. The storage functions of the urinary bladder depend entirely on the autonomic nervous system. Thus, the poorly regenerated neuronal network within the artificial urinary bladder wall can lead to urinary bladder dysfunction after such a surgery. Our previous study demonstrated poor innervation of a reconstructed urinary bladder in an animal model [3]. Neurons from urinary bladder wall and omentum cannot elongate and branch into the graft site because of the scarring processes that dominate in urinary tract healing and lack of a favorable environment for neuron regeneration. Neuronal regeneration is a very sophisticated process that needs very particular conditions to be effective [4].
Tissue engineering offers technologies for inducing urinary bladder wall innervation. Direct cellular supplementation with Schwann cells is the most promising among them.
The studies results indicated that Schwann cell implantation to the area of the regenerating peripheral nerve gap could prevent glial scar formation and induced proximal segment elongation to bridge the gap [5]. The idea of the use of Schwann cells in urology, in order to regenerate nervous fibers within urinary bladder, has not been reported. The proper amount of cells is needed for transplantation, which can be only obtained during their in vitro propagation. This is an introductive study to present the method of isolation and in vitro cultivation of Schwann cells.
MATERIALS AND METHODS
Thirty male rats (350 gr.) were used in the study. Animals were divided into equal groups (n =15), depended on time of nerve digestion. In all groups primary cultures were established from the sciatic nerve after transverse cut at 5, 6-, 7-, 8-, and 9-days after the nerve injury. The time of digestion with collagenase and trypsin in first group was 2.5 h and in second one 3.5 h.
Animal model of Wallerian degeneration
All procedures were carried out with the consent of the Local Ethics Committee. The left sciatic nerve was cut at the femur high under general anesthesia in order to induct degeneration in vivo. The animals were sacrificed and then 1 cm of distal part of nerve was collected. The control group was a nerve from the leg that was not operated on. The pieces of nerves were left for 1 h in medium (Dulbecco's Modified Eagle's Medium).
Cell culture
Then perineuria were mechanically dissociated using microsurgical tweezers. The nerve was cut into pieces 1-2 mm in length, and treated with an enzyme mixture of 1 mg/ml collagenase and 0.05 mg/ml trypsin at 37°C. After centrifugation, 5 ml medium with 10% FBS (Fetal Bovine Serum) was added to the precipitated cells. From each part of the nerve, two primary cultures of 105 were established. Cells were seeded into T-flasks (Falcon, 25 cm2). The medium was changed every two days. The number of cells was evaluated using trypan blue staining. Purity of primary cultures was evaluated as the mean percentage of Schwann cells with respect to the total number of cells counted on last day of cultivation.
Cell identification
On the seventh day, the culture was placed on microscope plates for immunohistochemical analysis. Antibody anti-S-100 was used to identify Schwann cells in culture (according to the manufacturer's recipe - 1 : 100 DAKO).
Differences between means were compared with t–Student test for α = 0.05.
RESULTS
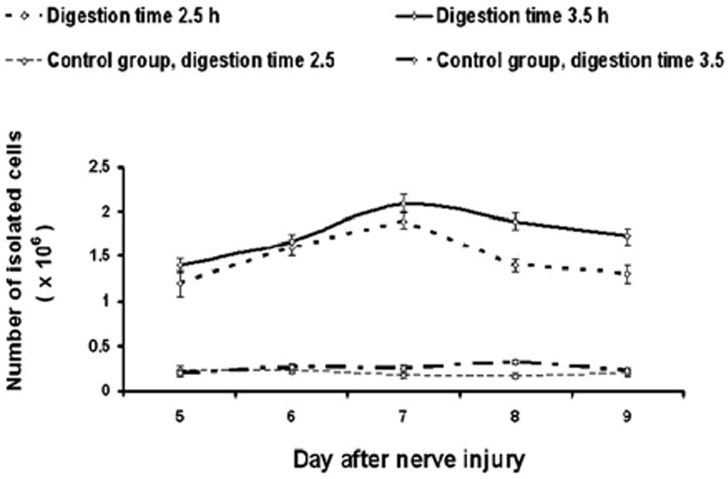
A larger number of cells were isolated from the degenerated sciatic nerve (Fig. 1; P2h = 0.0003, Fig. 1, P3, 5 h <0.0001). Colonies of cells that morphologically resembled Schwann cells were visible by light microscopy on the second day of in vitro cultivation (Fig. 1). After 7 days all dishes were coated. One centimeter of sciatic nerve gave 1.2-1.9 x 106 cells. The largest number of cells was obtained by carrying out the isolation on the 7th day of degeneration. The number of isolated cells increased from the 5th day. After the 7th day, the population of primary cultured cells decreased. The isolation efficiencies were similar on the 9th and 5th days of degeneration.
Fig. 1.
The number of cells isolated from degenerated nerves taken after different times from the injury.
Extending the digestion time from 2.5 h to 3.5 h resulted in a larger number of isolated cells (P = 0.01). There was no significant difference in the number of isolated cells between the control groups (P = 0.3).
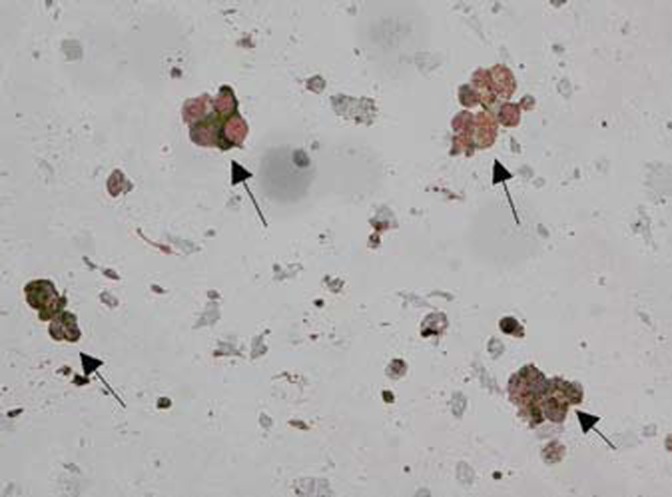
Homogeneity of the primary cultures increased in the last day of cultivation to 60% (Figs. 2–3). On the 7th day they reached around 60%. Homogeneity of culture did not depend on the peripheral nerve degeneration. Cultures starting on 5th day from nerve injury were characterized by a smaller number of Schwann cells. In this case, on the 3rd day the homogeneity of cultures was about 33% and increased on the 7th day to about 40%. The digestion time did not affect the homogeneity of the primary culture (P = 0.5). Marked cells with the marker S-100 showed the presence of Schwann cells, which formed oval connecting colonies (Fig. 4).
Fig. 2.

Homogeneity of Schwann cell primary culture on third and last day of cultivation. Cultures were established from nerves taken after different times from injury. Digestion time was 2.5 h.
Fig. 3.

Homogeneity of Schwann cell primary culture on third and last day of cultivation. Cultures were established from nerves taken after different times from injury. Digestion time was 3.5 h.
Fig. 4.
Immunostained Schwann cell is marked with black arrow. The characteristic multispinous morphology is visible.
Fig. 5.
Immunostained Schwann cell colonies are marked with black arrows.
DISCUSSION
The isolation of Schwann cells from the in vivo degenerated peripheral nerve is an effective protocol. Such a method is suitable for obtaining Schwann cells for in vitro cultures [6].
Neurons undergo Wallerian degeneration in peripheral and central nervous systems after axonal injury. During this process the myelin sheath is degraded, the distal stump broken apart, and the proximal stump is elongated [7]. Schwann cells are major cells that regulate Wallerian degeneration and act as the major support cells during neuron regeneration. Schwann cells can oscillate between mature and immature states after axonal injury. Mature Schwann cells form myelin sheaths and are static, non-proliferative cells without significant paracrine activity. After nerve injury Schwann cells are activated and dedifferentiate to immature cells. Immature Schwann cells are highly proliferating cells that characterize paracrine activity and immunomodulatory properties. Immature Schwann cells are the main source of growth factors and cytokines needed to induce neuron regeneration and reduce inflammatory processes to protect the intact proximal part of the axon [8].
Immature Schwann cells are harvested to be expanded in in vitro culture. Under conditions of Wallerian degeneration the Schwann cells separates from the basement membrane and endoneurium and gains ability [9]. The highest number of cells were isolated on the 7th day after sciatic nerve injury in the presented study. However, other groups of researchers reported that the most suitable time to establish neurolemmocyte culture ranges from 5th to 14th day of Wallerain degeneration [10, 11]. These differences are associated with changeable environment within nerve injury especially the scarring time that limits Schwann cells proliferation. Connective tissue deposition impacts Schwann cell isolation efficiency. The digestion time depends on peripheral nerve stromal structure. Thus, the proper enzyme concentration must be used and digestion time must be long enough to separate cells. From the other hand, too long digestion decreases the number of isolated cells because of damage by the enzymatic mixture. The number of cells received from the degenerated in vivo peripheral nerve is sufficient to established in vitro culture. However, the major problem of such an isolation method is the high number of fibroblasts in primary culture. The fibroblasts often outnumber Schwann cells. It is a consequence of the faster proliferation rate of fibroblasts. Homogeneity of the primary cultures in this study was increasing during cultivation to 60% in the last day. In this study any additional neurotrophic factors were not used in order to evaluate the natural in vitro proliferation ability of Schwann cells. Increasing homogeneity of the neurolemmocyte culture can be explained by production of trophic factors resulting in paracrine cell stimulation. Antibodies against fibroblasts are the most effective method of removing fibroblasts from culture. It is the best method to received 99-100% homogeneous population of Schwann cells. Unfortunately, it is very expensive. Other methods to hold the proliferation of fibroblasts are: reduction to a 2.5% concentration of serum, cytotoxic drugs (mainly cytosine arabinoside), or separation using a magnetic field [12]. Homogeneity of culture can be also increased by using NGF, GGF, progesterone, forskolin, or insulin enriched medium.
Tissue engineering methods allow replacement of urinary tract with the use of biomaterial seeded with stem cells. Regenerative templates composed of biodegradable material and autologous cells that were isolated and expanded ex vivo stimulate native-like organ tissue regeneration after implantation. Therapies based on cell transplantations are safe for patients.
It was proven in the clinical practice in the field of hematology/ oncology. There is only hypothetical risk of cancer transformation after implanting cells expanded in vitro. However, no research group reported cancer tumor formation after such a procedure. Germinal malignant tumor formations were reported only after transplantation of embryonal stem cells (ESC) in animal models.
Currently the main efforts are focused on restoring smooth muscle layer in urinary bladder wall grafts [13]. However, proper restoration of normal urinary tract function requires regeneration of the peripheral nervous system. Neurons within urinary tracts form very complicated and still undefined networks that regulate and integrate function of the upper and lower urinary tract. Schwann cells were documented to be effective in inducing regeneration of peripheral nerves after injury. Direct cellular supplementation with Schwann cells resulted in axon elongation and bridging the gap in injured peripheral nerves. There is no reason to not believe that transplantation of Schwann cells to urinary bladder graft would initiate correct innervation of reconstructed bladder by stimulation of neuronal branching from the intact wall. Schwann cells are the best source of neurotrophs such as Neuronal Growth Factor (NGF), Neurotrophin-3 and -4 (Nt-3, Nt-4), and Brain Derived Neural growth Factor (BDNF). Moreover, Schwann cells interact with axons and adopt phenotype and paracrine activity to requirements of neuronal cells [14]. Thus, transplantation of Schwann cells seems to be a better idea for inducing neuronal regeneration than supplementation with a single neurotrophic factor or covering the augmented bladder wall with omentum.
REFERENCES
- 1.Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Baptista PM, Orlando G, Mirmalek-Sani SH, et al. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–6529. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 3.Drewa T, Adamowicz J, Łysik J, et al. Chitosan scaffold enhances nerve regeneration within the in vitro reconstructed bladder wall: an animal study. Urol Intern. 2008;81:330–334. doi: 10.1159/000151414. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Yannas IV. Peripheral nerve regeneration. Adv Biochem Eng Biotechnol. 2005;94:67–89. doi: 10.1007/b100000. [DOI] [PubMed] [Google Scholar]
- 5.Sinis N, Schaller HE, Becker ST. Long nerve gaps limit the regenerative potential of bioartificial nerve conduits filled with Schwann cells. Restor Neurol Neurosci. 2007;25:131–141. [PubMed] [Google Scholar]
- 6.Brookes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells, Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–108. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 7.Verdú E, Rodríguez FJ, Gudińo-Cabrera G, et al. Expansion of adult Schwann cells from mouse predegenerated peripheral nerves. J Neurosci Methods. 2000;99:111–117. doi: 10.1016/s0165-0270(00)00221-1. [DOI] [PubMed] [Google Scholar]
- 8.Zurita M, Vaquero J, Oya S, et al. Neurotrophic Schwann-cell factors induce neural differentiation of bone marrow stromal cells. Neuroreport. 2007;18:1713–1717. doi: 10.1097/WNR.0b013e3282f0d3b0. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Velardez MO, Warot X, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;22:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoke R, Redett H, Hameed R, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9665. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinis N, Schaller HE, Schulte-Eversum C, et al. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. Neurosurg. 2005;103:1067–1076. doi: 10.3171/jns.2005.103.6.1067. [DOI] [PubMed] [Google Scholar]
- 12.Manent J, Oguievetskaia K, Bayer J, et al. Magnetic cell sorting for enriching Schwann cells from adult mouse peripheral nerves. J Neurosci Methods. 2003;15:167–173. doi: 10.1016/s0165-0270(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 13.Drewa T. Using hair-follicle stem cells for urinary bladder-wall regeneration. Regen Med. 2008;3:939–944. doi: 10.2217/17460751.3.6.939. [DOI] [PubMed] [Google Scholar]
- 14.Radtke C, Akiyama Y, Lankford KL, et al. Integration of engrafted Schwann cells into injured peripheral nerve: axonal association and nodal formation on regenerated axons. Neurosci Lett. 2005;387:85–89. doi: 10.1016/j.neulet.2005.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]