Abstract
Cystitis glandularis (CG) is defined as glandular metaplasia of bladder urothelium. In most cases the course of CG is asymptomatic. However, some patients complain of hematuria and lower urinary tract symptoms (LUTS) of varying degrees. We present a case of 45-year-old man with an extensive CG causing acute urinary retention. Although it was initially treated as an infection, prompt ultrasound and cystoscopy helped to establish the diagnosis. Transurethral resection of the cyst with biopsy of the bladder mucosa was then performed. Immediately after surgery the patient noticed significant improvement in urine passing. During the 2-month follow-up there was no relapse.
Keywords: cystitis glandularis, urinary retention, LUTS
INTRODUCTION
Cystitis glandularis (CG) is defined as glandular metaplasia of bladder mucosal and submucosal urothelium. Small changes of CG are commonly found in specimens from the bladder wall taken from cancer and other diseases or at autopsy. In most cases, these changes are microscopic and clinically insignificant. However in some patients it may lead to a significant proliferation of epithelial cells with the formation of macroscopically visible cysts of different size (cystitis cystica) or changes similar to bladder cancer (cystitis glandularis). The etiology remains unknown. It is thought that chronic irritation of the bladder mucosa (infection, bladder stones, indwelling catheterization, bladder exstrophy) is a major risk factor for CG. However, in some patients it is difficult to establish the etiological factor. Patients with advanced CG usually complain of hematuria, lower urinary tract symptoms (LUTS), or symptoms associated with the obstruction of the upper urinary tract. Cystoscopy allows giving the initial diagnosis of CG, but the final diagnosis requires pathological examination. Treatment of focal lesions generally causes no major problems and usually involves removal by transurethral resection. In the case of extended lesions with recurrent hematuria and urinary tract obstruction the only solution may be to perform a cystectomy with orthotopic neobladder.
In this paper we present a case of 45-year-old man with the extensive form of CG causing complete urinary retention.
CASE REPORT
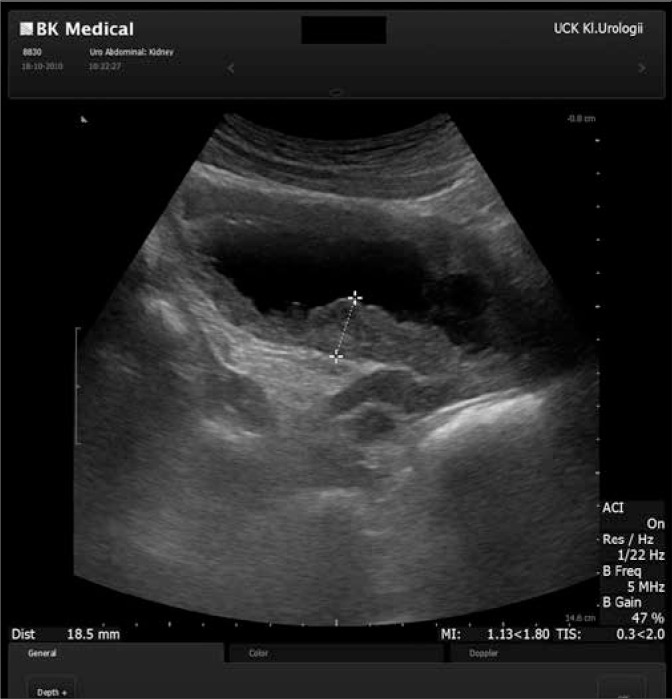
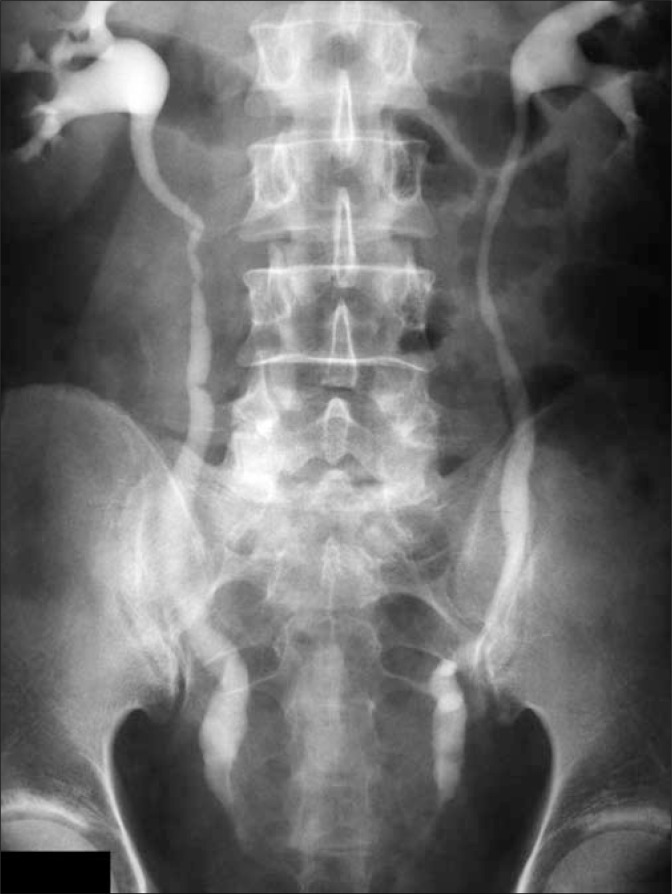
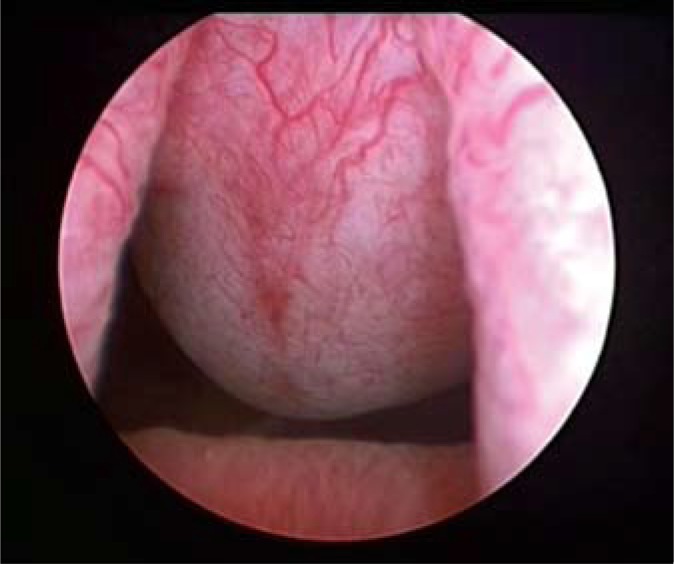
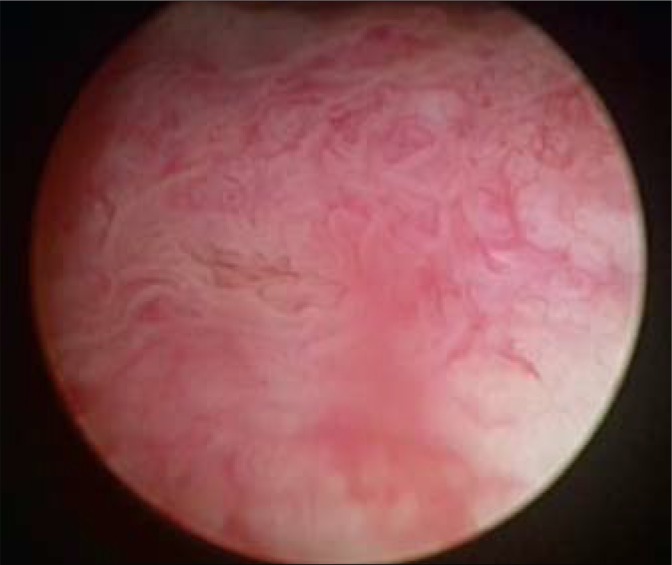
A 45-year-old male came to the Regional Urological Clinic with a diagnosis of “recurrent cystitis”. He complained of severe LUTS – pollakiuria, urgency (every 20 minutes), nocturia (every hour), feeling of incomplete emptying of the bladder, and a significant decreasing in urine flow. Previously, the patient received a 7-day course of oral antibiotic therapy (Ciprofloxacin 2 x 500 mg) with no improvement. The symptoms gradually increased over three weeks. Ultrasonography showed extensive irregular thickening of the bladder walls, up to 20 mm in greatest dimension, with the presence of two cystic lesions (32 mm and 21 mm) with anechoic content and a thin hyperechogenic wall (Fig. 1). They were localized in the bladder neck on the anterosuperior wall. Distal parts of the ureters were dilated without dilation of pyelocaliceal system. Residual urine volume was about 300 ml. Urine examination showed only erythrocyturia (up to 15 RBC/hpf). Urine culture was sterile. The level of creatinine was 1.04 mg/dl. Urography showed a normal course of both ureters with moderate dilation in the distal part (Fig. 2). During the cystoscopy two cystic changes were found in the neck of the bladder on the anterosuperior wall. The first with a diameter of about 30 mm and the second with a diameter of about 15 mm. The larger one almost completely closed the internal orifice of the urethra (Fig. 3). Mucosa throughout the bladder was significantly swollen, hemorrhagic, and particularly irregular within the trigone of the bladder (Fig. 4). During cystoscopy, biopsy of the bladder mucosa was performed. During waiting for pathological results, a significant increase of LUTS and acute urinary retention occurred. Urinary catheter was inserted and the patient was referred to the department of urology. Transurethral resection of all exophytic cystic lesions with biopsy of the bladder mucosa was performed. The bladder neck was also found to be affected by the disease. The postoperative period was uneventful. The catheter was removed on the third day after surgery. Pathological examination of both specimens (cyst and bladder mucosa) showed cystitis cystica glandularis (Fig. 5). Two weeks after surgery the patient noticed significant improvement in urinary flow without feeling of incomplete emptying of the bladder. Pollakiuria and urgency were less frequent. Maximum urinary flow in uroflowmetry performed 6-weeks after surgery was 34.8 ml/s (VV = 142 ml) and residual urine volume was about 15 ml. Bladder ultrasound showed persistent thickening of the walls up to 15 mm, without cystic changes.
Fig. 1.
Ultrasound picture of the bladder.
Fig. 2.
Urography.
Fig. 3.
Cystoscopy (bladder neck).
Fig. 4.
Cystoscopy (bladder mucosa).
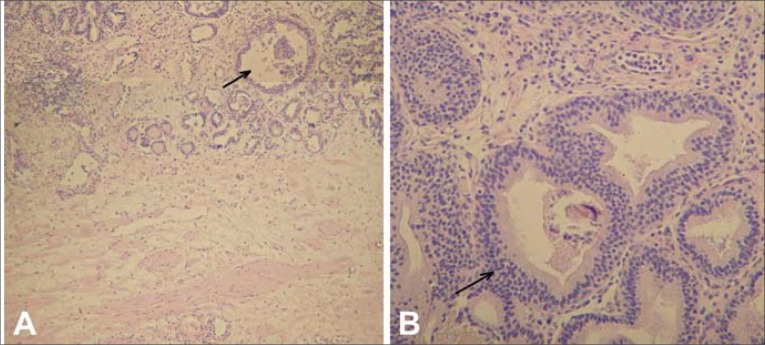
Fig. 5.
Pathological picture: cystitis glandularis of the typical type. A. The lesion is well circumscribed in the edematous and inflamed vesical mucosa and contains some cystically dilated structures. B. Larger magnification shows columnar lining cells devoid of mucus and goblet cells.
DISCUSSION
The etiology of CG is not clearly defined. Possible risk factors are: chronic irritation of the bladder mucosa due to infection or mechanical irritation (bladder stones, indwelling catheters, bladder exstrophy). It is thought that hormonal disturbances, the response to toxic substances in the urine, and deficiency of vitamins may also play a role in the development of CG. CG has been shown to be more frequent in patients with pelvic lipomatosis. It is probably caused by lymphostasis in the pelvis and the increased concentration of proteins in the intercellular fluid of the bladder mucosa. In some patients, however, the etiological factors cannot be determined [1].
Resulting from actions of the above-mentioned factors, von Brunn's nests appear in mucosal and submucosal layers of the bladder. They consist of foci of invaginated hyperproliferative urothelial cells, which further undergo cystic or glandular transformation. Most authors discern two types of CG – typical type and intestinal type. Typical CG is characterized by the presence of structures covered by cubic or columnar epithelium and unchanged urothelial cells. These lesions are usually located more superficially. Intestinal CG is characterized by the presence of metaplastic cells resembling intestinal cells. They are not covered by urothelium. A general characteristic for the both types is the ability to over-produce mucins, which create the mucous-filled structures. In typical CG, the expression of MUC1 protein was revealed. MUC1 is commonly present in the unaltered urothelium and plays an important role in the interaction of cells. On the other hand, in intestinal CG MUC5AC and MUC2 were expressed. MUC5AC is one of the major mucin secretors of gastric mucosa, and MUC2 is present in the small and large intestine [2].
It is believed that CG is benign. A few case reports regarding neoplastic transformation of CG into adenocarcinoma or urothelial carcinoma were published [3]. It seems that the intestinal CG has a higher risk of malignant transformation. On the other hand, foci of CG quite often coexist with bladder cancer (urothelial carcinoma, adenocarcinoma, squamous cell carcinoma) and are diagnosed in tumor biopsy specimens [4].
In most cases the course of CG is asymptomatic. However, some patients complain of hematuria and LUTS of varying degrees like pollakiuria, urgency, and dysuria. In case of a trigonal location of the lesions, obstructive symptoms may appear. The extensive form of CG can also cause compression of the intramural ureteral segments with dilation of the upper urinary tract and development of ureterohydronephrosis and chronic renal failure. Such cases often present symptoms associated with pyelonephritis resulting from a primary or secondary infection of the urinary system [5].
Most of the CG lesions, both asymptomatic and symptomatic, are detected during urinary tract ultrasound. However, due to lack of characteristic features of CG (exophytic papillary lesions, irregular thickening of the bladder wall), the ultrasound only allows recommendation for further evaluation. All patients need to have cystoscopy performed with concurrent biopsy. Definitive diagnosis is only possible on the basis of pathological examination. The additional examinations include urography, which allows assessing the degree of obstruction of the upper urinary tract and secretory function of the kidneys, and CT and MRI, which allow assessing wall thickening of the bladder and exclude the presence of pelvic lipomatosis [6].
Treatment is based on the elimination of possible etiological factors that cause chronic irritation of the mucosa of the bladder: long-term antibiotic therapy for urinary tract infections, treatment of bladder calculi, and intermittent catheterization in patients with neurogenic bladder.
Cases of endovesicular administration of hydrocortisone were described and, although it caused a reduction in symptoms, changes in the bladder persisted [7]. The long-term oral indomethacin therapy also results in only partial improvement [5].
In the presence of obvious exophytic lesions the method of choice is to perform transurethral resection (TUR) of the lesions [6, 8]. This results in a complete disappearance or significant relief of symptoms and patients usually do not require additional treatment.
In patients with extensive form of CG the treatment is more difficult. Ablation of the entire bladder mucosa using a neodymium: YAG laser (Nd: YAG) was performed [9]. However, treatment is partially effective and may be complicated by cirrhosis of the bladder. In the case of recurrent CG, which leads to obstruction of the upper urinary tract and the only one solution may be to perform cystectomy with orthotopic neobladder [10].
There are no recommendations regarding follow-up of patients with CG in the literature. Due to the low risk of malignant transformation, patients do not require control cystoscopy, but only routine urological examinations. Cystoscopy is indicated only in case of symptoms relapse.
This paper presents a case describing treatment of a patient with symptomatic CG. Due to the location of the exophytic lesions (neck of the bladder), the patient had significant LUTS, which gradually increased, and were the cause of acute urinary retention. TUR was uneventful. Immediately after surgery the patient noticed significant improvement in urine flow, and uroflowmetry performed 2-weeks after surgery confirmed the normal flow of urine and lack of residual volume. During the 2-month follow-up there was no relapse in the bladder neck, although irregular thickening of the wall still remained. LUTS, in the majority of young patients, are caused by urinary tract infection and disappear after antibiotic treatment. However, if symptoms persist a cystoscopy can be very useful. In our case, cystoscopy with simultaneous biopsy helped to determine the nature of cystic lesions and plan further treatment. There are more than 100 clinical cases of CG described in the literature. We did not find any cases of CG that would cause acute urinary retention.
REFERENCES
- 1.Parker C. Cystitis cystica and glandularis: a study of 40 cases. Proc R Soc Med. 1970;63(3):239–242. [PMC free article] [PubMed] [Google Scholar]
- 2.Jankovic Velickovic L, Katic V, et al. Differences in the expression of mucins in various forms of cystitis glandularis. Pathol Res Pract. 2007;203(9):653–658. doi: 10.1016/j.prp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Trasher JB, Rajan RR, Perez LM, et al. Cystitis glandularis transition to adenocarcinoma of the urinary bladder. N C Med J. 1994;55:562–564. [PubMed] [Google Scholar]
- 4.Smith A, Hansel DE, Jones S. Role of cystitis cystica et glandularis and intestinal metaplasia in development of bladder carcinoma. Urology. 2008;71(5):915–918. doi: 10.1016/j.urology.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 5.Touffahi M, Fredj N, Lefi M, et al. To analyse diagnosis, management and prognosis of florid cystitis glandularis (pseudoneoplastic entity) Prog Urol. 2007;17(5):968–972. doi: 10.1016/s1166-7087(07)92399-4. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg DA, Motta J, Brodherson MS. Cystitis glandularis. Urology. 1998;51(1):112–113. doi: 10.1016/s0090-4295(97)00502-5. [DOI] [PubMed] [Google Scholar]
- 7.Holder P, Plail R, Walker MM, Witherow RO. Cystitis glandularis--reversal with intravesical steroid therapy. Br J Urol. 1990;65(5):547–548. doi: 10.1111/j.1464-410x.1990.tb14811.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaya C, Akpinar IN, Aker F, Turkeri LN. Large Cystitis glandularis: a very rare cause of severe obstructive urinary symptoms in an adult. Int Urol Nephrol. 2007;39(2):441–444. doi: 10.1007/s11255-006-9042-4. [DOI] [PubMed] [Google Scholar]
- 9.Stillwell TJ, Patterson DE, Rife CC, Farrow GM. Neodymium:YAG laser treatment of cystitis glandularis. J Urol. 1988;139(6):1298–1299. doi: 10.1016/s0022-5347(17)42899-0. [DOI] [PubMed] [Google Scholar]
- 10.Black PC, Lange PH. Cystoprostatectomy and neobladder construction for florid cystitis glandularis. Urology. 2005;65(1):174. doi: 10.1016/j.urology.2004.07.026. [DOI] [PubMed] [Google Scholar]