Abstract
Introduction
Currently there is no universally accepted approach for the management of radiation-recurrent prostate cancer. The aim of this study was to detail our experience performing salvage radical prostatectomy for patients who failed primary treatment of prostate cancer with radiation therapy.
Material and methods
We retrospectively queried our institutional database of radical prostatectomy cases for patients who underwent salvage surgery for radiation-recurrent prostate cancer. Patients were assessed for the risk of complications and oncologic outcomes following salvage surgery.
Results
Twenty-four patients with a mean age of 65 years (range 51-74) underwent salvage radical prostatectomy. Fourteen of these patients (58%) received androgen deprivation therapy prior to surgery. Intraoperatively, mean blood loss was estimated at 415 mL (range 100-1000) and 19 (79%) patients received autologous blood. No patient required an allogeneic transfusion or experienced a rectal injury. Postoperative bladder neck contracture and urinary incontinence developed in 17% and 39% of men, respectively. Two (29%) of seven patients remained potent after salvage surgery. No patient developed a fistula. Overall and recurrence-free survival at 5-years was 90% and 39%, respectively. On multivariate analysis, extracapsular extension was the only significant predictor of biochemical recurrence (HR 6.9, 95% CI 1.9-25.3 p = 0.003).
Conclusion
In carefully selected patients, salvage radical prostatectomy for radiation-recurrent prostate cancer is a treatment option with acceptable oncologic outcomes and a moderate complication rate.
Keywords: biochemical recurrence, prostate cancer, salvage radical prostatectomy, radiation therapy
INTRODUCTION
Among newly diagnosed cases of clinically localized prostate cancer (PCa), approximately 35% of patients undergo primary treatment with radiation therapy (RT) [1]. Up to 30% of these patients will develop biochemical recurrence (BCR) and be considered for some form of salvage therapy [2, 3]. Local treatment options for RT-recurrent PCa (RR-PCa) include salvage radical prostatectomy (SRP), additional RT, cryotherapy, and high intensity focused ultrasound [4, 5, 6, 8, 9]. To date, there is no universally accepted approach for the management of RR-PCa. Historically, SRP was felt to be a technically challenging procedure with a high risk of complications such as rectal injury, fistula formation and the development of bladder neck contracture (BNC) [7, 8]. Accordingly, only a minority of patients with RR-PCa are treated with SRP. The aim of this study was to review our outcomes of patients who underwent SRP over the last 19 years with an emphasis on postoperative morbidity.
MATERIAL AND METHODS
Between January 1992 and January 2011, 2,166 men underwent open radical prostatectomy by a single surgeon (M.S.S.). We retrospectively reviewed our comprehensive Institutional Review Board approved database of prostatectomy cases for men who underwent SRP for RR-PCa. From among the 2,166 cases, 24 (1.1%) underwent SRP. Clinical, pathological, functional and oncological data was obtained for these patients.
Data was analyzed with PASW 18.0 (IBM Corporation, Somers, NY). Biochemical recurrence (BCR) after SRP was defined as a PSA ≥0.2 ng/mL, continence as using no pads, and potency as ability to have sexual intercourse with or without use of phosphodiesterase type-5 inhibitors on >50% of attempts [9]. BCR-free and overall survival were estimated with Kaplan-Meier analysis. Uni- and multivariate Cox proportional hazards analyses were performed to identify parameters associated with BCR after SRP. Any parameter with a p value of <0.20 was entered into the multivariate analysis. A p value of <0.05 was considered statistically significant.
RESULTS
Twenty-four patients with a mean age of 65 years (range 51-74) underwent SRP with curative intent for biopsy-proven RR-PCa. Of these patients, 13 (54%) had a history of external beam radiation therapy and 11 brachytherapy (46%). Patients had a mean preoperative PSA of 9 ng/mL (range 4-20). Prior to surgery, all patients had a projected life expectancy of at least 10 years and a negative metastatic workup with bone scan and contrast enhanced computed tomography of the abdomen and pelvis. Fourteen patients (58%) received androgen deprivation therapy prior to surgery. Patient characteristics, including preoperative and final pathology data, are detailed in Table 1.
Table 1.
Patient characteristics
| Parameter | n = 24 |
|---|---|
| Age, mean (range) | 64.5 (51-74) |
| Preoperative PSA, mean (range) | 8.7 (3.5-19.47) |
| Biopsy Gleason sum, n (%) ≤ 6 = 7 ≥ 8 |
10 (41.7) 5 (20.8) 9 (37.5) |
| Pathologic Gleason sum, n (%) ≤ 6 = 7 ≥ 8 |
6 (25.0) 9 (37.5) 9 (37.5) |
| Neoadjuvant androgen deprivation Yes No |
14 (58.3) 10 (41.7) |
| Nerve sparing, n (%) Bilateral Unilateral None |
3 (12.3) 2 (8.3) 19 (79.2) |
| Pelvic lymph node dissection, n (%) Bilateral Unilateral None |
13 (54.3) 2 (8.3) 9 (37.5) |
| Pathologic T stage, n (%) T2 T3a T3b |
11 (45.8) 5 (20.8) 8 (33.3) |
| Margin status, n (%) Positive Negative |
11 (45.8) 13 (54.2) |
| Positive lymph nodes Yes No |
2 (13.3) 13 (86.7) |
Intraoperatively, patients experienced a mean blood loss of 415 mL (range 100-1000). This volume of blood loss was not statistically different than those patients in our database who underwent a primary radical prostatectomy (mean 506 mL; p = 0.133). Autologous blood was given to 19 (79%) patients and none received an allogeneic transfusion. Five (21%) patients underwent a nerve sparing procedure and 15 (63%) a pelvic lymph node dissection (Table 1). No patient had a rectal injury.
Patients were followed for a mean of 63 (range 4-176) months. Postoperatively, four (17%) patients developed a BNC and were successfully managed endoscopically. Prior to surgery, 23 of 24 patients were fully continent. Following SRP, eight (35%) patients developed some degree of incontinence and were initially managed with pads. One patient ultimately required the placement of an artificial sphincter. Only seven patients were potent prior to surgery and two (29%) developed erectile dysfunction after surgery. No patient developed a fistula.
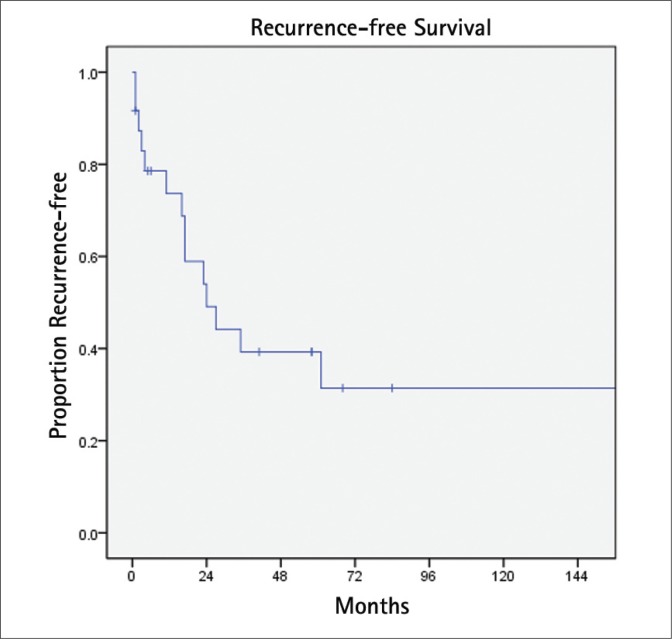
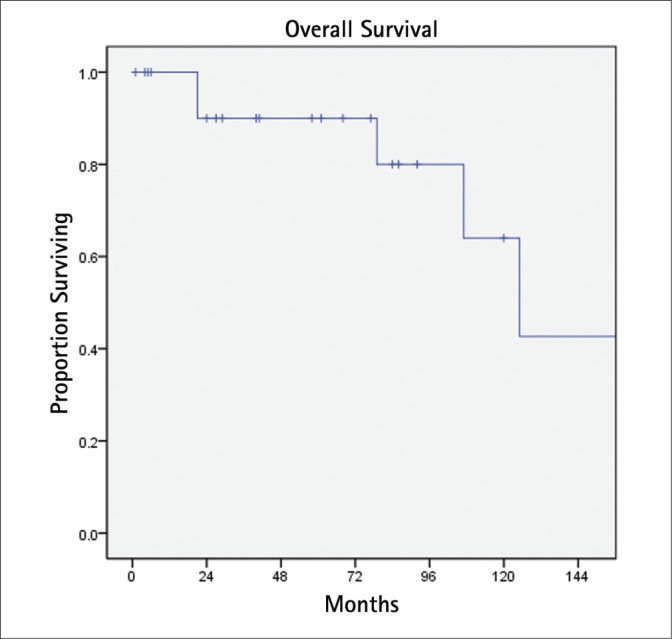
BCR was detected in 14 patients (58%) at a median interval of 24 months (Fig. 1). Eight of these patients were treated with androgen deprivation. On univariate Cox proportional hazards analysis (Table 2), extracapsular extension was the only significant predictor of BCR (HR 6.9, 95% CI 1.9-25.3 p = 0.003). This result was unchanged on multivariate analysis. During follow-up, six (25%) patients died, two (8%) due to PCa (Fig. 2). One patient died with metastatic PCa at 18 months after SRP with a pathologic Gleason score of 9 and positive lymph nodes. The other died nine years after surgery with Gleason 7 PCa and extraprostatic extension.
Fig. 1.
Biochemical recurrence-free survival.
Table 2.
Univariate analysis of parameters associated with recurrence after SRP
| Parameter | Hazard ratio (95% CI) | p value |
|---|---|---|
| Extracapsular extension | 6.9 (1.9-25.3) | 0.003 |
| Gleason score | – | 0.806 |
| Neoadjuvant androgen deprivation | – | 0.845 |
| Node metastasis | – | 0.441 |
| Positive margin | – | 0.263 |
| Radiotherapy type | – | 0.433 |
| Seminal vesicle invasion | – | 0.147 |
| Total PSA | – | 0.980 |
Fig. 2.
Overall survival.
DISCUSSION
Although the result of salvage RT following failed radical prostatectomy has been extensively studied [10–12], SRP for RR-PCa is limited to relatively few reports. To date, SRP has been the most reported treatment approach with the longest follow-up. In a large single center series by Ward and coworkers, the authors studied their experience treating 138 men with SRP between the years of 1967 to 2000 [4]. At a median follow-up of seven years, patients had a 10-year disease-specific survival of 77% and median progression-free survival of 8.7 years. In a more contemporary multiinstitutional report of 404 open SRP cases (1985 to 2009), 10-year BCR-free, metastasis-free, and cancer-specific survival probabilities were 37%, 77%, and 83%, respectively [5]. In our series, we found a 10-year BCR-free and overall survival of 31% and 64%, respectively.
On multivariate analysis, we found extracapsular extension to be the only parameter associated with BCR following SRP (HR 6.9, 95% CI 1.9-25.3, p = 0.003). In contrast, Chade and coworkers [5] found PSA and Gleason sum to be predictors of BCR. This difference is likely the result of our study's small sample size as evident by the large confidence interval. Of note, Chade et al. also evaluated the risk of progressing to metastatic disease. These authors reported that PSA, Gleason sum, and positive lymph nodes predicted metastases. Their report is the first to specifically evaluate metastatic disease as others have focused only on BCR, disease-specific, and overall survival.
With regard to complications, a review of contemporary SRP series cited an incidence of urinary incontinence, BNC and rectal injury at 24 - 67%, 12 - 30%, and 0 - 15%, respectively [7]. Our data is consistent with these reports as we observed incidences of 39%, 17%, and 0%, respectively. We believe our relatively low complication rate is the result of careful patient selection. An important component of patient selection was the digital rectal exam. Any patient with a flat prostate resulting from radiation changes was excluded from surgery. Only those with a relatively normal prostate size and shape were selected for SRP. In addition to careful patient selection, we believe our modified surgical approach in which the prostate was mobilized lateral to medial with sharp dissection was also responsible for the low complication rate. This differs from the standard dissection that proceeds medial to lateral. Using our modified approach, no patient had a rectal injury. Moreover, in the postoperative period no patient developed a fistula.
Taken together, our data, as well as that of others, suggests a reasonable oncologic outcome of SRP with an acceptable complication rate. The complications of urinary incontinence and BNC can be successfully managed conservatively or endoscopically as was the case for all of our patients with BNC.
In addition to SRP, recent reports have focused on novel ablative technologies for the treatment of RR-PCa. Cryotherapy is the best studied ablative approach with reports ranging in size from 18 to 279 patients [8]. In a retrospective comparative study, SRP proved to offer superior oncologic outcomes to that of cryotherapy [13]. Using a PSA of greater than 0.4 ng/mL as the definition of BCR, the authors found a 5-year BCR-free survival of 21% for patients treated with cryotherapy as compared to 61% for those treated with SRP (p <0.001). However, no significant difference was observed for 5-year disease specific survival. This report did not offer any comparison of procedure-related complications.
High-intensity focused ultrasound is another ablative technology, which has gained attention for the treatment of prostate cancer [14, 15]. While it remains too early to judge the oncologic efficacy of this approach in the setting of salvage treatment, current data suggests a high complication rate related to this procedure. In one study, three of 46 (6.5%) patients developed a post-treatment urinary fistula [16]. In contrast, none of the patients in our study developed this complication.
While less extensively reported on than cryotherapy, salvage RT for RR-PCa has also been studied. The 5-year disease-free survival of case series on salvage brachytherapy ranges from 20-70% [8]. This wide range is the result of the many varied definitions of treatment failure used in these reports. Thus it is difficult to interpret these data. In a feasibility study, Vavassori and coworkers treated six patients with RR-PCa with the CyberKnife image-guided radiosurgical system (Accuray Incorporated, Sunnyvale, CA) [6]. At a median follow-up of 11.2 months, all patients were alive without evidence of urinary or rectal morbidity. Four patients (67%), however, had disease recurrence.
One limitation common to reports on salvage treatment for RRPCa is the lack of diagnostic tests with sufficient sensitivity to a detect treatment failure. At present no studies have validated the use of PSA as a marker for recurrence following salvage treatment. This is in contrast to the well established use of a PSA ≥0.2 ng/mL in the setting of primary prostatectomy or the Phoenix criteria for primary RT [9, 17]. Future work must focus on defining failure following salvage treatment. This is of particular concern when studying non-surgical approaches such as cryotherapy or salvage-RT. Other limitations of this report include its retrospective design and small sample size. In the future urologists should consider a collaborative randomized trial comparing SRP to other well-established management strategies.
CONCLUSION
For men with RR-PCa, SRP is a reasonable treatment option with reproducible oncologic outcomes. Common complications of SRP include urinary incontinence, BNC, and erectile dysfunction. Careful patient selection is crucial for minimizing the risk of these complications.
Acknowledgments
CURED and Vincent A. Rodriguez
REFERENCES
- 1.Hamilton AS, Albertson PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107:576–584. doi: 10.1111/j.1464-410X.2010.09514.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, Thames HD, Levy LB, et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys. 2003;57:915–928. doi: 10.1016/s0360-3016(03)00632-1. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR, Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE) Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 4.Ward JF, Sebo TJ, Blute ML, Zincke H. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol. 2005;173:1156–1160. doi: 10.1097/01.ju.0000155534.54711.60. [DOI] [PubMed] [Google Scholar]
- 5.Chade DC, Shariat SF, Cronin AM, et al. Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Multi-institutional Collaboration. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.03.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vavassori A, Jereczek-Fossa BA, Beltramo G, et al. Image-guided robotic radiosurgery as salvage therapy for locally recurrent prostate cancer after external beam irradiation: retrospective feasibility study on six cases. Tumori. 2010;96:71–75. doi: 10.1177/030089161009600112. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich A, Ohlmann CH, Polyakov S. Salvage Radical Prostatectomy. Eur Urol Suppl. 2005;4:47–52. doi: 10.1016/j.eururo.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Kimura M, Mouraviev V, Tsivian M, et al. Current salvage methods for recurrent prostate cancer after failure of primary radiotherapy. BJU Int. 2010;105:191–201. doi: 10.1111/j.1464-410X.2009.08715.x. [DOI] [PubMed] [Google Scholar]
- 9.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Amico AV, Chen MH, Sun L, et al. Adjuvant versus salvage radiation therapy for prostate cancer and the risk of death. BJU Int. 2010;106:1618–1622. doi: 10.1111/j.1464-410X.2010.09447.x. [DOI] [PubMed] [Google Scholar]
- 12.Ost P, Lumen N, Goessaert AS, et al. High-Dose Salvage Intensity-Modulated Radiotherapy With or Without Androgen Deprivation After Radical Prostatectomy for Rising or Persisting Prostate-Specific Antigen: 5-Year Results. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.04.021. in press. [DOI] [PubMed] [Google Scholar]
- 13.Pisters LL, Leibovici D, Blute M, et al. Locally recurrent prostate cancer after initial radiation therapy: a comparison of salvage radical prostatectomy versus cryotherapy. J Urol. 2009;182:517–525. doi: 10.1016/j.juro.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Rebillard X, Soulie M, Chartier-Kastler E, et al. High-intensity focused ultrasound in prostate cancer; a systematic literature review of the French Association of Urology. BJU Int. 2008;101:1205–1213. doi: 10.1111/j.1464-410X.2008.07504.x. [DOI] [PubMed] [Google Scholar]
- 15.Murat FJ, Gelet A. Current status of high-intensity focused ultrasound for prostate cancer: technology, clinical outcomes, and future. Curr Urol Rep. 2008;9:113–121. doi: 10.1007/s11934-008-0022-3. [DOI] [PubMed] [Google Scholar]
- 16.Berge V, Baco E, Karlsen SJ. A prospective study of salvage high-intensity focused ultrasound for locally radiorecurrent prostate cancer: early results. Scand J Urol Nephrol. 2010;44:223–227. doi: 10.3109/00365591003727551. [DOI] [PubMed] [Google Scholar]
- 17.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]