Abstract
Introduction
Photodynamic therapy (PDT), an alternative treatment modality for superficial bladder tumors is based on the interaction of a photosensitizer with light. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising candidate for anticancer therapy due to its ability to selectively induce apoptosis in cancer cells. However, not all tumor cells are sensitive to TRAIL. TRAIL-resistant cancer cells can be sensitized to TRAIL induced apoptosis by anticancer agents.
Objective
We investigated the combined cytotoxic effect of TRAIL and PDT with 5-aminolevulinic acid (ALA) on bladder cancer cells.
Materials and methods
Three human bladder transitional cancer cell lines: T24, 647V, and SW780 were treated with TRAIL and/or ALA-mediated PDT. Cytotoxicity was determined by MTT and LDH assay.
Results
Our study confirmed that T24 and 647V bladder cancer cells were resistant to TRAIL, whereas SW780 cells were sensitive to TRAIL. We therefore examined the cytotoxic effect of TRAIL in combination with ALA-mediated PDT on bladder cancer cells. We showed for the first time that pretreatment with low dose of PDT significantly sensitizes bladder cancer cells to TRAIL induced cytotoxicity.
Conclusion
ALA-mediated PDT augments the cytotoxic effect of TRAIL on transitional cancer cells of bladder. The obtained results suggest that combined treatment of TRAIL and PDT may provide the basis for a new strategy to induce cytotoxicity in bladder cancer cells.
Keywords: bladder cancer, apoptosis, TRAIL, photodynamic therapy
INTRODUCTION
Photodynamic therapy (PDT) is becoming widely accepted as a potential treatment for several types of cancer [1]. The therapy is based on the selective accumulation of photosensitizing molecules in tumor tissues and its local activation by exposure to visible light. Several reports have suggested that interaction of light with photosensitizer leads to oxidative damage of a variety of cellular organelles, which results in cancer cell death [2]. Apoptosis has been reported as the main mode of PDT-mediated cell death, but multiple signaling pathways are likely to be involved in the death process [3]. The course of changes observed in cells under the influence of PDT depends on the type and concentration of photosensitizer or intensity of light. The 5-aminolevulinic acid (ALA)-based PDT is a variant of classical PDT in which administered ALA enters cancer cells and is metabolized to the active sensitizer, protoporphyrin IX (PpIX), via the heme synthesis pathway [4].
Bladder cancer is the fourth most common malignancy in men and eighth most common malignancy in women [5]. Transitional cell carcinoma (TCC) is observed in over 90% of pathologic subtypes of bladder tumors. Approximately 75-80% of bladder tumors are non-muscle invasive (superficial) disease. Although most cases of bladder cancer are superficial, up to 70% of patients have recurrence and 30% progress to a higher grade or stage [6]. For patients with bladder cancer, photodynamic therapy, first reported in 1975, represents an alternative treatment modality for superficial transitional cell carcinoma [7]. ALA-PDT is particularly effective for bladder cancer because the photosensitizer can be applied intravesically through the endoscopic access directly at the tumor site with minimal systemic toxicity. Also, this usually superficial, multifocal disease of the bladder allows for good light penetration during the irradiation [4, 8].
The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or Apo2 ligand) is a death ligand that belongs to the TNF superfamily of cytokines. TRAIL was identified as a powerful activator of programmed cell death in tumor cells with no toxicity against normal tissues [9, 10]. TRAIL induces apoptosis in cancer cells following binding to the two death receptors, TRAIL-R1 and TRAIL-R2 [11]. The recombinant form of TRAIL (rhsTRAIL) or monoclonal antibodies against the TRAIL-R1 and/or TRAIL-R2 (mapatumumab, lexatumumab, apomab) induce cell death in a wide variety of tumor cell lines and xenografts without causing toxicity to normal cells and are therefore potentially attractive anticancer agents [12, 13]. Agonistic monoclonal antibodies against TRAIL-R1 and/or TRAIL-R2, as well as rhsTRAIL are currently in early clinical development. The phase I and phase II studies showed limited toxicity and significant tumor responses after application of TRAIL [10–14].
However some tumor cells are resistant to TRAIL-induced apoptosis. The decreased expression of death receptors (TRAIL-R1 and/or TRAIL-R2) or increased expression of antiapoptotic protein in cancer cells were involved in TRAIL-resistance [13, 15]. Ongoing research especially focuses on combined treatment with TRAIL and other cytotoxic therapies [16–20]. A number of in vitro studies showed that chemotherapeutic agents or ionizing radiation sensitize bladder cancer cells to TRAIL induced apoptosis [16, 18, 19]. This evidence supports the therapeutic potential of the combination of TRAIL with photodynamic therapy in bladder cancer cells [21].
Nevertheless, relatively little is understood the cellular effect of low dose PDT on cancer cells sensitivity to TRAIL-induced cell death. PDT and TRAIL mediate apoptosis and may share common intracellular signaling pathways leading to apoptosis [3, 9]. We reasoned that TRAIL-resistant cancer cells may be sensitized by photodynamic therapy and therefore, we investigated the cytotoxic effect of TRAIL in combination with ALA-mediated PDT on bladder cancer cells.
MATERIALS AND METHODS
Bladder cancer cells
The tests were performed on three human bladder transitional cell cancer (TCC) lines derived from bladder tumors obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH - German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and ATCC (American Type Culture Collection, Manassas, VA, USA):
SW780 cell line, cat. no CRL-2169 – well differentiated transitional cells (G1),
647 cell line, cat. no ACC-414 – moderately differentiated transitional cells (G2),
T24 cell line, cat. no ACC-376 – poorly differentiated transitional cells (G3).
The bladder cancer cells were grown in monolayer cultures in plastic bottles of 70 ml and 500 ml (Nunc A/S Roskilde, Denmark): SW780 cells in Leibovitz's, 647V and T24 cells in DMEM. All mediums were supplemented with 10% of heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ ml streptomycin. The cells were incubated at 37°C, SW780 cells in 100% air atmosphere, 647V and T24 cells in 95% air atmosphere and 5% CO2 [11, 19–21]. Reagents for bladder cancer cell culture were purchased from PAA The Cell Culture Company (Pasching, Austria).
Cells adhering to the container bottom were trypsinized and suspensions were prepared for use during subsequent experiments. The number of bladder cancer cells tested in each experiment was 1x106 per 1 ml of the medium.
PDT
Photosensitizer
5-aminolevulinic acid (ALA) was obtained from Calbiochem (San Diego, CA, USA). Stock solutions of ALA were prepared in deionized water. Before incubation with bladder cancer cells, further dilutions were made with medium to obtain final concentrations as indicated [21, 22].
In vitro PDT
Bladder cancer cell lines were seeded onto 96-well plates for 24-hours. Under lowlight conditions the cells were incubated with ALA at the final concentrations of 5-50 μM for 4-hours, then the medium was removed and the cells were washed two times with PBS. The cells were irradiated with VIS (400-750 nm, 7.5 J/cm2) delivered from the incoherent light source PDT TP-1 (Cosmedico Medizintechnik GmbH, Schwenningen, Germany). After 3-hours the culture medium was again removed. The cytotoxicity assays were performed 18 hours later [21, 22].
TRAIL
Soluble, human recombinant TRAIL called “SuperKillerTRAIL” [rhsTRAIL (CC-mutant)] and dilution buffer “KillerTRAIL Storage and Dilution Buffer” were purchased from Alexis (San Diego, CA, USA).
Cytotoxicity assays
Mitochondrial dehydrogenase activity (MTT test)
The cytotoxic effect of tested agents (TRAIL and/or PDT) on bladder cancer cells were assessed with a MTT test (bromo-3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium) involving the measurement of mitochondrial dehydrogenase activity [23, 24]. The reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Lactate dehydrogenase activity (LDH test)
The cytotoxic effect of the TRAIL and/or PDT on bladder cancer cells were assessed by measuring lactate dehydrogenase (LDH) activity in the LDH test purchased from Roche Molecular Biochemicals (Mannheim, Germany) [25, 26]. Lactate dehydrogenase is released from the cytoplasm into the culture medium as a result of cell membrane damage and cell lysis. The LDH activity increase in cell culture supernatants correlates with the rate of necrotic cells.
Statistical analysis of results
The tests were performed in the same experimental conditions. The results were obtained from four independent experiments.
The significance level was p ≤0.05. The following standard tests were applied:
the cytotoxicity assessment with regard to the different TRAIL levels – the Kruskal-Wallis one-factor analysis of variance,
specific statistical analysis of the differences in cytotoxicity between two groups – the U Mann-Whitney test,
the analysis of simultaneous effects of two factors (cytotoxicity dependence on two factors: TRAIL and PDT) – a test based on a two-factor analysis of variance (ANOVA).
RESULTS
Cytotoxic effect of TRAIL on bladder cancer cells
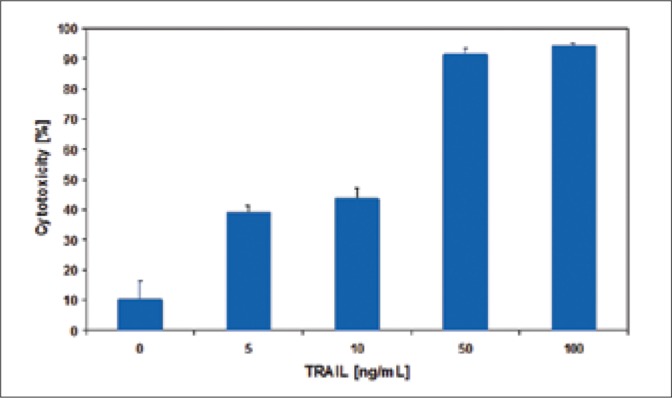
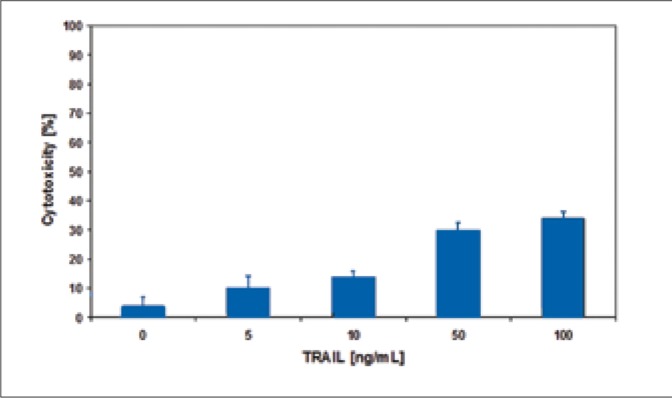
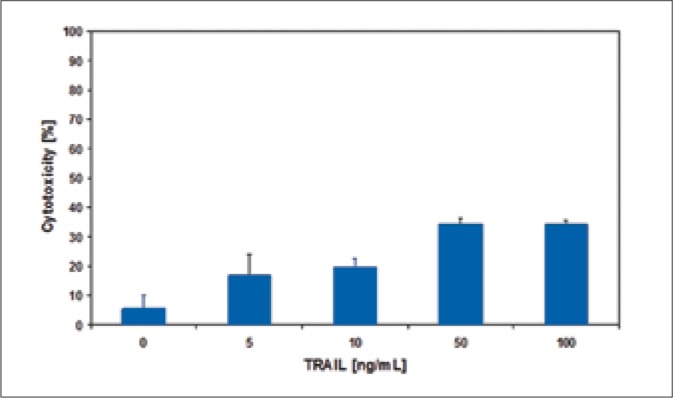
The bladder cancer cells were incubated with 5-100 ng/ml TRAIL for 18-hours. TRAIL induced cytotoxic effect on bladder cancer cells in a dose-dependent manner (Figs. 1-3). The SW780 cells were sensitive to TRAIL. In contrast the two other bladder cancer cell lines, 647V and T24, were resistant to TRAIL-mediated cytotoxicity. The cytotoxic activity of TRAIL at the concentrations of 5-50 ng/ml was: against SW780 cells 39.1 ±2.3 – 91.4 ±2.0%, against 647V cells 16.7 ±7.5 – 34.3 ±1.9% and against T24 cells 10.1 ±3.9 – 29.9 ±2.8%. The ligand concentration higher than 50 ng/ml had no significant influence on its cytotoxicity increase in relation to bladder cancer cells that were tested. During further tests, TRAIL at concentrations of 5-50 ng/ml was used. TRAIL at concentrations of 5-100 ng/ml did not induce the lysis of bladder cancer cells determined in the LDH test.
Fig. 1.
Cytotoxic effect of TRAIL at concentrations of 5 – 100 ng/ml on SW780 bladder cancer cells.
Fig. 3.
Cytotoxic effect of TRAIL at the concentrations of 5 – 100 ng/ml on T24 bladder cancer cells.
Fig. 2.
Cytotoxic effect of TRAIL at concentrations of 5 – 100 ng/ml on 647V bladder cancer cells.
Cytotoxic effect of ALA-mediated PDT on bladder cancer cells
Treatment of bladder cancer cells with ALA (5-50 μM) and sub-sequent irradiation with VIS (400-750 nm, 7.5 J/cm2) showed dose dependent inhibition of cell proliferation, whereas ALA or VIS alone had no effect. The photoactivated ALA demonstrated similar results of cytotoxicity in all TCC cell lines. The photocytotoxic effect was: 8.8 ±3.6 – 21.3 ±3.2% for SW780 cells, 6.2 ±1.7 – 21.8 ±3.0% for 647V cells, and 6.5 ±2.1 – 22.4 ±1.5% for T24 cells (Table 1). During further tests, PDT with ALA at a concentration of 25 μM was used. The necrotic cell death percentage of all bladder cancer cell lines incubated with ALA and treated VIS, examined by LDH release was near 0%.
Table 1.
Cytotoxic effect of ALA-mediated PDT on bladder cancer cells. The cells were incubated with ALA at the final concentrations of 5-50 μM for 4-hours and subsequently irradiated with VIS (400-750 nm, 7.5 J/cm2) delivered from the incoherent light source PDT TP-1
| PDT (400-750 nm, 7.5 J/cm2) ALA | Photocytotoxicity [%] | ||
|---|---|---|---|
| SW780 cells | 647V cells | T24 cells | |
| 5 μM | 8.81 ±3.64 | 6.17 ±1.72 | 6.46 ±2.11 |
| 10 μM | 14.46 ±2.06 | 8.32 ±0.40 | 8.85 ±1.16 |
| 25 μM | 26.50 ±2.65 | 16.53 ±3.15 | 17.27 ±2.94 |
| 50 μM | 28.27 ±2.18 | 21.76 ±2.00 | 22.15 ±1.45 |
Cytotoxic effect of TRAIL on bladder cancer cells pretreated with ALA-mediated PDT
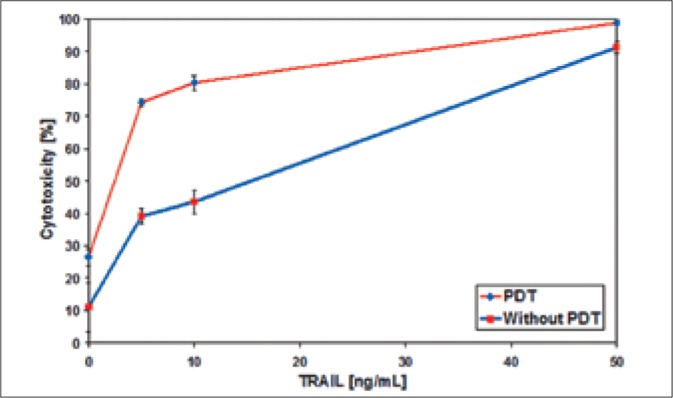
The bladder cancer cells were treated with low dose of PDT (ALA at concentration of 25 μM and VIS: 400-750 nm, 7.5 J/cm2) and 3 hours after irradiation subsequent incubation with TRAIL at concentration of 5-50 ng/ml for 18-hours was done. The cytotoxicity of TRAIL in combination with ALA-mediated PDT against bladder cancer cell lines is shown in Figures 4-6. PDT significantly augmented TRAIL-induced cell death in all bladder cancer lines. In TRAIL-resistant cancer cells, 647V and T24, PDT increased cytotoxic effect of TRAIL and sensitized both TCC lines to TRAIL-induced cell death. The cytotoxicity of combined treatment TRAIL at the concentrations of 50 ng/ml with PDT against bladder cancer cells was as follows: 98.9 ±0.6% for SW780, 67.2 ±4.9% for 647V, 45.5 ±1.5% for T24 cells. The photoactivated ALA also enhanced the cytotoxic effect of lower concentrations of TRAIL (5 ng/ml and 10 ng/ml) in TCC cells by 18-37% compared to PDT or TRAIL alone.
Fig. 4.
Cytotoxic effect of TRAIL on SW780 bladder cancer cells pretreated with ALA-mediated PDT.
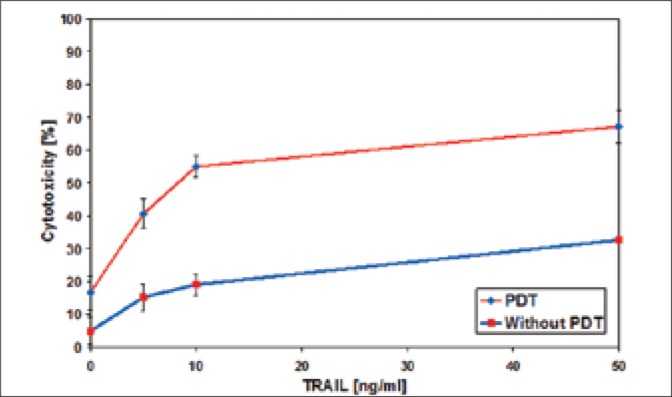
Fig. 6.
Cytotoxic effect of TRAIL on T24 bladder cancer cells pretreated with ALA-mediated PDT.
Fig. 5.
Cytotoxic effect of TRAIL on 647V bladder cancer cells pretreated with ALA-mediated PDT.
DISCUSSION
The adjuvant treatments of non-muscle invasive bladder cancer are performed after TURbt (transurethral resection of bladder tumor) to eradicate residual disease, prevent recurrence, and reduce the risk of progression [5, 6]. The bladder cancer cells resistance or severe side effects such as bladder irritability and lack of response in some patients limited the use of intravesical chemo- or immunotherapy [27].
TRAIL is a promising anticancer agent able to induce apoptosis in cancer cells with no toxicity to normal cells [9–14]. We tested the sensitivity of bladder cancer cells to TRAIL. The moderately differentiated transitional 647V cells and poorly differentiated transitional T24 cells were resistant to TRAIL, in contrast to well-differentiated transitional SW780 cells sensitive to TRAIL-induced cytotoxicity.
Photodynamic therapy is clinically approved and rapidly developing cancer treatment regimen [2]. PDT has been investigated as a new, minimally invasive, alternative modality for bladder tumors [8]. The in vivo and in vitro studies reported that PDT could be an effective and safe treatment of superficial bladder cancer [4, 8, 22]. ALA is the most widely used photosensitizer for PDT of superficial bladder cancer in clinical trials [27, 28]. The TCC bladder cancer cell lines, SW780, 647V, and T24 are an interesting in vitro cellular model to explore PDT with ALA, because this photosensitizer deposits in the bladder cancer lesion [18]. Therefore ALA-mediated PDT of bladder tumors may be a good therapeutic prospect. The 5-aminolevulinic acid, an endogenous substance of heme biosynthesis, is metabolized to its active form protoporhyrin IX (PPIX) [29]. Irradiation of PPIX results in a photocytotoxic effect in bladder cancer cells [22].
TRAIL has proved to be a potent antitumor agent in preclinical and clinical studies. However, TRAIL-resistance of some cancer cells increases number of investigations into the possibility of combined treatment of TRAIL with other anticancer agents. It has been reported that chemotherapeutic agents or ionizing radiation sensitize bladder cancer cells to TRAIL induced apoptosis [16, 18, 19]. We therefore examined the cytotoxic effect of TRAIL in combination with ALA-mediated PDT on bladder cancer cells. We showed for the first time that pretreatment of bladder cancer cells with low dose of PDT significantly sensitizes bladder cancer cells to TRAIL-induced cytotoxicity. PDT with ALA reversed resistance to TRAIL-mediated cytotoxicity in 647 cells and T24 cells. The present study demonstrates that the combined treatment of TRAIL and PDT resulted in synergistic cytotoxic activity against bladder cancer cells.
The enhancement of TRAIL by photodynamic therapy was previously described only once by Granville et al. in human Jurkat lymphoma cells. Authors of this work indicated that TRAIL augments the effect of PDT on the induction of apoptosis in Jurkat cells [30]. Nonetheless, the effect of combined treatment using TRAIL with PDT on solid cancer cells was investigated. Our results confirmed that PDT enhanced the potential of TRAIL in bladder cancer cells by about 25% compared to PDT or TRAIL alone. Pretreatment bladder cancer cells with low dose of PDT augments the effect of TRAIL and sensitizes TRAIL-resistant cancer cells to TRAIL induced cytotoxicity. It suggest that use of combined therapy of TRAIL with PDT in the future might be a potential method for treatment of superficial bladder cancer, preventing recurrence and cancer progression. The combination of TRAIL with PDT could also reduce the effective dosage for these agents and lower their side effects on normal cells.
The mechanism by which photoactivated ALA acts on TRAIL-induced cell death of bladder cancer is not clear at present. Therefore, further studies will be required to examine the signaling pathways by which PDT overcomes the resistance of TCC cells to TRAIL and sensitizes bladder cancer cells to TRAIL-induced apoptosis.
CONCLUSIONS
ALA-mediated PDT augments the cytotoxic effect of TRAIL against TCC cells of bladder. The obtained results suggest that the combined treatment with TRAIL and PDT may provide the basis for a new therapeutic approach to induce cytotoxicity in bladder cancer cells with reducing the effective dosage of TRAIL and PDT and lowering the toxic effects on normal cells.
REFERENCES
- 1.Pass HI. Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst. 1993;85:443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- 2.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 3.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochem Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.French AJ, Datta SN, Allman R, Matthews PN. Investigation of sequential mitomycin C and photodynamic therapy in a mitomycin-resistant bladder cell-line model. BJU Int. 2004;93:156–161. doi: 10.1111/j.1464-410x.2004.04576.x. [DOI] [PubMed] [Google Scholar]
- 5.Grasso M. Bladder cancer: a major public health issue. Eur Urol. 2008;7:510–515. [Google Scholar]
- 6.Colombel M, Soloway M, Akaza H, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol. 2008;7:618–626. [Google Scholar]
- 7.Kelly JF, Snell ME. Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol. 1976;115:150–151. doi: 10.1016/s0022-5347(17)59108-9. [DOI] [PubMed] [Google Scholar]
- 8.Weidelich R, Beyer W, Knuchel R, et al. Whole bladder photodynamic therapy with 5-aminolevulic acid using a white light source. Urology. 2003;61:332–337. doi: 10.1016/s0090-4295(02)02164-7. [DOI] [PubMed] [Google Scholar]
- 9.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Huerta-Yepez S, Vega M, et al. The NO TRAIL to YES TRAIL in cancer therapy (review) Int J Oncol. 2007;31:685–691. [PubMed] [Google Scholar]
- 11.Szliszka E, Czuba ZP, Mazur B, Zydowicz G, Krol W. TRAIL-induced apoptosis and expression of death receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia Histochem Cytobiol. 2009;47:579–585. doi: 10.2478/v10042-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 12.Duiker EW, Mom CH, Jong S, et al. The clinical trail of TRAIL. Eur J Cancer. 2006;42:2233–2240. doi: 10.1016/j.ejca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn A, Behbakht K, Ford H. TRAIL-receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resist Updat. 2008;11:17–24. doi: 10.1016/j.drup.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruyt F. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–227. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani Y, Nakao M, Ogawa O, et al. Enhanced sensitivity of bladder cancer cells to tumor necrosis factor related apoptosis inducing ligand mediated apoptosis by cisplatin and carboplatin. J Urol. 2001;165:263–270. doi: 10.1097/00005392-200101000-00076. [DOI] [PubMed] [Google Scholar]
- 17.Shankar S, Srivastava RK. Enhancement oh therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Baierlein SA, Distel L, Sieber L. Combined effect of tumor necrosis factor and ionizing radiation on the induction of apoptosis in 5637 bladder carcinoma cells. Strahlenther Oncol. 2006;182:467–472. doi: 10.1007/s00066-006-1475-2. [DOI] [PubMed] [Google Scholar]
- 19.Szliszka E, Majcher A, Domino M, et al. Cytotoxic activity of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) against bladder cancer cells after using chemotherapeutic drugs. Urol Pol. 2007;60:138–142. [Google Scholar]
- 20.Szliszka E, Gebka J, Bronikowska J, Krol W. Dietary flavones enhance the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on bladder cancer cells. CEJUrol. 2010;63:138–143. [Google Scholar]
- 21.Szliszka E. Cytotoxic activity of tumor necrosis factor-related apoptosisinducing ligand (TRAIL) against bladder cancer cells after photodynamic therapy pretreatment. SUM Katowice; 2005. Doktoral Thesis. [Google Scholar]
- 22.Manivasager V, Heng P, Hao J, et al. A study of 5-aminolevulic acid and its methyl ester used in in vitro and in vivo systems of human bladder cancer. Int J Oncol. 2003;22:313–318. [PubMed] [Google Scholar]
- 23.Szliszka E, Czuba ZP, Domino M, et al. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules. 2009;14:738–754. doi: 10.3390/molecules14020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szliszka E, Czuba ZP, Mazur B, et al. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int J Mol Sci. 2010;11:1–13. doi: 10.3390/ijms11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szliszka E, Czuba ZP, Mazur B, et al. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules. 2010;15:5336–5353. doi: 10.3390/molecules15085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronikowska J, Szliszka E, Czuba ZP, et al. The combination of TRAIL and isoflavones enhance apoptosis in cancer cells. Molecules. 2010;15:2000–2015. doi: 10.3390/molecules15032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell MA. Advances in the management of superficial bladder cancer. Semin Oncol. 2007;34:85–97. doi: 10.1053/j.seminoncol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Fotinos N, Campo MA, Popowycz F, et al. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem Photobiol. 2006;82:994–115. doi: 10.1562/2006-02-03-IR-794. [DOI] [PubMed] [Google Scholar]
- 29.Wu SM, Ren QG, Zhou MO, et al. Protoporhyrin IX production and its photodynamics effects on glioma cells, neuroblastoma cells and normal cerebellar granule cells in vitro with 5-aminolevulic acid and its hexyl ester. Cancer Lett. 2003;2:123–131. doi: 10.1016/s0304-3835(03)00271-4. [DOI] [PubMed] [Google Scholar]
- 30.Granville DJ, Jiang H, McManus BM, et al. Fas ligand and TRAIL augment the effect of photodynamic therapy on the induction of apoptosis in JURKAT cells. Int Immunopharmacol. 2001;1:1831–1840. doi: 10.1016/s1567-5769(01)00107-2. [DOI] [PubMed] [Google Scholar]