Abstract
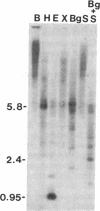
We describe a 5- to 6-kilobase-pair repetitive family in human DNA. One member of this family is linked to the beta-globin gene cluster and is close to the 3' breakpoints of three different naturally occurring deletions involving this gene cluster. Sequence analysis indicates that this element includes terminal direct repeats of 415 base pairs that exhibit the features of long terminal repeats (LTRs) of retroviruses. A potential histidine tRNA primer binding site occurs just 3' to the 5' direct repeat. This retrovirus-like element interrupts a member of the Kpn I family of repeated DNA and is bracketed by a 5-base-pair directly repeated sequence. When attempts are made to clone the element in bacteriophage, homologous recombination between the LTR-like sequences is very frequently observed. Copy number estimates by two methods indicate that the element is repeated 800-1000 times in the human genome. We term this Homo sapiens family of retrovirus-like elements having a histidine tRNA primer binding site the hsRTVL-H family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Tronick S., Schlom J. Detection and cloning of human DNA sequences related to the mouse mammary tumor virus genome. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5503–5507. doi: 10.1073/pnas.79.18.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida H., Miyata T. Unusual evolutionary conservation and frequent DNA segment exchange in class I genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 May;80(9):2671–2675. doi: 10.1073/pnas.80.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson C. P., Elder P. K., Ono T., Foster D. N., Getz M. J. Structure and expression of mouse VL30 genes. Mol Cell Biol. 1983 Dec;3(12):2221–2231. doi: 10.1128/mcb.3.12.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Restriction mapping of a new deletion responsible for G gamma (delta beta)o thalassemia. Nucleic Acids Res. 1981 Dec 21;9(24):6813–6825. doi: 10.1093/nar/9.24.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Keshet E., Shaul Y. Terminal direct repeats in a retrovirus-like repeated mouse gene family. Nature. 1981 Jan 1;289(5793):83–85. doi: 10.1038/289083a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Biro P. A. Genomic representation of the Hind II 1.9 kb repeated DNA. Nucleic Acids Res. 1982 May 25;10(10):3221–3239. doi: 10.1093/nar/10.10.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. Nucleotide sequence definition of a major human repeated DNA, the Hind III 1.9 kb family. Nucleic Acids Res. 1982 May 25;10(10):3211–3219. doi: 10.1093/nar/10.10.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClements W. L., Enquist L. W., Oskarsson M., Sullivan M., Vande Woude G. F. Frequent site-specific deletion of coliphage lambda murine sarcoma virus recombinants and its use in the identification of a retrovirus integration site. J Virol. 1980 Aug;35(2):488–497. doi: 10.1128/jvi.35.2.488-497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Kurihara M., Takano T. Retrovirus-related sequences in human DNA: detection and cloning of sequences which hybridize with the long terminal repeat of baboon endogenous virus. Nucleic Acids Res. 1982 May 11;10(9):2865–2878. doi: 10.1093/nar/10.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Ono M., Kitasato H., Ohishi H., Motobayashi-Nakajima Y. Molecular cloning and long terminal repeat sequences of intracisternal A-particle genes in Mus caroli. J Virol. 1984 May;50(2):352–358. doi: 10.1128/jvi.50.2.352-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Steele P. E., Garon C. F., Martin M. A. mRNA transcripts related to full-length endogenous retroviral DNA in human cells. Nature. 1983 Dec 8;306(5943):604–607. doi: 10.1038/306604a0. [DOI] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Steele P. E., Martin M. A. Characterization and partial nucleotide sequence of endogenous type C retrovirus segments in human chromosomal DNA. Proc Natl Acad Sci U S A. 1983 Feb;80(3):678–682. doi: 10.1073/pnas.80.3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Hendrick J. P., Jr, Lerner M. R., Steitz J. A., Reichlin M. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983 Feb 11;11(3):853–870. doi: 10.1093/nar/11.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Yoshida M. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6899–6902. doi: 10.1073/pnas.79.22.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Golde D. W., Miwa M., Sugimura T., Chen I. S. Nucleotide sequence analysis of the long terminal repeat of human T-cell leukemia virus type II. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1079–1083. doi: 10.1073/pnas.81.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]