Abstract
Introduction
The prostate cancer is difficult to predict, and treatment failure is associated with local infiltration, as well as distant metastases. Adhesion and migration abilities to of cancer cells play a major role in formation of metastasis. The participation of β-catenin in pathogene-sis of many types of cancer and benign processes has been an important discovery of recent years.
Material and methods
The studied material was obtained by transrectal, sextant core biopsy from 102 patients hospitalized in Department of Urology, Regional Hospital in Kalisz (2001-2004).
The aim of our study was to determine the predictive value of β-catenin immunoexpression in prostate cancer, to analyze the prognostic aspect of some histopathological features and finally to assess the relationship between β-catenin immunoreactivity and the microscopic image of the tumor.
Relationships between the investigated variables were analyzed using the Chi2 test of compatibility. We used the Kaplan-Meier curves to assess survival differences between groups of patients. Finally we established which of the studied factors significantly affect the patient outcome, using the method of Cox proportional hazard regression.
Results
In prostate cancer in comparison with the normal epithelium, both the location and the strength of β-catenin immunoexpression are impaired.
Conclusions
Our results indicate that the presence of disorders in β-catenin immunoexpression in prostate cancer cells indicates a high risk of death due to tumor progression and makes it imperative for immediate treatment procedures.
Keywords: β-catenin, immunoexpression, prostatic cancer, prognostic factor, unfavorable prognosis, Gleason index
INTRODUCTION
In the last few years, prostate cancer (PCa) has become one of the main research challenges for many medical studies [1]. In Europe alone, there are over a quarter million new cases recorded annually. On a worldwide scale, significant regional differences in the incidence of this cancer exist [2, 3]. Poland has recently experienced a significant increase in the number of men diagnosed with PCa, but fortunately the number of deaths from this disease has not grown so dramatically. Nevertheless, it has been estimated that in 2008 more than 3.8 thousand men died in Poland because of PCa [4].
The development of PCa is a multi-step process, the result of a sequence of molecular changes. The course of the disease is difficult to predict, and treatment failure is associated with the local infiltration of the gland and surrounding structures as well as distant metastases. Metastases are the most common cause of death in PCa [1, 5, 6].
The adhesion of cancer cells and their ability to migrate play a major role in formation of metastasis. A clear correlation between the level of adhesion molecules and the ability of these cells to metastasize was discovered in the mid-twentieth century [7, 8].
The important role of β-catenin associated with the cytoplasmic domain of E-cadherin for the functioning of intercellular adhesion complexes is widely known [7]. The β-catenin-cadherin complex recruits α-catenin, which in turn binds the actin of the cytoskeleton. These interactions permit the formation of intercellular adherens junctions. They are necessary for the creation and maintenance of epithelial cell layers by regulating cell growth and adhesion. β-Catenin is also responsible for transmitting the contact inhibition signal that causes cells to stop dividing once the epithelial sheet is complete [9].
Secondly, β-catenin acts as a component of the Wnt signal transduction pathway. This pathway is a network of proteins best known for their roles in embryogenesis, but also their involvement in normal physiological processes in adult animals. It regulates cell proliferation and differentiation. The level of free β-catenin is controlled by a complex that facilitates its breakdown. The complex includes axin (scaffolding protein), glycogen synthase kinase 3b (GSK-3β), and the adenomatous polyposis coli (APC) protein. In the absence of the Wnt-signal, the creation of the complex described above results in a substantial increase in the phosphorylation of β-catenin by facilitating the action of GSK3β. This leads to ubiquitination and proteasomal degradation of β-catenin through the β-TrCP/SKP complex. When Wnt-signaling proteins bind their receptors, they inactivate GSK-3β, allowing β-catenin to accumulate in both the cytoplasm and nucleus. Within the nucleus, β-catenin activates the TCF/LEF transcription factors, which in turn act on genes including c-Myc, TCF-1, and cyclin D [10].
During development, the Wnt/β-catenin pathway integrates signals from many other pathways including retinoic acid, FGF, TGF-β, and BMP in many different cell-types and tissues [11]. In addition, GSK-3β is also involved in glycogen metabolism and other key pathways, which has made its inhibition relevant to diabetes and neurodegenerative disorders [10].
The participation of β-catenin as a central protein in the Wnt signaling pathway in the pathogenesis of many types of malignant and benign neoplasms has been a significant discovery of recent years [8]. β-catenin expression is regulated by the adenomatous polyposis coli (APC) gene, which can function as an oncogene. Importantly, point-mutations in β-catenin lead to its deregulated stabilization. Mutations of the β-catenin gene were detected in colonic, ovarian, pancreatic, and prostatic carcinomas as well as nonepithelial neoplasms such as synovial sarcoma, osteosarcoma, liposarcoma, and malignant fibrous histiocytoma [8, 12–15]. Dysregulation of β-catenin also occurs in Gardner's syndrome, where it leads to both familial adenomatous polyposis and fibromatosis. Expression of β-catenin is increased in aggressive fibromatosis [16]. Recent evidence suggests that β-catenin plays an important role in various aspects of liver biology, including liver development (both embryonic and postnatal), liver regeneration following partial hepatectomy, HGF-induced hepatomegaly, and pathogenesis of liver cancer [17].
Interest in the Wnt / β-catenin pathway in the context of therapy (non-steroidal drugs, exisulind, vitamin A, endostatin, monoclonal antibodies, and low molecular weight inhibitors) has resulted in an increasing number of studies indicating this complex as a crucial element in the etiology of many diseases. Pharmacological inhibition of the Wnt / β-catenin signaling pathway can be an effective weapon in the battle against cancer by inducing apoptosis and inhibiting the proliferation of cancer cells [18].
In normal epithelial cells, β-catenin is found at the plasma membrane where it provides a mechanical linkage between cell-to-cell junctional and cytoskeletal proteins. In tumor cells, however, β-catenin is often found in the cytoplasm and nucleus where it is associated with TCF family members to form a complex that activates transcription of pro-mitotic proteins [9]. Relocalization of β-catenin also occurs as part of the epithelial-mesenchymal transition (EMT) process and body axis specification, which are essential to organ development in the embryo [11, 19].
The results of our study indicate that the presence of disorders in β-catenin immunoexpression in PCa cells corresponds with a high risk of death due to tumor progression and urges doctors to apply immediate and radical treatment procedures. Changes in immunohistochemical staining of β-catenin, in conjunction with the high value of the Gleason score, appear to be a valuable prognostic parameter allowing the selection of a group of patients with aggressive forms of PCa based on microscopic examination of tissue obtained from prostate biopsy.
MATERIALS AND METHODS
The materials were obtained by transrectal sextant core biopsy from 102 patients, who were hospitalized in the Department of Urology of the Regional Hospital in Kalisz in the years 2001-2004 because of the suspicion of PCa. Core biopsies were performed according to a uniform procedure under the control of transrectal ultrasonography (TRUS). Patients’ age ranged from 52 to 84 (mean age 69.8). Their outcomes (death, survival time) were known. Statistical data from the Hospital Information System and the Registry Office in Kalisz allowed us to establish that PCa was the cause of death in 25 patients.
All biopsy specimens were fixed in 4% buffered formalin solution and then embedded in paraffin according to standard procedures. Serial sections (2µm thickness) were used for hematoxylin and eosin staining and immunohistochemistry.
An Olympus BX/41 light microscope was used for detailed microscopic evaluation. The histopathological analysis of specimens was based on the following features: microscopic malignancy according to Mostofi; Gleason score; mitotic index; volume percent of neoplastic infiltration in tissue specimen; the presence of prostatic intraepithelial neoplasia (PIN) in prostatic adjacent tissues; infiltration of nerves, blood vessels and/or muscles by tumor cells; inflammation in and around the cancer foci; and the presence of tumor necrosis.
Immunohistochemical studies were performed using Novocastra antibodies against β-catenin (Dilution 1:300, Clone 17C2) according to the manufacturer's protocol.
In evaluating of the results of immunohistochemical staining in PCa cells, we estimated them according to location and strength of reaction using a semi-quantitative analysis method. Normal glandular epithelium from the vicinity of the cancer was used as a tissue of reference in estimating the strength of the reaction as reduced, comparable, or increased. As the reference point we established positive membrane staining when clearly visible in greater than 70% of cells. The presence of cytoplasmic staining and/or expression of cell membrane in a percentage less than 70% were qualified to the group of tumors with an impaired immunoexpression of β-catenin.
All data were collected in a database for statistical evaluation. A simple relationship between the investigated variables and the parameter of survival in patients with PCa were analyzed using the compatibility Chi-square test. We used the Kaplan-Meier curves to assess survival differences between the groups of patients. Differences between curves were verified by the log-rank test. Cox proportional hazard regression statistical analysis was used to determine which of the factors significantly affect patients’ survival time. To assess the differences between the two groups derived from the ordinal scale we used the nonparametric Mann-Whitney U test, as in the case of comparing two groups with an interval scale. However, to compare more groups derived from an ordinal scale, the nonparametric Kruskal-Wallis test was used. All tests were analyzed at a significance level of p = 0.05.
RESULTS
Histopathological features of PCa
Gleason score greater than seven increased the risk of death by more than threefold (RR = 3.12). The Kaplan-Meier analysis showed a significant difference in possible survival between men suffering from PCa with Gleason score of seven and those with Gleason score above seven (p = 0.00048). This confirms the value of the Gleason score as an objective prognostic factor for PCa.
PIN associated with the cancer was found in 64% of cases (the dominated type was HG-PIN). We did not notice the correlation between the occurrence or lack of PIN, as well as the presence of HG-PIN and survival (P = 0.5832). Also, no statistical relationship was revealed with the value of Gleason score (P = 0.3025).
Nerve infiltration was found in more than half of tumors (53%) and, although the risk of death for patients with this feature increased almost twofold, we did not notice a statistical significance (P = 0.0825). There was also no significant dependence of value of Gleason score (P = 0.0687).
The infiltration of blood vessels was discovered in fewer cases (23%). In turn, inflammation was noticed in almost half of tumors (48%). We did not show a significant correlation for neither of these microscopic changes and the risk of death of patients or Gleason score.
The presence of necrosis in tumor tissue and value of Gleason score was significant (P = 0.0252). This feature was noted more than five times more often in poorly differentiated carcinomas (Gleason score above seven) than in the group with moderately differentiated tumors (Gleason score 5-7).
The infiltration of muscles was disclosed in 51% of specimens, but there was no significant relationship between this parameter and patient survival (P = 0.1339). However, we found the strong correlation between value of Gleason score and muscles invasion (P = 0.0008). This feature was observed in the majority of cancers with Gleason score above seven (73%) and in only 38% of the group with Gleason score 5-7.
We found the mitotic index of the tumors to be highly significant when patients who died were compared with those still alive: 2.0 vs. 1.36 (P = 0.0108). Also, the volume percent of tumor infiltration in the studied biopsies among the deceased patients was greater (mean 65.6%) than in the living (52.6%), however there was no significant correlation (P = 0.054).
We also demonstrated a significant difference between the value of Gleason score in terms of mitotic activity (p = 0.0009) and in the volume percent of tumor infiltration (p = 0.0028). In poorly differentiated prostate tumors (Gleason score greater than seven), both the volume percent of tumor infiltration and the value of mitotic index were generally higher (67.9% vs. 48.5%, and 2.62 vs. 0.89).
β-catenin immunoexpression and survival of patients
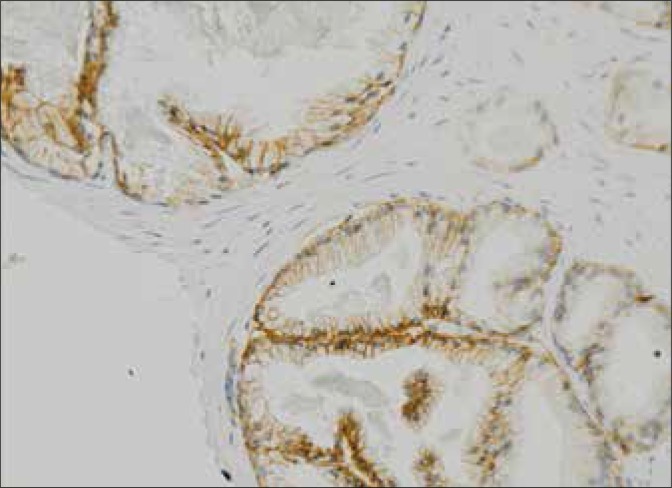
In PCa, both the localization and the intensity of β-catenin immunoexpression are impaired in comparison to normal prostatic epithelium. Disturbances in β-catenin immunoexpression in association with the Gleason score in the studied cases were characterized by a very strong statistical significance. The majority (94%) of moderately differentiated PCa (Gleason index 5-7) were characterized by normal immunoexpression of the tested protein. On the contrary, almost all tumors (97%) in the group with a poor differentiation (Gleason 8-10) were characterized by a reduced intensity of immunohistochemical staining for β-catenin compared to normal prostate epithelium (Fig. 1).
Fig. 1.
Normal immunohistochemical staining for β-catenin in prostate glandular epithelium (magnification 200x).
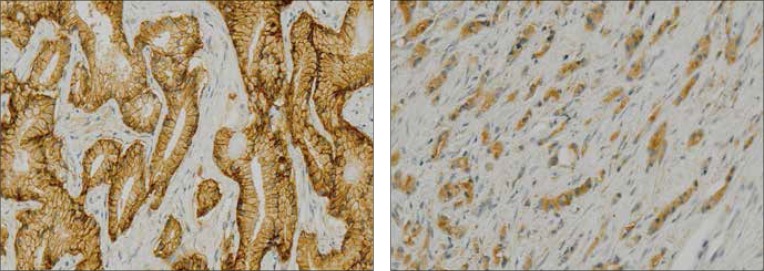
Increased mortality in patients with PCa was associated with significantly impaired localization and intensity of β-catenin immunohistochemical expression in PCa cells. The risk of death in patients with abnormal β-catenin immunoexpression in the tumor (cytoplasmic staining) increased threefold (RR = 3.26), while the risk of death in patients with reduced intensity of immunohistochemical reaction of β-catenin increased over 6-fold (RR = 6.39) when compared to normal expression (Tables 1 and 2). Comparison of Kaplan-Meier analyses confirmed a significant difference in the probable survival for the group of patients with abnormal β-catenin immunoexpression (p = 0.00019) and with reduced β-catenin immunohistochemical reaction in PCa cells (p = 0.0001) (Figs. 2 and 3).
Table 1.
Localization of β-catenin immunoreaction and patients survival
| β-CATENIN IMMUNOEXPRESSION | PATIENTS DIED | PATIENTS LIVING |
|---|---|---|
| Impaired (cytoplasmic) | 16 (44%) | 20 (56%) |
| Normal (membrane) | 9 (13%) | 57 (87%) |
| TEST Chi2 | 95% CI = 1.60 – 6.62 RR = 3.26 P <0.0001 |
|
Table 2.
Intensity of β-catenin immunoreaction and patients survival
| β-CATENIN IMMUNOEXPRESSION | PATIENTS DIED | PATIENTS LIVING |
|---|---|---|
| Impaired (reduced) | 21 (46%) | 25 (54%) |
| Normal (comparable) | 4 (7%) | 52 (93%) |
| TEST Chi2 | 95% CI = 2.36-17.30 RR = 6.39 P <0.0001 |
|
Fig. 2 and 3.
Distorted β-catenin immunoexpression in prostate cancer cells (magnification 200x).
The relationship between microscopic features of PCa and β-catenin immunoexpression
Cox regression proportional hazard analysis for all tested parameters also revealed that the reduced intensity of β-catenin immunoexpression significantly affects patients’ survival (p = 0.007). These patients presented with more than seven-fold higher (7.40x) risk of dying from PCa compared with men with normal tumor expression of this protein. Thus, we consider these parameters (the localization and intensity of β-catenin immunoexpression) as possible predictors of survival in patients with PCa (Table 3).
Table 3.
Model of Cox proportional hazards regression
| EXAMINED VARIABLES | RISK INDEX | 95% CI | P-VALUE |
|---|---|---|---|
| Age | 0.99 | 0.94-1.00 | 0.8230 |
| Gleason score | 0.47 | -0.19-2.05 | 0.2905 |
| Accompanying PIN | 1.54 | 0.70-2.85 | 0.1221 |
| Infiltration of nerves | 2.27 | -0.32-4.54 | 0.1589 |
| Angioinvasion | 0.63 | -0.08-1.35 | 0.4261 |
| Inflammation | 1.31 | 0.21-1.64 | 0.5302 |
| Presence of necrosis | 1.94 | -0.76-3.15 | 0.3507 |
| Mitotic index | 1.03 | 0.86-1.04 | 0.7484 |
| Extent of tumor infiltration% | 1.00 | 0.98-1.00 | 0.9157 |
| Infiltration of the muscles | 1.07 | -0.08-1.09 | 0.8957 |
| β-catenin-disturbed location of reaction | 0.35 | -0.15-3.31 | 0.1494 |
| β-catenin-reduced reaction intensity | 7.40 | -3.37-1798 | 0.0070 |
| The significance of the model | Chi2 | degrees of freedom df | p-value |
| 27.89 | 12 | 0.0057 |
We showed a significant correlation (P <0.0001) between Mostofi histological grading of PCa and β-catenin immunoreactivity (both localization and intensity). Aberrant (localization) β-catenin immunoexpression increased from 5% for G1 to 67% for G3 tumors. In the group of neoplasms with reduced immunohistochemical staining we also observed a similar trend: 15% for G1 and 88% for G3.
We noted a significant correlation between the presence of PIN and aberrant staining of β-catenin (localization – P = 0.0373) (intensity – P = 0.0243). Characteristically, tumors without accompanying PIN, were most represented in the group with reduced staining and incorrect localization of β-catenin.
In turn, we did not detect a significant relationship between β-catenin immunoexpression and nerve invasion (P = 0.1018 and P = 0.0639), blood vessels invasion (P = 0.3506 and P = 0.2109), or inflammatory reaction (P = 0.9999 and P = 0.0213).
The presence of necrosis in PCa was correlated with the intensity of β-catenin reaction (P = 0.0213), but we did not notice the connection with aberrant localization of immunohistochemical staining (P = 0.9999).
Analysis of the relationship between muscles invasion on PCa cells and the results of β-catenin immunohistochemical staining showed a significant correlation for both, localization (P = 0.0192) and intensity (P = 0.0026). Infiltration of muscles was observed twice as often in the group with decreased reaction, and nearly twice as often among patients with aberrant localization of β-catenin immunostaining.
We also showed a significant correlation between the mitotic index (P = 0.0079), volume percent of tumor infiltration (P = 0.0241), and location of β-catenin immunohistochemical staining. Physiological localization of the β-catenin immunoreaction was associated with a mitotic index at half of mean value of (1.09 vs. 2.31) and a lower volume of malignant invasion (50.3% vs. 65.3%) compared with tumors with aberrant expression of the protein. The decreased intensity of immunohistochemical staining was associated with nearly three times higher mitotic index than the mean value (2.30 vs. 0.88, P = 0.0031) and a highly significant volume of neoplastic infiltration in the studied biopsies (65.5% vs. 47.5, P = 0.0034).
DISCUSSION
PCa usually develops slowly, and direct causes of death in patients with this tumor vary, but the majority of treatment failures are associated with the dissemination of the disease and the presence of distant metastases. The cause of metastasis in PCa appears to be a disorder in the structure and functioning of the molecular mechanisms responsible for cellular adhesion [5, 6, 15]. In the last decade, only a few articles concentrated on the results of PCa studies conducted in terms of the importance of adhesion molecules and other elements of the signaling pathway of Wnt / β-catenins in the growth of this tumor. Polish studies on this subject are also not abundant, and their results must be described as ambiguous [20]. They demonstrated the prognostic value of impaired immunoexpression of E-cadherin and ezrin, especially in poorly differentiated cancers (Gleason score greater than seven) [21].
At the 40th Congress of the American Society of Clinical Oncology in New Orleans, Horvath et al. presented the results of their study of the prognostic value of low levels of β-catenin immunoexpression in the nuclei of cancer cells in its early form as an independent parameter indicating a very poor prognosis for those patients whose initial prognosis seemed good and the tumor was qualified for radical surgery [22].
Jaggi et al. assessed the expression of major disturbance in the adhesion complex components such as E-cadherin and β-catenin. They examined serial sections from postoperative specimens after radical prostatectomy (17 for β-catenin and 16 for E-cadherin) by immunohistochemical analysis. The authors found that in poorly differentiated PCa, the disorder of immunoexpression of β-catenin and E-cadherin may be a useful biomarker of tumor aggressiveness. Immunopositive reactions for β-catenin and E-cadherin in PCa were evaluated for their location and strength (using a semi-quantitative scale), comparing them with normal glandular epithelium. Statistical analysis was applied to the microscopic malignancy expressed in Gleason score, stage of disease (TNM), and results of immunohistochemical analysis. The strength of immunoreactions for β-catenin and E-cadherin were decreased in PCa compared with normal glandular epithelium and correlated with the increased malignancy expressed in Gleason score, particularly at a ratio of greater than seven [23].
Similarly, Aaltomaa et al. examined the immunohistochemical staining of α-and β-catenin in cancer cells and their correlation to patients’ survival (observation period up to 7.3 years) in 181 men after radical prostatectomy. They concluded that an unaffected location of the β-catenin immunoreaction (no cytoplasmic/nuclear staining) was associated with the low clinical stage of the tumor, and a reduced strength of immunohistochemical expression of both catenins correlated with shorter patients’ survival [24].
Bismar et al. published the results of β-catenin immunoexpression in 101 patients with PCa and high grade PIN (HG-PIN) and compared them with normal prostate epithelium as well as 24 cases of colorectal tumors. Furthermore, they studied the reactions of other proteins: cytokeratins 7 and 20 as well as PSA. In 83% of samples from colorectal cancer they noted nuclear β-catenin immunoreactivity, whereas membrane staining predominated in PCa (88%). As for strength, they observed the reaction comparable to that of normal epithelium in 80% of PCa, decreased in 4% and increased in 4% of tumors. In our study we did not find the increase in strength of reaction in any cancer and the reduction of immunoexpression was observed in a greater number of tumors (45%). The authors, however, failed to assess the relationship between outcomes and the degree of clinical progression and malignancy of the examined tumors. β-catenin immunoreactivity in HG-PIN was similar to that observed in normal prostate epithelium. Finally, Bismar et al., due to the results of immunoexpression of other examined reagents, proved that there are differences in the role of the Wnt/β-catenin signaling pathway in the pathogenesis of prostate and colorectal cancers [25].
In recent years, in vitro studies of PCa have brought much to know about the molecular mechanism of tumor progression, including the formation of distant metastases. However, many aspects of this process remain unsolved and require further study. In locally advanced prostate tumors cell clones capable to metastasize arise, due to aberrations of structure and function of molecules involved in adhesion mechanisms, including: catenins, E-cadherin, and ezrin. Van Oort et al. studied the predictive value of disorders of the E-cadherin immunoexpression and molecules associated with a cadherin complex (α, β, γ-catenins and p120 protein) in PCa. In 65 specimens obtained by radical prostatectomy or TURP, they examined the strength of immunoexpression for each of the elements of the cadherin complex and evaluated survival curves using the Kaplan-Meier analysis and multivariate Cox proportional hazards regression. Their investigations have shown the correct expression of β-catenin in 61.5% of cases, when compared to results obtained in our research (65%). A significant correlation between expressions of individual studied molecules was also confirmed. Five-year survival rate of patients with impaired β-catenin staining was 27.3% and it was significantly lower in comparison to the 73.1% in patients with normal immunoreaction of β-catenin [21]. As with our analysis, the difference between normal and impaired protein immunoexpression compared to survival (log rank P <0.0001) was statistically significant. However, the strength of α-catenin expression proved to be a little more valuable as a prognostic factor in correlation with tumor stage and value of Gleason score. Multivariate analysis using proportional hazards regression revealed the predictive value for α-and β-catenin with slight advantage of the former [26].
Some research has been based on the latest molecular techniques. Potti et al. examined specimens from 67 patients with metastatic and hormone-resistant PCa, derived from the primary tumor and lymph node and bone metastases. These tissue samples were subjected to immunohistochemical analysis using antibodies against the urokinase receptor (uPAR), Wnt-1, and β-catenin. In regard to β-catenin immunoexpression in the primary tumor, 34% of cancers showed the disturbed location of reaction (44% in our study), and the percentage of abnormal immunohistochemical staining increased to 77% for cancer metastasis to lymph nodes and 85% for bone metastases. Convergent results were recorded in the assessment of reactions to uPAR and Wnt-1. The authors confirmed the prognostic usefulness of these markers and showed the direction of the therapeutic strategy by targeting gene interaction, because invasive cancer is regulated by their protein products [27].
Genes promoting tumor invasiveness encode, among others, enzymes hydrolyzing basement membrane and extracellular matrix, thereby increasing the ability of cells to migrate [15]. Recently, despite costs, studies using tissue microarrays are gaining popularity. Whitaker et al. analyzed 77 specimens starting from benign prostatic hyperplasia to low, moderate, and high-grade carcinomas. They noted a gradual decline in β-catenin immunoexpression with increasing malignancy of the tumors estimated by Gleason score. Interestingly, there were no changes in expression of β-catenin in tumors treated with androgen ablation and in those without hormone therapy. The authors concluded that the decline of β-catenin immunoexpression seems to be a better prognostic marker than the absolute value of its expression in PCa cells [28]. Unfortunately, the tissue matrix method is too expensive for clinical practice.
Saha et al. compared immunohistochemical coexpression of β-catenin and E-cadherin in primary PCa with metastases to bone, in association with the results from benign prostatic hyperplasia (BPH). They revealed a clear decrease in β-catenin immunoexpression in cancer cells, as in our study. It is interesting that there are increases in the immunohistochemical reactions of both adhesion molecules in the cells obtained from bone metastases to values comparable to that observed in BPH. These authors first showed an increased expression of these proteins in cancer cells from bone metastases suggesting a close relationship of these immunoexpressions with cell adhesion in metastases [29]. It seems to be important to find a useful biological marker and a method for its identification in order to assess the aggressiveness of PCa at the time of clinical diagnosis. Still in the first place, is the pathological examination of the tissue obtained by prostate core biopsy, which decides the scope, nature, and even time of implementation of the clinical treatment.
CONCLUSIONS
The results of this study suggest that β-catenin is involved in PCa tumorigenesis and progression. The aberrant or decreased expression of β-catenin appears to be one of most promising markers of poor prognosis in localized PCa. Gleason score is also an objective prognostic factor for this neoplasm.
In addition, we established the survival in patients with PCa depends on histopathological features, such as: the presence of necrosis, the value of mitotic index, and volume percent of tumor infiltration in transrectal core biopsy material.
REFERENCES
- 1.Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic and therapeutic implications. Hum Pathol. 2010;41:781–793. doi: 10.1016/j.humpath.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(Suppl 6A):3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Zbiór Krajowego Rejestru Nowotworów (KRN) [Collection of the National Cancer Registry] http://85.128.14.124/krn/
- 5.Ahmad A, Hart IR. Mechanism of metastasis. Critical Reviews in Oncology/Hematology. 1997;26:163–173. doi: 10.1016/s1040-8428(97)10002-6. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WG, DeMarzo AM, DeWeese TL. The molecular pathogenesis of prostate cancer: focus on the earliest steps. Eur Urol. 2001;39(Suppl 4):8–11. doi: 10.1159/000052574. [DOI] [PubMed] [Google Scholar]
- 7.Davies G, Jiang WG, Mason MD. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J Urol. 2000;163:985–992. [PubMed] [Google Scholar]
- 8.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 9.Hayashida Y, Honda K, Idogawa , et al. E-cadherin regulates the association between β-catenin and actinin-4. Cancer Res. 2005;65:8836–8845. doi: 10.1158/0008-5472.CAN-05-0718. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 12.Ng TL, Gown AM, Barry TS. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 13.Kuhnen C, Herter P, Muller O. Beta-catenin in soft tissue sarcomas: expression is related to proliferative activity in high-grade sarcomas. Mod Pathol. 2000;13:1005–1013. doi: 10.1038/modpathol.3880181. [DOI] [PubMed] [Google Scholar]
- 14.Lamparska-Przybysz M, Wieczorek M, Majorek M, Guzenda P. Rola szlaku Wnt/β-katenina w molekularnym mechanizmie procesów nowotworowych [Role of Wnt/β-catenin pathway in molecular mechanism of tumorigenesis] Wsp Onk. 2006;10:497–501. [Google Scholar]
- 15.Mason M, Gaynor D, Wen G. Cell adhesion molecules and adhesion abnormalities in prostate cancer. Critical Reviews in Oncology/Hematology. 2002;41:11–28. doi: 10.1016/s1040-8428(01)00171-8. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C. Nuclear beta-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29:653–659. doi: 10.1097/01.pas.0000157938.95785.da. [DOI] [PubMed] [Google Scholar]
- 17.Thompson MD, Monga SP. WNT/β-catenin signaling in liver health and disease. Hepatology. 2007;45(5):1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 18.Janssens N, Janicot M, Perera T. The Wnt-dependent signaling pathways as target in oncology drug discovery. Invest New Drugs. 2006;24:263–280. doi: 10.1007/s10637-005-5199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139(6):1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Pietruszewska W, Kobos J, Gryczyński M. Ekspresja białka beta-katenina w raku płaskon abłonkowym krtani [Expression of β-catenin protein in squamous cell carcinoma of the larynx] Otol Pol. 2004;5:949–956. [PubMed] [Google Scholar]
- 21.Musiał J, Sporny S, Nowicki A. Prognostic significance of E-cadherin and ezrin immunohistochemical expression in prostate cancer. Pol J Pathol. 2007;58:235–243. [PubMed] [Google Scholar]
- 22.2004. Jun 5-8, p. 9570. [Google Scholar]
- 23.Jaggi M, Johansson SL, Baker JJ, et al. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23:402–406. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Aaltomaa S, Kärjä V, Lipponen P, et al. Reduced alpha- and beta-catenin expression predicts shortened survival in local prostate cancer. Anticancer Res. 2005;25:4707–4712. [PubMed] [Google Scholar]
- 25.Bismar TA, Humphrey PA, Grignon DJ, Wang HL. Expression of beta-catenin in prostatic adenocarcinomas: a comparison with colorectal adenocarcinomas. Am J Clin Pathol. 2004;121:557–563. doi: 10.1309/4470-49GV-52H7-D258. [DOI] [PubMed] [Google Scholar]
- 26.Van Oort M, Tomita K, van Bokhoven A, et al. The prognostic value of E-cadherin and the cadherin-associated molecules α-, β -, γ-catenin and p120ctn in prostate cancer specific survival: A long-term follow-up study. Prostate. 2007;67:1432–1438. doi: 10.1002/pros.20626. [DOI] [PubMed] [Google Scholar]
- 27.Potti A, Chen G, Shukeir N. Molecular markers of metastases in advanced stage adenocarcinoma of the prostate. J Clin Oncol; ASCO Annual Meeting Proceedings 9672.2005. [Google Scholar]
- 28.Whitaker HC, Girling J, Warren AY, et al. Alterations in beta-catenin expression and localization in prostate cancer. Prostate. 2008;68:1196–1205. doi: 10.1002/pros.20780. [DOI] [PubMed] [Google Scholar]
- 29.Saha B, Arase A, Imam SS, et al. Overexpression of E-cadherin and beta-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68:78–84. doi: 10.1002/pros.20670. [DOI] [PubMed] [Google Scholar]