Abstract
Purpose
To prove the long-term efficacy of BTX-A injection in the management of children with neurogenic detrusor hyperactivity.
Materials and methods
28 out of 145 children with neurogenic bladder (15 male and 13 female, mean age 10.7 years) who were treated between 2002 and 2010 and became non-responders to conservative treatment were included into the retrospective study.
We injected 10-12 U/kg of BTX-A (Botox®) into the detrusor at 20-30 sites, sparing the trigone. The mean follow-up was 48 months (range 6-84 months).
Results
Group 1. 14 patients had a single injection of BTX-A. Five of them were successful.
Mean bladder reflex volume increased (from 62.9 to 117.5 ml), maximum detrusor pressure decreased (from 59 to 37.5 cm H2O), detrusor compliance increased (from 4.8 to 9.5 ml/cm H2O), and leak-point-pressure decreased (from 46.5 to 24.2 cm H2O). Four patients did not respond and were treated by ileocystoplasty. Another five were lost to follow-up. Group 2. 14 patients had repeated (mean 2.5) injections of BTX-A with a mean interval of 13.7 months. In thirteen patients, urodynamic parameters of the first and last injection were similar to those obtained in Group 1, showing a good response. One patient received an ileocystoplasty.
Conclusion
BTX-A is a safe alternative in the treatment of detrusor hyperactivity in children with myelomeningocele (MMC). The efficacy lasted a mean of 12 months and urodynamic response was unchanged even after several injections. In our series, 21.7% of children with severe low-compliance bladders were non-responders.
Keywords: neurogenic detrusor hyperactivity, children, BTX-A
INTRODUCTION
Myelomeningocele (MMC), tethering of the cord, and sacral anomalies are the most common cause of pediatric neurogenic bladder [1]. Traumatic spinal lesions in children are less frequent.
Detrusor sphincter dyssynergia (DSD) is a major urological problem in newborns with MMC and occurs in almost 50% of MMC patients [2]. Hydronephrosis develops in 72% of MMC patients with DSD, while vesicorenal reflux (VRR) is found in about 81% of patients with leak point pressure of 40 cm H2O or more [2, 3]. A bladder pressure >40 cm H2O is the essential urodynamic warning for damage to the upper urinary tract [4]. Treatment of bladder dysfunction is, therefore, primarily aimed at preservation of the upper urinary tract and secondarily at gaining continence and improving the quality of life. Management aims for this type of neurogenic bladder are to achieve a continent low-pressure reservoir with adequate capacity and good compliance, and without VRR in order to avoid the well-known consequences of renal scarring and renal failure.
Early intermittent catheterization (IC) and administration of anticholinergic medications are the basic treatment in most cases [5, 6]. Failure of this conservative approach is revealed by recurrent leakage, recurrent urinary tract infections, and/or worsening of hydronephrosis, and suggests the need of an alternative treatment. About 10% of patients are non-responders to anticholinergic medication or suffer side effects from anticholinergic drugs, even if administered intravesically [7].
Recently published reports have discussed the successful outcome of intravesical injections of botulinum toxin A (BTX-A) as an effective second line treatment for MMC children with neurogenic detrusor hyperactivity [8–23].
Our previous study showed that BTX-A is a safe alternative in the management of detrusor hyperactivity in MMC children. Both urodynamic parameters and continence were improved after the therapy [8].
We present the long-term follow-up of children with MMC treated with BTX-A due to neurogenic detrusor hyperactivity during 2002-2010.
MATERIAL AND METHODS
In this retrospective study, the data related to 28 children, 15 boys and 13 girls, with neurogenic detrusor hyperactivity who received BTX-A injections between 2002 and 2010 was analyzed. The mean follow-up time was 48 months (range: 6-84 months). The children’s average age at the time of first injection was 6.45 years (range 1-16 years).
Twenty-four of the 28 children had MMC, one had spina bifida occulta, one had a laminectomy to remove an intradural lipoma, and one had a spinal cord injury. Eighteen children were wheelchair-bound and 10 could walk (non-wheelchair-bound patients).
The children were separated into two groups. Group-1 (14 children) received only one BTX-A injection, while group-2 (14 children) received repeated BTX-A injections ranging from two injections to a maximum of six with an average of 2.5, and the interval between these was 13.7 months on average.
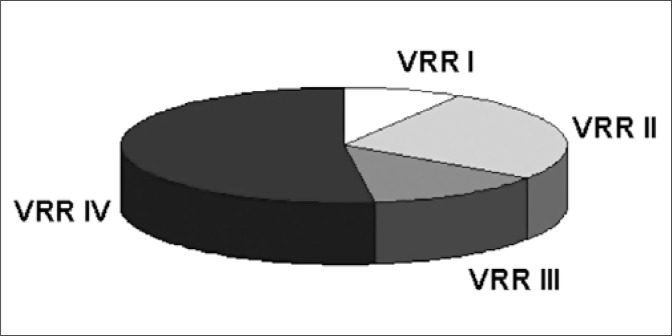
All patients had neurogenic detrusor hyperactivity and 11 of them also had a low-compliance bladder. Fifteen patients had VRR – seven unilateral and eight bilateral. In the 23 refluxive ureters the reflux grade was as follows: two had grade I, six had grade II, three had grade III, and 12 had grade IV (Fig. 1).
Fig. 1.
VRR grade in 15 MMC children, who received BT-a injection.
All patients were on anticholinergic medication (oxybutynin chloride 0.3 mg/ kg bid, or tolterodine chloride 0.1 mg/ kg bid) and IC was used to achieve bladder emptying four to five times daily.
Twenty-six patients were non-responders to oral or intravesical anticholinergic medications – all children had a high leak point pressure (more than 40 cm H2O) despite using high doses of anticholinergics. Two patients had intolerable side effects to anticholinergic medication.
Medical history was assessed in all patients. Unrelated to age of the children, the daily incontinence score was recorded on a scale ranging from 0-3, as already described by Schruch et al. (9) – 0 = completely dry; 1 = wet once a day, usually at night; 2 = wet for less than 50% of time between catheterizations; and 3 = wet for more than 50% of time between catheterization. We evaluated patients’ bladder diaries for at least three days by documenting the catheterization protocol (timing and volumes) and assessing urine losses by repeated measurement of the weight of absorptive pads (Pad testing). If deemed necessary, diaper tests were also performed by placing an alarm sensor into the pads.
Physical examination was performed on all patients and urine samples were obtained for urinalysis and bacterial cultures. Renal clearance and renal spilt function were determined by Technetium-99m dimercaptosuccinic acid (DMSA) in case of febrile urinary tract infections (UTI), and the urinary tract was assessed by ultrasound.
The urodynamic parameters that were measured included: reflex volume (RV, the filling volume when the first uninhibited detrusor contraction occurs), maximal detrusor pressure (MDP), maximal cystometric bladder capacity (MCBC), bladder compliance (BC), and leak point pressure (LPP). Slow bladder filling (10 ml/min) with 24% contrast medium was used to enable simultaneous morphologic evaluation in order to identify the presence of VRR and to evaluate incontinence.
Using a rigid cystoscope and a 25 cm long 3.7 F needle (Williams Needel, Cook, Ireland), BTX-A (Botox®, Allergan) was injected into the detrusor muscle at 20-30 random sites over the bladder, including the dome but sparing the trigone. The dosage depended on the patient’s body weight and ranged between 100 and 300 U (10-12 U/kg). One hundred units of toxin were diluted in 10 ml of normal saline. The whole injection procedure was performed under general anesthesia. The bladder was emptied after the endoscopic treatment and parents were told to restart clean intermittent catheterization (CIC) after 4 hours.
Additional surgical interventions were performed during some of BTX-A injection procedures as follows: 11 endoscopic subureteral injections to treat the reflux (15 ureters) using dextranomer/hyaluronic acid copolymer (Deflux®) during the first injection, one case of bilateral ureteral reimplantation, and one BTX-A injection in the sphincter of a patient with detrusor sphincter dyssynergia.
Urodynamic evaluation was performed at three, nine, and 12 months after treatment and then at least once yearly. During each follow-up visit, ultrasound and urinalysis were repeated. Treatment efficacy was evaluated according to the urodynamic parameters and incontinence score.
Anticholinergics were discontinued after injection therapy and were resumed after three months as an adjuvant treatment if needed. Reinjection was decided according to symptoms (incontinence, UTI) or urodynamic worsening. In group-1, the urodynamic parameters were compared pre- and post-injection, while in group-2 the outcomes were compared before and after the first and the last injection sessions.
Statistical data were analyzed by standard software. Means were compared by paired Student’s t test. Statistical significance was set at p <0.05.
RESULTS
Group-1
Five out of fourteen children did not require any reinjection, their clinical symptoms and urodynamic parameters remained stable during the follow-up of 56 months (range: 21- 84 months), although with the use of adjuvant antimuscarinics. Two of them were non-responders to oxybutynin chloride 0.3 mg/kg twice a day, but a changeover to tolterodine chloride 0.1 mg/ kg twice a day, initially as adjuvant therapy, was successful. The other three children were on CIC and suffered from recurrent UTI, hence were converted to aseptic IC, which helped them become responders to adjuvant antimuscarinics.
Four out of fourteen children had undergone an ileocystoplasty and rectus fascial sling procedure due to persistent low-compliance bladder, severe incontinence, and in two of them due to persisting high-grade VRR.
We lost five patients from this group to follow-up, because they were admitted from abroad.
Continence-score: Three out of the five stable patients became completely dry between CIC. The other two patients improved from score 3 to 1.
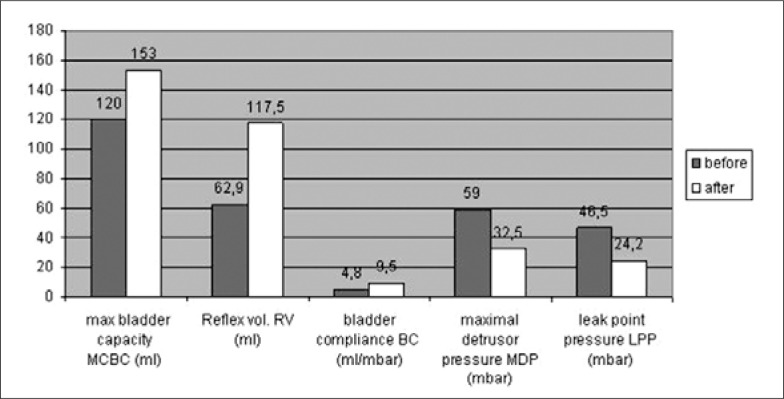
Urodynamic parameters: According to the results obtained three months after BTX-A Injection the mean reflex volume increased from 62.9 ml to117.5 ml (P <0.01). The maximum bladder capacity increased from 120 ml to 153 ml (P <0.01), the maximum detrusor pressure decreased from 59 mbar to 32.5 mbar (P = 0.05). Bladder compliance increased from 4.8 ml/ mbar to 9.5 ml/ mbar (P = 0.05). The leak point pressure decreased from 46.5 mbar to 24.2 mbar (P = 0.01). (Fig. 2. Five patients did not require any reinjection).
Fig. 2.
Urodynamic parameters before and after BTX-A detrusor injection (group 1).
Group-2
All 14 patients had repeated injections (between 2- and 6 months, mean 2.5), the reinjection was decided when the symptoms (incontinence, UTIs) or urodynamic parameters deteriorated. The intervals between the injections were between 6- and 24 months, mean 13.7 months.
Continence-score: six patients became completely continent between catheterizations and five patient's scores improved from 3 to 1, the others four patients remained with a score of 2-3.
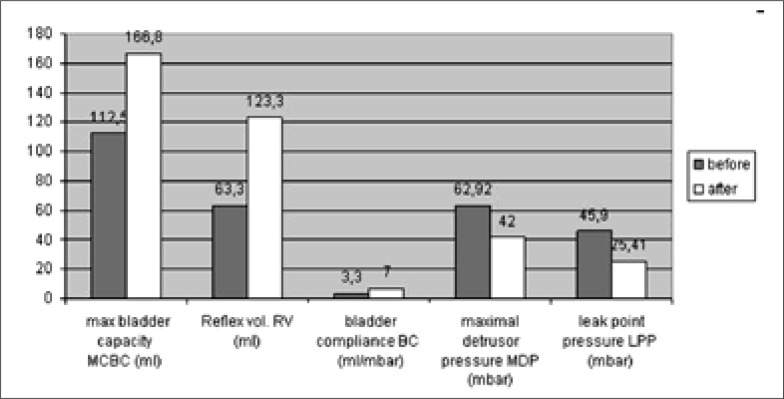
Urodynamic parameters: The urodynamic parameters three months after the first injection showed that the mean reflex volume increased from 63.3 ml to 123.3 ml (P <0.01). The maximum bladder capacity increased from 112.5 ml to 166.8 ml (P = 0.01). The maximum detrusor pressure decreased from 62.92 mbar to 42 mbar (P = 0.027). Bladder compliance increased from 3.3 ml/mbar to 7 ml/mbar (P <0.01). The leak point pressure decreased from 45.9 mbar to 25.4 1mbar (P = 0.05). (Fig. 3: all 14 patients).
Fig. 3.
Urodynamic parameters before and after BTX-A first detrusorinjection (group 2).
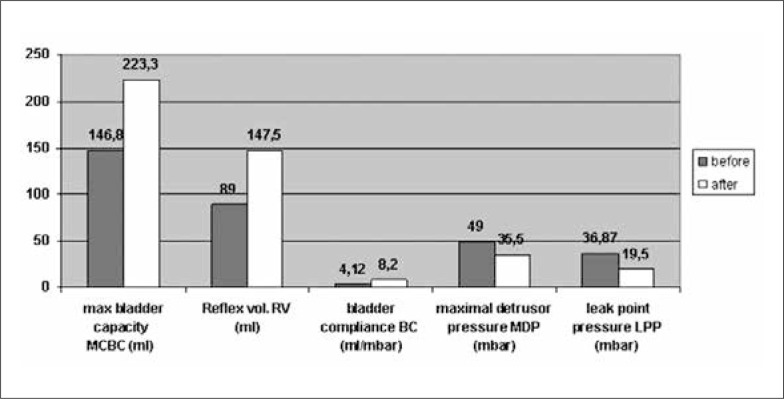
Comparing the urodynamic results three months after the last injections with those obtained after the first one, we found similar parameters (Fig. 4: all 14 patients). Twelve out of 14 patients received adjuvant anticholinergic therapy at this time. One patient underwent ileocystoplasty with rectus fascial sling procedure and Mitrofanoff stoma. Even this child was injected with BTX-A twice – he had high leak-point pressure >45 mbar and the bladder compliance fell to 3 ml/mbar with persisting VRR grade IV in the left kidney.
Fig. 4.
Urodynamic parameters before and after BTX-A last detrusorinjection (group 2).
Both groups
Five patients underwent an ileocystoplasty with rectus fascial sling procedure, two of them ended up with a Mitrofanoff stoma. They aged between seven and 13 years at the time of operations. In spite of the BTX-A injection the neurogenic incontinence persisted in all patients – with low compliant bladder <5 ml/mbar, maximum detrusor pressure >46 mbar, and leak-point pressure >34 mbar – and four patients had persisting VRR. After bladder augmentation, all children achieved adequate continence with a low-pressure reservoir. Two children had asymptomatic persistent VRR that did not require therapy.
Out of the 15 patients with VRR, the VRR resolved in two patients after BTX-A injection only, in seven it resolved after additional endoscopic treatment with Deflux®, two patients required reimplantation of ureter, and four patients underwent ileocystoplasty.
During our almost eight years of experience with BTX-A bladder injections, our patients have never experience any serious side effects.
DISCUSSION
The ideal treatment of neurogenic bladder dysfunction in children should prevent any renal function deterioration, taking into account the importance of controlling urinary incontinence to improve the quality of life of the neurologically handicapped children in the future.
Urinary incontinence in patients with myelomeningocele frequently has an underlying component of detrusor hyperactivity with or without poor bladder compliance. High intravesical pressure, which is often associated with detrusor-sphincter dyssynergia, may cause upper urinary tract deterioration. Other therapeutic approaches should be employed when the option of oral or intravesical anticholinergics is insufficient.
Neurological treatment options such as transurethral electrical bladder stimulation and selective sacral rhizotomy have been found to improve bladder capacity, decrease intravesical pressure, and eliminate uninhibited contractions [24, 25]. However, clinical experience to date is either limited or variable [26, 27].
Bladder augmentation remains a last resort for management of severe low-compliance bladder. A variety of augmentation techniques have been described, including standard enterocystoplasty, auto-augmentation, and seromuscular enterocystoplasty. However, the long-term complications associated with these techniques can be significant [28, 29].
In the last 10 years BTX-A has been reported as a promising approach to manage neuropathic and idiopathic detrusor hyperactivity in children. It is fairly easy to perform with an attractive safety profile [10–13].
Botulinum toxin (BTX-A), first isolated by van Ermengen in 1897, is the strongest biologically occurring lethal toxin that is known. It is produced by Clostridium botulinum, an anaerobic, rod-shaped, Gram-positive bacterium. The toxin acts by selective inhibition of calcium-mediated transport at the synaptic cholinergic junction through modulating a membrane-bound protein (SNAP-25). BTX-A causes a prolonged decrease in regional contractility by chemical denervation [30–33]. In 1989 it was used to treat strabismus and dystonia and this opened the door for its utilization in many other fields of medicine [34–37].
BTX-A was first used in urology in the late 1980s and it was injected into the urethral sphincter in patients with spinal cord injury having detrusor-sphincter dyssynergia [38].
In 2000, Schruch et al. reported their first results of treating high intravesical pressure caused by detrusor-sphincter dyssynergia in adults through the injection of BTX-A directly into the detrusor, showing encouraging results [9]. In 2002 the BTX-A detrusor injection procedure was applied in children by Schulte-Baukloh et al. [39] – they successfully treated 17 patients without adverse events.
Game et al. reviewed the BTX-A intradetrusor injections in children with neurogenic detrusor hyperactivity [20]. They reviewed a total of six articles evaluating the efficacy and safety of BTX-A – 108 patients were included in the six selected studies, their mean age was 9.8 years, and the underlying neurological disorder was MMC in 93%. The patients were followed up for a maximum of one year. All were non-responders to anticholinergic medications. The average (or the usual) dose of BTX-A used was 10-12 U/kg with a maximum dose of 300 U, which was distributed as 30 injections into the detrusor while excluding the trigone. According to the mentioned studies, there was a clear symptomatic improvement (65-87% became completely dry) as well as good effect on the urodynamic parameters (in most studies mean maximal detrusor pressure was reduced to at least 40 mbar and bladder compliance was significantly increased). However, the studies evaluated were limited by the lack of control groups in them and that most of them involved small numbers. The mean time interval between repeated BTX-A injections ranged between 6.3 and 9.6 months. Moreover, the regimen of antimuscarinics used in the patients included in these studies was not clearly described in the majority of them, so it was not possible to assess any impact related to adjuvant antimuscarinics in terms of the efficacy or the duration of the effect of BTX-A.
Among our patients, 64% were completely dry or had an improvement of the continence score from 3 to 1. We think that most of them had additional mixed degrees of urethral sphincter insufficiency combined with neurogenic incontinence. The maximal detrusor pressure was reduced to less than 40 mbar and also an acceptable increase in the bladder compliance was achieved in the patients who were good responders to BTX-A.
In our center, an adjuvant anticholinergic is usually initiated at a low dosage if it is needed. We do not routinely reinject BTX-A after a predefined time interval of 8-9 months, which is based on the literature data of duration of effect [9, 39]. However, reinjection is administered according to symptoms and urodynamic parameters, thus the intervals between injections in group-2 patients in our study seem to be longer compared to the literature data (at least 12 months).
Five children in group-1 required a single injection of BTX-A and remained stable during a long follow-up of 56 months. They became responders to adjuvant anticholinergic treatment and, thus, there was no reason to change their therapy regimen. Two of them were non-responders to oxybutynin chloride 0.3 mg/kg bid, but the changeover to tolterodine chloride 0.1 mg/ kg bid, first as adjuvant therapy, was successful. The other three children were on CIC with recurrent UTI and conversion to aseptic IC helped them become responders to adjuvant antimuscarinics. Regarding the incidence of UTI and urethral strictures, aseptic IC seems to be superior to CIC [40, 41].
We also found it important to undertake a surgical intervention in order to protect the upper urinary tract in the patients who became non-responders to anticholinergics and showed no clinical or urodynamic improvement after BTX-A injection. These children had initially severe low-compliant bladders with high leak-point pressure and high-grade VRR (five children in both groups).
Grosse et al. studied 66 adult patients who received repeated BTX-A injections. They reported a significant improvement in bladder function from baseline up to the third injection. It was noted that the repeated injections did not induce any drug tolerance.
In their studies, Akbar et al., [16] Altaweel et al., [13] and Schulte-Baukloh et al. [12] discussed repeated BTX-A detrusor injections in treatment of children with neurogenic detrusor hyperactivity. They found a significant improvement in clinical symptoms and urodynamic parameters. Moreover, it was noted that the repeated injections reproduced the initial effect.
Haferkamp et al. [42] have studied detrusor biopsies taken from patients after BTX-A injections, they could not find any changes in the muscular structure of the detrusor or any fibrosis. Similar results were demonstrated by Schruch et al. [43] and Grosse et al. [44].
Many studies [11, 42, 45, 46, 47] found that the effect of BTX-A in the bladder is much longer compared to striated muscles, other cholinergic non-striated muscle organs, and sweet glands. In the classic application of BTX-A to spastic muscles, BTX-A injections need to be repeated at approximately the third month.
Formation of neutralizing antibodies (NAB) against the toxin is a potential problem with repeated BTX-A injections, which may reduce the treatment effect [48, 49]. Risk factors for formation of NAB are a high BTX-A dose and short injection intervals [50, 51]. For the detrusor, the relatively long injection intervals and the moderate dose are positive factors, reducing the risk of NAB formation.
In our study, patients of group-2 who received repeated BTX-A injections had similar results as reported by other authors [12, 13, 16]. Importantly, the clinical and urodynamic response remains unchanged even after several injections.
Muscle weakness was never seen in our patients. Recent analysis of a comprehensive database with long-term follow-up has shown that the correct use of higher doses of BTX-A during multi-muscle treatment is rarely associated with systemic side effects. That is because the total dose is distributed over multiple muscles and over multiple injection sites per muscle [52]. No lethal complications after treatment with BTX-A have ever been reported. Its median lethal dose (LD50) has been determined across multiple animal species. Based on the findings from primate studies, a human LD50 is estimated to be approximately 3000 units for a 70 kg adult [53].
Neel et al. [18, 22] reported a successful experience in total endoscopic management of children with non-compliant neuropathic bladder. There was a good effect on treating VRR when BTX-A injection was combined with endoscopic subureteral injection. In about 60% of our patients with VRR the total endoscopic therapy approach was successful, and in the other complicated cases open surgery was carried out.
CONCLUSIONS
BTX-A is a safe alternative in the treatment of detrusor hyperactivity in MMC children. The efficacy lasted a mean of 12 months and urodynamic response is unchanged even after several injections. Some patients remain on adjuvant anticholinergic treatment, but in lower doses. In our series, 21.7% of children were non-responders. Patients with severe resistant fixed low-compliance bladder with high LLP and VRR are non-responders to both standard conservative treatment and BTX-A injection. Bladder augmentation is still the last resort in these patients.
REFERENCES
- 1.Snodgrass WT, Adams R. Initial urologic management of myelomeningocele. Urol Clin North Am. 2004;31:427–434, viii. doi: 10.1016/j.ucl.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bauer SB, Hallett M, Khoshbin S, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA. 1984;252:650–652. [PubMed] [Google Scholar]
- 3.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–209. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 4.Cahlil RA, Kiely EA. The spectrum of urological disease in patients with spina bifida. Irish Med Sci. 2003;172:180–184. doi: 10.1007/BF02915286. [DOI] [PubMed] [Google Scholar]
- 5.Joseph DB, Bauer SB, Colodny AH, et al. Clean, intermittent catheterization of infants with neurogenic bladder. Pediatrics. 1989;84:78–82. [PubMed] [Google Scholar]
- 6.Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500–1504. [PubMed] [Google Scholar]
- 7.Hernandez RD, Hurwitz RS, Foote JE, et al. Nonsurgical management of threatened upper urinary tracts and incontinence in children with myelomeningocele. J Urol. 1994;152(5 Pt 1):1582–1585. doi: 10.1016/s0022-5347(17)32480-1. [DOI] [PubMed] [Google Scholar]
- 8.Riccabona M, Koen M, Schindler M, et al. Botulinum-A toxin injection into the detrusor: a safe alternative in the treatment of children with myelomeningocele with detrusor hyperreflexia. J Urol. 2004;171(2 Pt 1):845–848. doi: 10.1097/01.ju.0000108892.35041.2d. [DOI] [PubMed] [Google Scholar]
- 9.Schruch B, Stohrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyperre flexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 10.Schulte-Baukloh H, Michael T, Stürzebecher, Knispel HH. Botulinum-a toxin detrusor injection as a noval approach in the treatment of bladder spasticity in children with neuro genic bladder. Eur Urol. 2003;44(2):139–143. doi: 10.1016/s0302-2838(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 11.Schruch B, Corcos J. Botulinum toxin injections for paediatric incontinence. Curr Opin Urol. 2005;15(4):264–267. doi: 10.1097/01.mou.0000172401.92761.86. [DOI] [PubMed] [Google Scholar]
- 12.Schulte-Baukloh H, Knispel HH, Stolze T, et al. Repeated botulinum-A toxin injections in treatment of children with neurogenic detrusor overactivity. Urology. 2005;66(4):865–868. doi: 10.1016/j.urology.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Altaweel W, Jednack R, Bilodeau C, Corcos J. Repeated intradetrusor botulinum toxin type A in children with neurogenic bladder due to myelomeningocele. J Urol. 2006;175(3 Pt 1):1102–1105. doi: 10.1016/S0022-5347(05)00400-3. [DOI] [PubMed] [Google Scholar]
- 14.Hoebeke P, De Caestecker K, Vande Walle J, et al. The effect of botulinum-A toxin in incontinent children with therapy resistant overactive detrusor. J Urol. 2006;176(1):330–331. doi: 10.1016/S0022-5347(06)00301-6. [DOI] [PubMed] [Google Scholar]
- 15.Kajbafzadeh AM, Moosavi S, Tajik P, et al. Intravesical injection of botulinum toxin type A: management of neuropathic bladder and bowel dysfunction in children with myelomeningocele. Urology. 2006;68(5):1091–1097. doi: 10.1016/j.urology.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Akbar M, Abel R, Seyler TM, et al. Repeated botulinum-A toxin injections in the treatment of myelodysplastic children and patients with spinal cord injuries with neurogenic bladder dysfunction. BJU Int. 2007;100(3):639–645. doi: 10.1111/j.1464-410X.2007.06977.x. [DOI] [PubMed] [Google Scholar]
- 17.Franco I. Overactive bladder in children. Part 2: Management. J Urol. 2007;178:769–774. doi: 10.1016/j.juro.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 18.Neel KF, Soliman S, Salem M, et al. Botulinum-A toxin: solo treatment neuropathic noncompliant bladder. J Urol. 2007;178:2593–2598. doi: 10.1016/j.juro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Neel KF, Salem M, Solimann S. Total endoscopic management (TEM approach) of children with non-compliant neuropathic bladder: a preliminary report. J Pediatr Urol. 2008;4(2):124–126. doi: 10.1016/j.jpurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Game X, Mouracade P, Chartier-Kastler E, et al. Botulinum toxin-A (Botox) intradetrusor injections in children with neurogenic detrusor overactivity/ neurogenic overactive bladder: a systematic literature review. J Pediatr Urol. 2009;5(3):156–164. doi: 10.1016/j.jpurol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Dyer LL, Franco I. Botulinum Toxin-A therapy in pediatric urology: indications for the neurogenic and non- neurogenic neurogenic bladder. Scientific World Journal. 2009;18(9):1300–1305. doi: 10.1100/tsw.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safari S, Jamali S, Habibollahi P, et al. Intravesical injections of botulinum toxin type A for management of neuropathic bladder: a comparison of two methods. Urology. 2010;76(1):225–230. doi: 10.1016/j.urology.2009.09.087. [DOI] [PubMed] [Google Scholar]
- 23.Neel KF. Total endoscopic and anal irrigation management approach to noncompliant neuropathic bladder in children: a good alternative. J Urol. 2010;184(1):315–318. doi: 10.1016/j.juro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan WE, Richards I. Intravesical bladder stimulation in myelodysplasia. J Urol. 1988;140:1282. doi: 10.1016/s0022-5347(17)42025-8. [DOI] [PubMed] [Google Scholar]
- 25.Decter RM, Snyder P, Laudermilch C. Transurethral electrical bladder stimulation: a follow-up report. J Urol. 1995;152:812. doi: 10.1016/s0022-5347(17)32717-9. [DOI] [PubMed] [Google Scholar]
- 26.Franco I, Storrs B, Firlit C, et al. Selective sacral rhizotomy in children with high pressure neurogenic bladders: preliminary results. J Urol. 1992;148:648. doi: 10.1016/s0022-5347(17)36681-8. [DOI] [PubMed] [Google Scholar]
- 27.Schneidau T, Franco I, Zebold K, et al. Selective sacral rhizotomy for the management of neurogenic bladders in spina bifida patients: long- term follow up. J Urol. 1995;154:766. doi: 10.1097/00005392-199508000-00116. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell ME, Piser JA. Intestinocystoplasty and total bladder replacement in children and young adults: follow up in 129 cases. J Urol. 1987;138:579. doi: 10.1016/s0022-5347(17)43264-2. [DOI] [PubMed] [Google Scholar]
- 29.Skobejko-Wlodraska L, Strulak K, Nachulewicz P, Szymkiewicz C. Bladder autoaugmentation in myelodysplastic children. Br J Urol. 1998;(Suppl. 81):114. doi: 10.1046/j.1464-410x.1998.00022.x. [DOI] [PubMed] [Google Scholar]
- 30.Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol. 2003;44:165–174. doi: 10.1016/s0302-2838(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 31.Coffield JA, Cosidine RV, Simpson LL. The site and mechanism of action of botulinum neurotoxin. In: Jankovic J, Hallett M, editors. Therapy with Botulinum Toxin. New York, NY: Marcel Dekker Inc; 1994. pp. 3–13. [Google Scholar]
- 32.Drachman DB. Botulinum toxin as a tool for research on the nervous system. In: Simpson LL, editor. Neuropoisons: Their pathophysiological Actions. Vol. 1. New York: Plenum Press; 1971. pp. 325–347. Chap.15. [Google Scholar]
- 33.Duchen LW. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970;33:40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurvitz EA, Conti GE, Brown SH. Changes in movement characteristics of the spastic upper extremity after botulinum toxin injection. Arch Phys Med Rehabil. 2003;84:444–454. doi: 10.1053/apmr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 35.Niamtu J., 3rd Botulinum toxin A: a review of oral and maxillofacial patient treatments. J Oral Maxillofac Surg. 2003;61:317–324. doi: 10.1053/joms.2003.50069. [DOI] [PubMed] [Google Scholar]
- 36.Glogau RG. Review of the use of botulinum toxin for hyperhidrosis and cosmetic purposes. Clin J Pain. 2002;18:S191–197. doi: 10.1097/00002508-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien CF. Treatment of spasticity with botulinum toxin. Clin J Pain. 2002;18:S182–190. doi: 10.1097/00002508-200211001-00011. [DOI] [PubMed] [Google Scholar]
- 38.Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum-A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol. 1989;139:919–922. doi: 10.1016/s0022-5347(17)42717-0. [DOI] [PubMed] [Google Scholar]
- 39.Schulte-Baukloh H, Michael T, Schobert J, et al. Efficacy of botulinum-A toxin in children with detrusor hyperreflexia due to myelomeningeocele: preliminary results. Urology. 2002;59:325–327. doi: 10.1016/s0090-4295(01)01641-7. disc. 327–328. [DOI] [PubMed] [Google Scholar]
- 40.Grigoleit U, Pannek J, Stöhrer M. Single-use intermittent catheterisation. Urologe A. 2006;45(2):175–182. doi: 10.1007/s00120-006-1007-9. [DOI] [PubMed] [Google Scholar]
- 41.Stöhrer M, Block B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56(1):81–88. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Haferkamp A, Schruch B, Reitz A, et al. Lack of ultrastructural detrusor changes following endocsopic injection of botulinum toxin type A in overactive neurogenic bladder. Eur Urol. 2004;46:784–791. doi: 10.1016/j.eururo.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Schruch B. The role of botulinum toxin in neurourology. Drugs Tody (Barc) 2004;40:205–212. doi: 10.1358/dot.2004.40.3.820084. [DOI] [PubMed] [Google Scholar]
- 44.Grosse J, Kramer G, Schruch B. Repeated detrusor injections of botulinum A toxin in patients with neurogenic lower urinary tract dysfunction do not cause increased drug tolerance. Neurourol Urodyn. 2002;21:386–387. [Google Scholar]
- 45.de Paiva A, Meunier FA, Molgo J, et al. Functional repair of motor endplates after botulinum neurotoxin type a poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminal. Proc Natl Acad Sci USA. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heckmann M, Ceballos-Baumann AO, Plewig G. Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating) N Engl J Med. 2001;344:448–493. doi: 10.1056/NEJM200102153440704. [DOI] [PubMed] [Google Scholar]
- 47.Heckmann M, Plewig G. Low- dose efficacy of botulinum toxin A for axillary hyperhidrosis: a randomized, side-by-side, open-label study. Arch Dermatol. 2005;141:1255–1259. doi: 10.1001/archderm.141.10.1255. [DOI] [PubMed] [Google Scholar]
- 48.Dressler D, Hallett M. Immunological aspects of Botox, Dyspot and Myobloc/Neuro Bloc. Eur J Neurol. 2006;13(Suppl.1):11–15. doi: 10.1111/j.1468-1331.2006.01439.x. [DOI] [PubMed] [Google Scholar]
- 49.Schulte-Baukloh , Bigalke H, Miller K, et al. Botulinum neurotoxin type A in urology: Antibodies as a cause of therapy failure. Intern J Urol. 2008;15:407–415. doi: 10.1111/j.1442-2042.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 50.Aoki KR. Pharmacology and immunology of botulinum toxin serotypes. J Neurol. 2001;248(Suppl 1):3–10. doi: 10.1007/pl00007816. [DOI] [PubMed] [Google Scholar]
- 51.Hermann J, Geth K, Mall V, et al. Clinical impact of antibody formation to botulinum toxin A in children. Ann Neurol. 2004;55:732–735. doi: 10.1002/ana.20098. [DOI] [PubMed] [Google Scholar]
- 52.Heinen F, Schroeder AS, Fietzek U, Breweck S. When it comes to botulinum toxin, children and adults are not the same: multimuscle option for children with cerebral palsy. Mov Disord. 2006;21:2029–2030. doi: 10.1002/mds.21097. [DOI] [PubMed] [Google Scholar]
- 53.Cayan S, Coskun B, Bozlu M, et al. Botulinum toxin type A may improve bladder function in rat chemical cystitis model. Urol Res. 2003;30:339–404. doi: 10.1007/s00240-002-0291-0. [DOI] [PubMed] [Google Scholar]