Abstract
Introduction
A prostate biopsy can result in such complications as: hematuria, rectal bleeding, pain in hypogastrium, perineum or urethra, fever, nausea, vomiting, retention of urine or other adverse events. The aim of this research was to estimate complication rates after a prostate biopsy based on the number of cores.
Material and methods
The complication rate was evaluated on the basis of questionnaires filled out by patients. Questions were related to the occurrence of mentioned complications on the first and second day after prostate biopsy. Patients were divided into two groups: 1st group (41 patients) 5-8 cores and 2nd group (73 patients) 12 or more cores.
Results
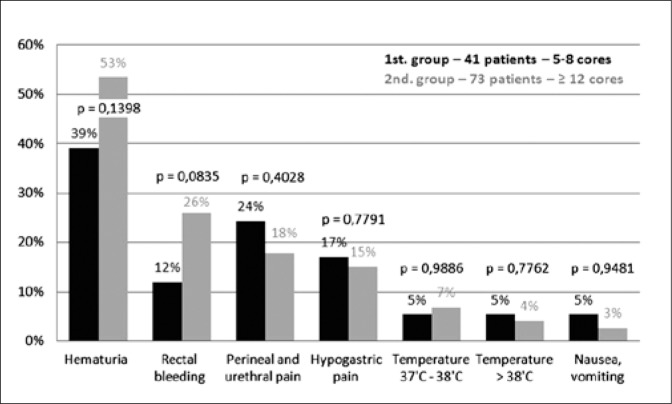
There was no significant statistical difference in the occurrence of complications mentioned in the questionnaires in both groups. The biggest difference was recorded for hematuria – 1st day: 39% in the 1st and 53% in the 2nd group (p = 0.1398); 2nd day: 15% in the 1st and 30% in the 2nd group (p = 0.0650). Rectal bleeding on the 1st day also seems to vary: 12% in the 1st and 26% in the 2nd group (p = 0.0835). Other complications occurred in 3-8% of patients. 32% of patients in the 1st and 29% in the 2nd group (p = 0.7419) had no complications at all.
Conclusions
The most common complications after a prostate biopsy are hematuria and rectal bleeding. Other complication rates are low. In general, complication rates after a prostate biopsy procedure are not related to the number of sampled cores.
Keywords: prostate, biopsy, complications
INTRODUCTION
The diagnosis of prostate cancer (PCa) is based on a prostate biopsy. The procedure is performed under the control of transrectal ultrasonography (TRUS). Main indications for a prostate biopsy are an elevated prostate specific antigen (PSA) level or abnormal digital rectal examination (DRE). There are several prostate biopsy strategies used today and they are distinguishable according to the number of biopsy cores. The traditional sextant prostate biopsy is not recommended anymore. By sampling more cores it is possible to improve the rate of cancer detection. According to the current EAU Guidelines (2011), 8-12 cores should be sampled from the peripheral zone of the prostate [1]. Sampling of more than 12 cores during a prostate biopsy is not significantly more conclusive [2].
A prostate biopsy can result in such minor complications as: hematuria, rectal bleeding, pain in hypogastrium, perineal and urethral pain, high body temperature, nausea and vomiting. Severe complications such as: post-procedural infections (also with urosepsis), urine retention, prostatitis, epididymitis, or other adverse events leading to repeated patient hospitalization have also been observed [3].
The aim of this research was to estimate complication rates after a prostate biopsy based on the number of cores.
MATERIAL AND METHODS
In our research we asked all patients admitted to the 2nd Clinic of Urology to undergo a prostate biopsy to complete a questionnaire regarding post-procedure complications. The questionnaires were distributed and collected from January 2010 to October 2010. About 50% of patients returned the questionnaires.
We obtained 114 completed questionnaires from patients who were divided into two groups according to the number of cores sampled during the biopsy. The first group (41 patients) had 5-8 cores sampled and the second group (73 patients) had 12 or more cores sampled.
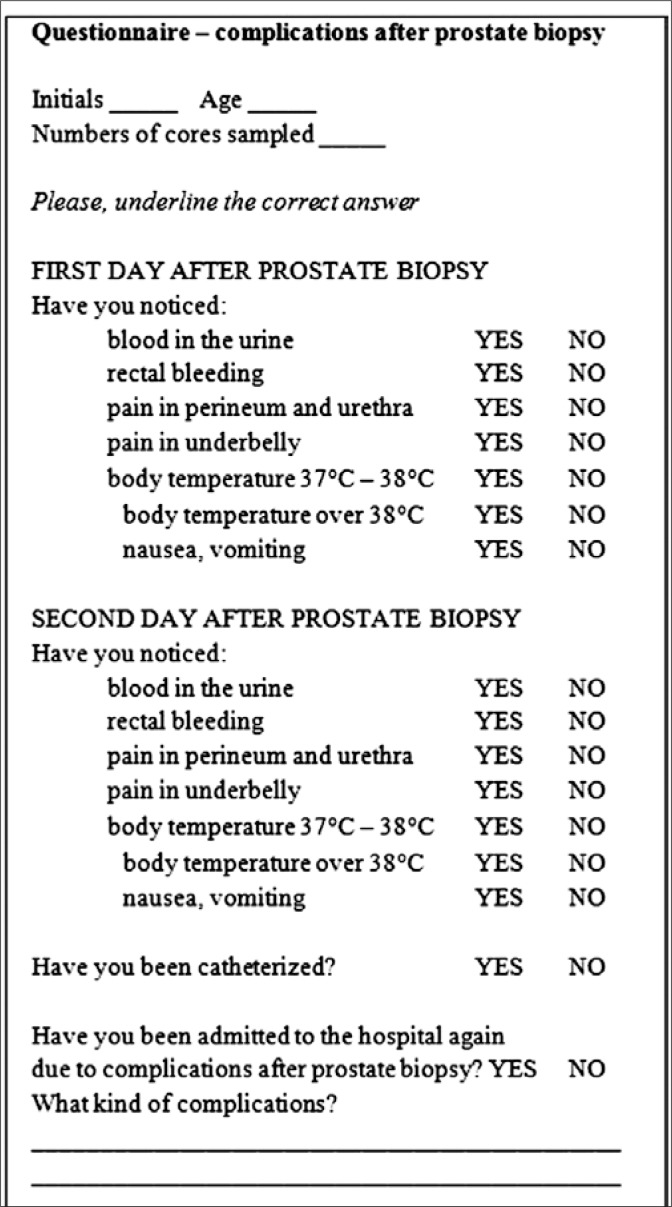
Patients filled out the questionnaire shown in Figure 1. The attending physician discussed all questions with the patients beforehand. The questionnaires were qualitative – patients were asked if the complication had occurred or not. Questions were related to the occurrence of adverse events within the first two days after the prostate biopsy, such as hematuria, rectal bleeding, hypogastric pain, perineal and urethral pain, body temperature 37-38°C, body temperature in excess of 38°C, nausea, vomiting, need of bladder catheterization, and repeated patient hospitalization. The complication rate was evaluated on the basis of questionnaires filled out by patients from both groups.
Fig. 1.
Questionnaire – complications after a prostate biopsy.
The prostate biopsy procedure in 2nd Clinic of Urology involves of a three-day hospitalization. On the first day, patients are admitted to the hospital and prepared for the procedure using intravenous antibiotic therapy (100 mg ciprofloxacin twice a day). On the second day, cleansing enema is administered to every patient and the biopsy procedure is performed. On the third day, patients are discharged from the hospital with a recommendation for oral antibiotic therapy within the following five days (500 mg ciprofloxacin twice a day). Contraindications for biopsy are prostatitis or other genitourinary tract infections and the use of anticoagulants. We ask patients to discontinue anticoagulant therapy for seven days prior to the biopsy procedure.
As a standard procedure in our Clinic, 12 cores are sampled during the prostate biopsy. If a patient does not tolerate local anesthesia or rectal bleeding is heavy, eight cores are sampled. Fewer cores are sampled in the case of a formal biopsy (for example for patients with excessive PSA, with positive DRE, or hypoechogenic zone in TRUS). More than 12 cores are sampled during saturation biopsies.
A statistical comparison between the two groups was performed using the chi-squared test, Yates’ chi-squared test and V-squared test. All analyses were performed using Statistica 9 statistical software (StatSoft, Cracow, Poland).
RESULTS
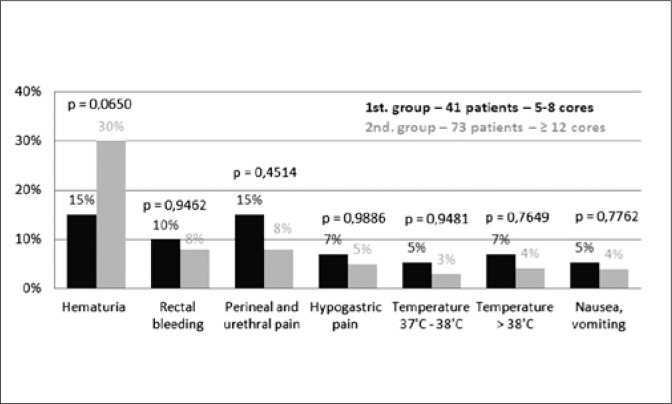
There was no significant statistical difference in the occurrence of complications mentioned in the questionnaires from both groups. The biggest difference was recorded for hematuria – on the 1st day: 39% in the 1st and 53% in the 2nd group (p = 0.1398); on the 2nd day: 15% in the 1st and 30% in the 2nd group (p = 0.0650). Rectal bleeding on the 1st day also seems to vary: 12% in the 1st and 26% in the 2nd group (p = 0.0835). Other results and p value are shown in Figures 2 and 3.
Fig. 2.
Complication rates on the first day after a prostate biopsy.
Fig. 3.
Complication rates on the second day after a prostate biopsy.
Thirty-two percent of patients in the 1st and 29% in the 2nd group (p = 0.7419) had no complications at all.
Five percent of patients in the 1st and 1% of patients in the 2nd group required bladder catheterization due to urine retention (p = 0.6077). Five percent of patients in the second group were admitted to the hospital again because of prostatitis and septic complications. In the first group no complications occurred (p = 0.3195). Those results also do not offer any statistical significance.
DISCUSSION
In our research we attempted to verify whether sampling more cores during a prostate biopsy will cause higher occurrence of post-biopsy complications. Usually, complications are connected with various factors, for example: the level of experience of the operator who performs the biopsy. In our study, all procedures were performed by the same operator.
One hundred fourteen patients participated in the research. They were divided into two groups of different sizes on the basis of the number of cores. A 5-8-core biopsy is performed rarely, because sampling less than 8 cores is not recommended anymore [2].
Based on the results of the research, there is no statistically significant difference in the occurrence of complications between biopsies with sampling 5-8 cores and 12 or more cores. The lowest p value was recorded in the case of hematuria on the 2nd day (p = 0.0650). Further statistical analysis indicates that the rate of occurrence of other complications was similar and was not related to the number of sampled cores.
There are several studies that evaluate complications in a larger group of patients. Berger et al. evaluated complications after almost 6,000 biopsies [4]. He identified hematospermia as the most common complication after a prostate biopsy, which was confirmed by other authors [5]. This complication was not estimated in our study because the questionnaire questions only focused on the first two days after the procedure. What is more, literature on the subject demonstrates a huge difference in the occurrence of hematospermia because of difficulties in the proper evaluation of ejaculation [6, 7].
According to subject literature, the second and third most common complications were hematuria and rectal bleeding, respectively. Hematuria occurrence ranged from 47% to 58% and rectal bleeding occurrence ranged from 10% to 37% [8, 9, 10]. Additionally, there were also some similarities in the low occurrence of other minor complications, such as: hypogastric pain, perineal and urethral pain, temporarily elevated body temperature 37-38°C, and nausea and vomiting [4, 8, 9, 10]. These complications usually do not require treatment.
In our research, we asked patients to discontinue anticoagulant therapy for seven days prior to the biopsy. Despite withdrawing anticoagulants, the most common complications were related to bleeding. Interestingly enough, in a paper by Maan et al., a relationship between anticoagulant intake and post-biopsy bleeding was not identified [11].
More severe adverse events, such as prostatitis and septic complications, occurred in 5% of patients from the group in which 12 or more cores were sampled. The low presence of septic complications is related to the antibiotic prophylaxis performed in our study. Different prophylactic regimens have been used in different studies, but the use of antibiotics in general significantly decreases the septic complication rate. Biopsies performed without this kind of prophylaxis can result in a high septic complication rate [9, 12, 13].
Before a prostate biopsy in our clinic, a cleansing enema is routinely used. This procedure has also been mentioned in literature [4, 7]. Some authors criticize the value of this procedure in reducing infectious complications. Zaytoun et al. concluded that the cleansing enema is redundant in the case of decreasing infectious complications, and it can even be harmful: the use of routine enemas could increase the level of bacteria in the rectum and has an adverse impact on ultrasound imaging [15]. Therefore, the administration of a cleansing enema in our clinic requires debate.
Sampling more cores during a prostate biopsy increases cancer detection and does not seem to be associated with a higher complication rate, as shown in our research and in the subject literature [2, 14].
CONCLUSION
The most common complications after a prostate biopsy are hematuria and rectal bleeding. Other complication rates are low. In general, complication rates after a prostate biopsy procedure are not related to the number of sampled cores.
REFERENCES
- 1.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175(5):1605–1612. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 3.Antoniewicz A, Zapała Ł, Borówka A, de Reijke T. Biopsy of the prostate – the urge to search for a new standard. CEJUrol. 2010;4:166–175. [Google Scholar]
- 4.Berger AP, Gozzi C, Steiner H, et al. Complication rate of transrectal ultrasound guided prostate biopsy: a comparison among 3 protocols with 6, 10 and 15 cores. J Urol. 2004;171(4):1478–1480. doi: 10.1097/01.ju.0000116449.01186.f7. [DOI] [PubMed] [Google Scholar]
- 5.Rietbergen JB, Kruger AE, Kranse R, Schröder FH. Complications of transrectal ultrasound-guided systematic sextant biopsies of the prostate: evaluation of complication rates and risk factors within a population-based screening program. Urology. 1997;49(6):875–880. doi: 10.1016/s0090-4295(97)00100-3. [DOI] [PubMed] [Google Scholar]
- 6.Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166(3):856–860. [PubMed] [Google Scholar]
- 7.Naughton CK, Ornstein DK, Smith DS, Catalona WJ. Pain and morbidity of transrectal ultrasound guided prostate biopsy: a prospective randomized trial of 6 versus 12 cores. J Urol. 2000;163(1):168–171. [PubMed] [Google Scholar]
- 8.Collins GN, Lloyd SN, Hehir M, McKelvie GB. Multiple transrectal ultrasound-guided prostatic biopsies—true morbidity and patient acceptance. Br J Urol. 1993;71(4):460–463. doi: 10.1111/j.1464-410x.1993.tb15993.x. [DOI] [PubMed] [Google Scholar]
- 9.Enlund AL, Varenhorst E. Morbidity of ultrasound guided transrectal core biopsy of the prostate without prophylactic antibiotic therapy. A prospective study in 415 cases. Br J Urol. 1997;79(5):777–780. doi: 10.1046/j.1464-410x.1997.00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol. 1998;160(6 Pt 1):2115–2120. doi: 10.1097/00005392-199812010-00045. [DOI] [PubMed] [Google Scholar]
- 11.Maan Z, Cutting CW, Patel U, et al. Morbidity of transrectal ultrasonography-guided prostate biopsies in patients after the continued use of low-dose aspirin. BJU Int. 2003;91(9):798–800. doi: 10.1046/j.1464-410x.2003.04238.x. [DOI] [PubMed] [Google Scholar]
- 12.Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60(5):826–830. doi: 10.1016/s0090-4295(02)01958-1. [DOI] [PubMed] [Google Scholar]
- 13.Aron M, Rajeev TP, Gupta NP. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: a randomized controlled study. BJU Int. 2000;85(6):682–685. doi: 10.1046/j.1464-410x.2000.00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Philip J, Ragavan N, Desouza J, et al. Effect of peripheral biopsies in maximising early prostate cancer detection in 8-, 10- or 12-core biopsy regimens. BJU Int. 2004;93(9):1218–1220. doi: 10.1111/j.1464-410X.2004.04857.x. [DOI] [PubMed] [Google Scholar]
- 15.Zaytoun OM, Anil T, Moussa AS, et al. Morbidity of prostate biopsy after simplified versus complex preparation protocols: assessment of risk factors. Urology. 2011;77(4):910–914. doi: 10.1016/j.urology.2010.12.033. [DOI] [PubMed] [Google Scholar]