Abstract
Significant advances have been achieved in the field of urologic laparo-endoscopic single-site surgery (LESS) since the first reported clinical series in 2007. The aim of the present review paper is to summarize and critically analyze the most recent advances in the field of urologic LESS. A literature review was performed using PubMed to retrieve publications related to LESS in urology over the last two years (from January 2011 to May 2012). In the free-text protocol, the following terms were applied: LESS; single port laparoscopy; single incision laparoscopy. Despite unsolved challenges, LESS can be regarded as an emerging trend in minimally invasive urologic surgery and it has significantly evolved, becoming a widely applicable technique in a relatively short time. Outcomes demonstrate that a broad range of procedures can be effectively and safely done, given a solid laparoscopic surgical background and stringent patient-selection criteria. The recent introduction of a purpose-built instrumentation is likely to further foster the application of robotics to LESS. Further improvements are needed before this technique might reach a widespread adoption. Future advances in the field of robotic technology are expected to overcome the current limitations of LESS.
Keywords: laparo-endoscopic single-site surgery, single-port laparoscopy, LESS, robotics, scarless surgery, urology
INTRODUCTION
The concept of laparo-endoscopic single-site surgery (LESS) was introduced in the urology field five years ago with the description of the first nephrectomy cases [1, 2]. Soon after, large series were reported, showing its feasibility in a broad spectrum of urological procedures [3].
Despite evolving from the techniques of standard laparoscopy, LESS defies some basic laparoscopic principles, including instrument and external port spacing to decrease clashing. Thus, new laparoscopic access devices, optics, and instrumentation specifically designed for facilitating LESS have been developed in the last few years [4]. Moreover, the application of robotics to LESS has been hypothesized to overcome the limitations of the technique [5, 6].
Overall, LESS has proved to be immediately applicable in the clinical field, being safe and feasible in the hands of experienced laparoscopic surgeons in well-selected patients. However, despite promising early outcomes, the benefits of LESS are yet to be demonstrated.
A comprehensive literature overview was reported almost two years ago by Autorino et al. on the status of natural orifice transluminal endoscopic surgery (NOTES) and LESS [7]. The authors provided a detailed analysis of the clinical and experimental data available at that time. Since then, further advances have been achieved in our surgical specialty, given the commitment of investigators from all over the world.
The aim of the present review paper is to summarize and critically analyze the most recent advances in the field of urologic LESS.
Literature search
A literature review was performed using PubMed to retrieve publications related to LESS in urology over the last two years (from January 2011 to May 2012). In the free-text protocol, the following terms were applied: laparo-endoscopic single-site surgery; single-port laparoscopy; single-incision laparoscopy. Review articles, editorials, commentaries and letters to the editor were included only if deemed to contain relevant information. In addition, cited references from the selected articles and from review articles retrieved in the search were assessed for significant manuscripts not previously included.
Novel purpose-built instrumentation for LESS
Access devices
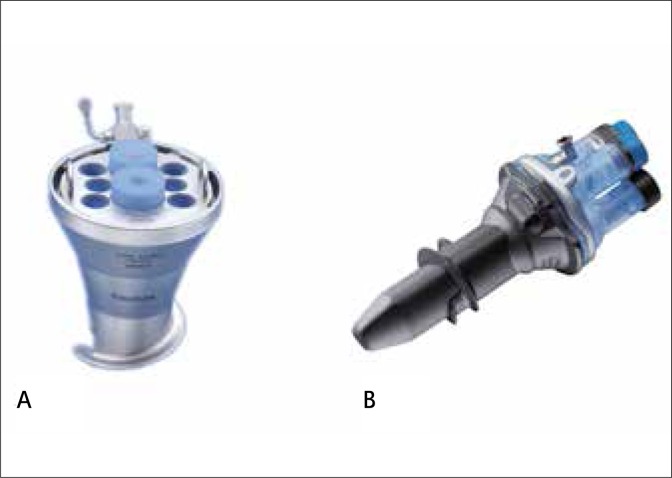
Several access devices have been developed for single port surgery to allow simultaneous use of multiple instruments and their clinical application has been shown. Each device presents specific features aiming to facilitate LESS. However, the ideal platform is yet to be defined [8]. In order to minimize the costs associated with LESS, investigators have focused on low cost devices, including reusable ones (Fig. 1).
Fig. 1.
Reusable access devices for LESS: (a) EndoCone® (Karl Storz); Keyport® (Richard Wolf)
A homemade single port device was initially popularized by several groups from Korea [9]. The idea uses a standard wound protector and a surgical glove, with a series of little accesses made on the tips of the glove-fingers to create a working channel for trocars/ instruments. This technique has been more recently adopted by others, which shows the feasibility of different procedures [10–13]. From the same idea, a device called the Gloveport® (Nelis, Korea) has been commercialized, and its clinical use demonstrated [14].
A reusable device from Karl Storz, the Endocone®, has been shown to be suitable for urologic applications, including LESS nephrectomy [15] and partial nephrectomy [16].
A new DuoRotate manual system developed by Richard Wolf (KeyPort®) was recently shown to be applicable for LESS radical prostatectomy by Caceres et al. [17]. They performed 31 procedures (10 with and 21 without neurovascular preservation, eight with and 23 without pelvic lymph node dissection) by using the tri-channel reusable KeyPort and one 3.5-mm extra port to facilitate urethrovesical anastomosis and drainage extraction. The authors concluded that the system allows performance of LESS radical prostatectomy with few complications, a low positive-margin rate, excellent aesthetic results, and very low postoperative pain levels.
SPIDER
The SPIDER (Single Port Instrument Delivery Extended Reach) surgical system (TransEnterix, Morrisville, NC, USA) is comprised of a multichannel port connected to flexible instrument delivery tubes. The main cannula has four instrument channels. The superior and inferior channels are 5mm in diameter and allow for the passage of a bariatric-length endoscope and rigid laparoscopic instruments. The two lateral channels contain the instrument delivery tubes, which are the conduits for the passage of specialized flexible instruments designed specifically for the SPIDER device. The lateral channels provide for true left- and right-handed control over instruments (Fig. 2).
Fig. 2.
SPIDER system for LESS (Transenterix).
Haber et al. reported an initial laboratory experience using the SPIDER surgical system and its first clinical application in urology [18]. The SPIDER system was tested in a laboratory setting and used for a clinical case of renal cyst decortication. Three tasks were performed during the dry lab session, and different urologic procedures were conducted in a porcine model. The surgeons had a positive experience with the SPIDER system, and were able to gain proficiency in performing tasks regardless of their level of expertise. The highest scores recorded were for ease of device insertion, instrument insertion and exchange, and triangulation. During the clinical case, the platform provided good triangulation without instrument clashing. However, retraction was challenging because of the lack of strength and precise maneuverability with the tip of the instruments fully deployed. The authors concluded the SPIDER system offers intuitive instrument maneuverability and restored triangulation without external instrument clashing.
After an initial experience in the porcine model [19], Leveillee et al. described an initial clinical case of simple nephrectomy with the SPIDER system in a patient with a nonfunctioning kidney secondary to a ureteropelvic junction obstruction [20]. The SPIDER-LESS nephrectomy was performed successfully without additional skin incisions for laparoscopic ports, instrument clashing, or the need for open conversion. Total operative time was 210 minutes with minimal blood loss and no perioperative complications.
daVinci Single-site instrumentation
Intuitive Surgical developed a novel set of single-site instruments and accessories specifically dedicated to LESS (Fig. 3). The set includes a multichannel access port with room for four cannulae and an insufflation valve. Two curved cannulae are for robotically controlled instruments. The other two cannulae are straight; one is 8.5mm and accommodates the high-definition and 3-dimensional endoscope and the other is a 5-mm bedside-assistant surgeon port. Triangulation is achieved by crossing the curved cannulae midway through the access port. Same-sided hand-eye-control of the instruments is maintained through the use of software, the Si system, that enables the surgeon's right hand to control the screen-right instrument even though the instrument is in the left robotic arm and reciprocally the left hand controls the screen-left instrument even though the instrument is in the right robotic arm. The second part of the platform is a set of semi-rigid, non-wristed instruments based with standard da Vinci instrument tips. The semi-rigid flexible shaft allows for insertion down the curved cannula and triangulation of the anatomy. Robotic arm collisions are minimized externally because the curved cannulae angle the robotic arms away from each other. Internal collisions with the camera are avoided because the camera is designed to be placed into the middle of the curved cannula zone and is not in a parallel arrangement. The Intuitive Surgical single-port instruments and accessories are intended to be used with the da Vinci® Si Surgical System. The single-site instruments and accessories are of similar construction to existing EndoWrist® instruments, except they do not have a wrist at the distal end of the instrument.
Fig. 3.
da Vinci Single Site® surgery instrumentation (Intuitive Surgical).
In 2010, Haber et al. described the first laboratory experience with VeSPA robotic instruments by assessing their feasibility and efficiency for urological applications [21]. Sixteen procedures (including 4 pyeloplasties, 4 partial nephrectomies, and 8 nephrectomies) were performed without additional ports or need for conversions. Mean total operative time was 110 minutes and no intraoperative complications occurred. There were no instances of extracorporeal conflict between robotic arms nor were there instances of intracorporeal conflict between the laparoscope and robotic instruments. Significant gas leakage was experienced in half of the procedures and partial tearing of the multichannel port was noted in six procedures. During this feasibility evaluation, limitations of the platform were noted, including the lack of articulation at the tip of the instruments compared with the Endowrist™ instruments afforded by the current daVinci Si, making intracorporeal suturing more challenging.
More recently, Kaouk et al. also reported the use of a second generation of daVinci single-site instruments for robotic LESS to perform different kidney procedures in the cadaver model [22]. Three types of left side kidney procedures were performed (one pyeloplasty, one partial nephrectomy, and one nephrectomy) in a female cadaver model by a surgeon. The curved cannulae for the robotic instruments were shorter than the previous ones (35 vs. 55 mm), in order to make them more suitable for urologic single-site surgery. Time for setup, including positioning, multichannel port insertion, robot docking, and insertion of instruments, was 40 minutes. The procedures were completed successfully without the addition of extra ports. Time to complete the ureteropelvic anastomosis during pyeloplasty was 39 minutes. For partial nephrectomy, simulated warm ischemia time was 21 minutes. For nephrectomy, time to complete the procedure was 13 minutes. No tearing of the multichannel port, no significant gas leakage, and no injuries to intra-abdominal organs or vessels occurred. The working space was not problematic for the surgeon, but the assistant experienced significant collision against the robotic arms, which at times restricted retraction and suction. A positive feature was the easy insertion and exchange of the instruments through the multichannel port and curved trocars. The lack of wrist articulation was confirmed as the main limitation of these instruments, particularly for procedures involving suturing.
Outcomes of LESS: recent evidence
Worldwide experience
Until recently, most of the evidence supporting LESS has been limited to small case series or case-control studies from selected centers [4]. A recent collaborative analysis was reported by Kaouk et al. with the aim of providing an analytical overview of indications, techniques, and outcomes of urologic LESS in various hospital settings worldwide [23]. Overall, 1,076 consecutive cases were done between 2007 and 2010 at 18 participating institutions that were included in this analysis. Each group had performed a variety of LESS procedures according to its own protocols, entry criteria, and techniques. The most common procedures were extirpative or ablative operations of the upper urinary tract. The da Vinci robot was used to operate on 143 patients (13%). A single-port technique was most commonly used and the umbilicus represented the most common access site. An additional port was used in 23% of cases. This study provided a global view of the evolution of LESS in the field of urologic surgery by showing that a broad range of procedures have been effectively performed, primarily in the academic setting, and that when LESS is performed by experienced laparoscopic surgeons, the risk of complications remains low when stringent patient-selection criteria are applied.
Complications
Regardless of all it advantages, LESS must also be scrutinized for its inherent risk of conversions and complications. To date, only a few studies focusing on this important aspect have been published.
Irwin et al. reported a study of complication and conversion rates in 125 LESS procedures of the upper urinary tract from a total of six institutions [24]. Conversion to laparoscopy was necessary in seven patients (5.6%), but none required open conversion. Complications, on the other hand, occurred in 15.2% of cases. Irwin et al. concluded that LESS appeared to be associated with a higher complication rate than in an experienced laparoscopic series, but conversion from LESS was rare and was thought to reflect stringent patient selection. The limitations of that study included the inability to standardize LESS patient selection criteria, instrumentation, and surgical technique, as well as the lack of available complete data on a control group for comparison. A risk analysis was more recently done by Greco et al, who looked at risk factors for complications in a multi-institutional series of LESS done for upper urinary tract disease [25]. The overall complication rate in this series was 17% with conversion to open surgery considered a complication. Multivariable analysis revealed that a higher ASA score and malignant disease on pathological evaluation were risk factors for complications. Greco et al. concluded that surgeons approaching LESS should start with benign diseases in patients at low surgical risk.
Best et al. reviewed their initial series of LESS pyeloplasty, focusing on the 30-day complication rate [26]. Seven patients (25%) experienced complications with 71% of complications in the initial 10 patients. Best et al. concluded that LESS pyeloplasty is a technically challenging procedure even for an experienced laparoscopic surgeon. Ramasamy et al. compared the postoperative complications of LESS and standard laparoscopic living donor nephrectomy using a standardized complication reporting system [27]. At 30-days there was no difference in the overall complication rate between the two groups (7.1% vs. 7.9%, p > 0.05). Multivariable binary logistic regression analysis revealed that estimated blood loss was the only predictor of fewer complications.
From the already mentioned worldwide multi-institutional project, Autorino et al. recently reported a detailed analysis of the incidence of and risk factors for complications and conversion of urological LESS, including upper tract and pelvic surgical procedures [28]. Included in analysis were 1,163 cases. Intraoperative complications occurred in 3.3% of cases. The overall conversion rate was 19.6% with 14.6%, 4.0%, and 1.1% of procedures converted to reduced port laparoscopy, conventional laparoscopic/robotic surgery, and open surgery, respectively. On multivariable analysis, the factors significantly associated with the risk of conversion were oncological surgical indication (p = 0.02), pelvic surgery (p <0.001), robotic approach (p <0.001), high difficulty score (p = 0.004), extended operative time (p = 0.03), and an intraoperative complication (p <0.001). A total of 120 postoperative complications occurred in 109 patients (9.4%) with major complications in only 2.4% of the entire cohort. Reconstructive procedure (p = 0.03), high difficulty score (p = 0.002) and extended operative time (p = 0.02) predicted high-grade complications. The authors concluded that urological LESS can be done with a low complication rate resembling that in laparoscopic series.
Randomized studies
Over the last two years, three small, randomized trials have been reported in the field of urologic LESS. The first one was reported by Tugcu et al. [29] in which twenty-seven patients were randomized to either LESS or standard laparoscopic simple nephrectomy. All procedures in both groups were performed by the same experienced surgeons. There was no difference in median operative time (117.5 vs. 114 min, p = 0.52), blood loss (50.71 vs. 47.15 mL, p = 0.60), transfusion rates (0% for both), and hospitalization time (2.07 vs. 2.11 days, p = 0.74) between the groups. Time to return to normal activities was shorter in the LESS group (10.7 vs. 13.5 days, p = 0.001). Both the visual analogue scale and the postoperative use of analgesics were significantly lower during postoperative days 1, 2, and 3 in patients who underwent LESS. There were no intraoperative or postoperative complications in both groups. LESS-SN was more expensive, but all patients undergoing LESS-SN were very pleased with the cosmetic outcome.
More recently, Kurien et al. enrolled fifty renal donors and randomized them into a standard laparoscopic and LESS donor nephrectomy group [30]. The primary end point of the study was patients’ postoperative pain. The operative times were similar in both groups (175.83 ±47.57 vs. 172.20 ±38.33 minutes, p = 0.38). The postoperative patient pain scores were similar until 48 hours following surgery (3.84 ±1.68 vs. 3.68 ±0.75, p = 0.33), but later the patients in the LESS group had improved pain scores (2.08 ± 0.91 vs. 1.24 ± 0.72, p = 0.0004). Analgesic requirements were similar in both groups (p = 0.47). The warm ischemia times in the LESS group (5.11 ±1.01 vs. 7.15 ±1.84 minutes, p <0.001) was longer but the total ischemia times in both groups were similar (62.55 ±9.46 vs. 62.71 ±12.14 minutes, p = 0.48). Intraoperative (8% vs. 16%, p = 0.2) and postoperative complications (20% vs. 16%, p = 0.99) were comparable. The patients in the LESS group had shorter hospital stay (4.56 ±0.82 vs. 3.92 ±0.76 days, p = 0.003). There was no graft loss in either group except for one recipient in the standard laparoscopy group who sustained sudden cardiac death. The estimated glomerular filtration rates of recipients at 1-year were comparable for both groups (80.87 ±22.12 vs. 81.51 ±29.01 mL/minute, p = 0.46). The donor's quality of life, body image, and cosmetic scores were comparable for both groups. Thus, LESS donor nephrectomy, although challenging and with longer warm ischemic times, was found to give early pain relief with shorter hospital stay and comparable graft function.
The last small, randomized trial was the one reported by Lee et al. to compare the outcomes of transperitoneal laparoscopic and transumbilical LESS varicocele ligation [31]. The study sample included 82 male patients with 92 clinically palpable varicoceles. The operating room time and hospital stay of the two study groups were comparable, but time to return to normal activity was significantly shorter in the LESS group. Both VAS and the postoperative use of analgesics were significantly lower during postoperative days 2 (p = 0.005) and 3 (p = 0.047) in patients who underwent LESS. Significant improvements in sperm count, motility, and morphology were observed in both groups (p <0.001; at each of the variables in both groups).
Advanced indications for LESS
Adrenalectomy has been regarded among one of the most challenging procedures for LESS due to the anatomical location of the adrenal gland. However, LESS adrenal surgery has been effectively performed for a number of indications with a wide variety of approaches (transperitoneal versus retroperitoneal, multichannel trocar versus multiple ports, trans- or extraumbilical) [32]. The procedure seems to be safe, taking more time than the standard laparoscopic counterpart, but appears to offer the patient less postoperative discomfort. Technical difficulties of the procedure include the requirement of more time for adjustment of articulating instruments, longer one-handed manipulation time, and a high perioperative tissue re-grasping rate. Only long-term follow-up outcomes will prove its benefits over conventional laparoscopy and define the role and the oncological safety of LESS adrenal surgery.
Laparoscopic living-donor nephrectomy has become an established alternative to open surgery, with equivalent allograft outcomes, quicker recovery, and superior cosmesis [33]. Gill et al. first reported the successful completion of single-port transumbilical live-donor nephrectomy [34]. Recently, the largest single center experience with this procedure was reported by Wang et al. [35], who studied 100 consecutive LESS donor nephrectomies performed by a single surgeon through a periumbilical incision using the GelPoint® system. No extraumbilical incisions or punctures were made. A comparison was made using a matched cohort of conventional live donor nephrectomies done by the same surgeon. Mean operative time was longer in the LESS group, but there was no difference in estimated blood loss or warm ischemia time as well as no difference in the complication rates between the groups. Mean hospital stay and visual analog pain scores were similar in the groups, but the LESS group showed improved convalescence with faster return to work, normal activity, and 100% recovery. Recipient graft function was equivalent in the two groups. The authors concluded that, despite the benefits of LESS over conventional laparoscopy being limited, they may nevertheless prove beneficial to decrease barriers to live kidney donation.
Increasing experience and the proven safety and feasibility of LESS have allowed for the expansion of indications to include complex reconstructive procedures [36]. Recently, Khanna et al. reported intermediate-term outcomes for patients undergoing reconstructive LESS procedures at a single institution. Thirty-two reconstructive procedures were identified, including pyeloplasty (n = 25), ileal interposition (3), ureteroneocystostomy (3), and retrocaval ureter repair (1). Median follow-up was 24.4 months for pyeloplasty, 35 months for ileal interposition, 29.4 months for ureteroneocystostomy, and 20 months for retrocaval ureter repair. At last follow-up, 24 of 25 patients treated with pyeloplasty, two of three with ileal ureter, three of three with ureteroneocystostomy, and one of one with retrocaval ureter reported being asymptomatic or improvement after the procedure. Delayed incisional hernia repair was required in one patient.
After the first report by Kaouk et al. [37], another group from China described the outcomes of a small series (five cases) of LESS radical cystectomy at a single institution. They used a homemade single-port device composed of an inverted cone device of polycarbonate and a powder-free surgical glove [10]. All of the procedures were completed successfully. The mean operative time was 208.2 minutes, estimated blood loss was 270 mL, bowel recovery time was 9.75 days, and postoperative hospital stay was 19.5 days. The pathologic evaluation revealed negative margins and negative lymph node involvement. After the operations, one patient had a bowel obstruction, while another patient died from cardiac disease. Mean follow-up time was short (143 days).
Robotic applications to LESS
The amount of available clinical outcomes of robotic LESS has grown considerably since the pioneering description of the first successful clinical series of single-port robotic procedures [5]. So far, a cumulative number of roughly 150 robotic urologic LESS cases have been reported by different institutions across the globe with a variety of techniques and port configurations (Table 1).
Table 1.
Robotic LESS procedures: major* clinical series in urology
| Reference | Procedure (n of cases) | Access device | OR, min | Conversions, n (to what) | Major (Clavien grade >3) postop. complications, n (%) | Comments |
|---|---|---|---|---|---|---|
| 38 | RN (10) | SILS or Gelpoint | 167.5^ | 0 | 0 | With SILS port, robotic trocars placed inside the same skin incision, and tunneled before piercing the fascia. With the Gelpoint, robotic trocars inserted at the most cephalad and caudal aspects of the device |
| 39 | PN (14) | Homemade | 205^ | 2 (open PN) | 0 | Median tumor size 3.2 cm. Median WIT 30 min. All margins negative. Additional port used. |
| 40 | PN (35) | Homemade | 187.5^^ | 0 | 1 (3) | WIT 29.5 min. One positive margin. Additional port used (two-port technique). |
| 43 | Pyelo (10) | Gelpoint | 226^^ | 0 | 1 (10) | Two 5-mm robotic ports, a 12-mm camera port, and a 12-mm assistant trocar through GelPOINT in a diamond-shaped configuration. Robotic instruments crisscrossing at umbilicus. |
| 45 | RP (20) | SILS | 187.6^^ | 1 (RALP) | 1 (5) | Skin incision measuring 3–4.5 cm, mostly concealed within the umbilicus. Instruments not crossed. Use of “marionette” sutures. |
| 46 | STEP (9) | Gelport | 234^ | 1 (open SP) | 4 (44) | Initial transurethral incision of the prostatic apex. Two patients requiring digital rectal assistance for enucleation. No suturing. |
| 50 | Pyelo (9) | daVinci Single-site instrument | 160^ | 0 | 0 |
of at least 5 cases
Median value
Mean value
Legends. RN=Radical Nephrectomy; PN=Partial Nephrectomy; Pyelo=Pyeloplasty; RP=Radical Prostatectomy; STEP=Suprapubic Transvesical enucleation of the prostate; RALP=Robot Assisted Laparoscopic Prostatectomy; SP=Simple prostatectomy
White et al. detailed the technique of robotic LESS radical nephrectomy and reported the comparative outcomes versus the current gold standard laparoscopic procedure [38]. Two single-port devices, the SILS port and the GelPort or GelPoint, were used equally and the da Vinci S or da VinciSi System (in a three-arm approach) was employed. A three to 7 cm skin incision was concealed within the umbilicus. There was no difference between R-LESS and conventional laparoscopy in terms of operative time, estimated blood loss, visual analogue scale, or complication rate. The robotic LESS group had a lower median narcotic requirement during hospitalization (25.3 vs. 37.5 morphine equivalents; p = 0.049) and a shorter length of stay (2.5 vs. 3.0 days; p = 0.03). The study's limitations included a small sample size, short follow-up period, and the retrospective design of the study.
Han et al. described their experience with robotic LESS partial nephrectomy through a homemade transumbilical port to treat 14 cases of renal cell carcinoma. All surgical margins after partial nephrectomy were negative for malignancy. No port-related complications were reported. Two cases required conversion to mini-incisional partial nephrectomy. We note our use of an extra 12-mm trocar below the subxyphoid or alongside the homemade single port to facilitate suturing [39]. The same group later reported a matched pair comparison with standard robotic partial nephrectomy [40]. Main surgical outcomes were comparable in both groups. Moreover, pain scores, in-hospital morphine requirement, and length of hospital stay were likewise similar in both groups. The authors suggested that a two-port technique is a viable option until a more advanced robotic platform specifically designed for LESS is developed and the need for extra ports can be safely overcome.
Park et al. were the first to recently report a case of retroperitoneoscopic robotic LESS adrenalectomy for an adrenal cortical adenoma [41]. A 3 cm transverse skin incision was made just below the 12th costal arch and, after exposing the retroperitoneal space, a Glove port was applied to the skin incision and CO2 insufflated to create an adequate working space. A 10 mm robotic camera with 30° up view and three 5mm robotic ports were inserted through the Glove port. The total operation time was 188 minutes and the patient recovered uneventfully.
By adopting the principle of the “chopstick technique” [42], Olweny et al. used a setup including a Gelpoint access device, a 30° up robotic scope, and the da Vinci Si surgical robot to enhance the applicability of the robotic platform to LESS pyeloplasty and reduce its learning curve [43]. The authors compared their initial 10 cases of robotic LESS pyeloplasty with the last 10 cases of conventional LESS pyeloplasty done by a single surgeon. Mean operative time was significantly longer for robotic LESS, but this was probably related to the stent insertion time. Conventional LESS alone required an accessory port for the anastomosis in all the cases. Two conversions to standard laparoscopy and two postoperative complications occurred in 30% of LESS patients, whereas there were no conversions and one postoperative complication in the robotic LESS group.
When first reporting an initial feasibility study of LESS radical prostatectomy in humans, Kaouk et al. acknowledged the limitations of embarking on this procedure due to challenges related to ergonomics and intracorporeal suturing. They reported the first claim of a potential application of robotics [44].
White et al. detailed the surgical technique and reported the outcomes of 20 robotic LESS radical prostatectomies [45]. Most of the study population was represented by low/intermediate risk patients with baseline erectile dysfunction. A 3-4.5 cm incision was created intraumbilically, and a 2 cm incision through the linea alba. The initial robotic 8mm port was placed at the most caudal portion of the incision on the right side and directed as far laterally as possible; this was repeated on the opposite side with a 5mm pediatric or standard 8 mm robotic port. A SILS port was inserted and the patient positioned in steep Trendelenburg. The da VinciS or Si system in a three-arm approach was docked and the robotic 12 mm scope introduced through the SILS port with a 5 mm channel free for suction or sutures to be passed through. The steps of the procedures resembled those of the standard robotic procedure. During the bladder neck dissection, a suture was placed through the abdominal wall and then through the distal bladder neck or prostatic base and then exited out of the abdominal wall to serve as a retractor in a “marionette” fashion. The vas deferens and seminal vesicles were mobilized with the 5 mm harmonic scalpel in a non-nerve-sparing approach and athermally with Hem-o-lok clips in a nerve-sparing approach, which was adopted in three cases. Prostatic dissection was obtained using a 5-mm harmonic scalpel in a non-nerve-sparing procedure. Otherwise, an interfascial nerve-sparing approach was accomplished with a combination of sharp dissection and robotically applied Hem-o-lok clips. Complete dissection of the prostate apex was accomplished in a retrograde fashion. A standard lymph node dissection was performed including external iliac nodal tissues, as well as nodes from the obturator fossa. Vesicourethral anastomosis was done with two sutures in a semicircular “running” fashion. A positive margin was found in four cases, two of them being in the first three cases, so it is likely the result of the learning curve. The limited follow-up did not allow a reliable oncologic assessment, but early postoperative continence rates were encouraging.
Within the field of prostate surgery, Fareed et al. reported the perioperative and short-term outcomes of their initial series of robotic single port transvesical enucleation of the prostate (R-STEP) [46]. Nine patients with symptomatic BPH were scheduled for the procedure. A 3 cm lower midline incision was made, a cystotomy created, and a GelPort positioned in the bladder. The da Vinci S operating system was docked through the GelPort platform. There was significant postoperative improvement in the flow rates. But a high-grade (Clavien III-IV) complication was observed in three patients (37.5%). The authors concluded that despite providing adequate relief of bladder outlet obstruction, the procedure carries a high risk of complications and its role remains to be determined.
Khanna et al. reviewed the Cleveland Clinic's experience with robot-assisted LESS for renal surgery [47]. Twenty-eight procedures were analyzed, including radical nephrectomies (n = 11), partial nephrectomies (5), nephroureterectomies (3), pyeloplasties (7), simple nephrectomy (1), and renal cyst decortication (1). In four cases (14%) a conversion was needed to complete the procedure. Patients who underwent radical nephrectomy, partial nephrectomy, and nephroureterectomy all had negative surgical margins and have remained disease free during the follow-up period. Six of seven patients who underwent pyeloplasty reported resolution or significant improvement of symptoms. From the same institution, White et al. presented their cumulative experience [48]. Overall, 50 patients were scheduled to undergo robotic urological LESS during the study period, representing 36% of the total patients undergoing LESS. Specifically, 24 patients underwent renal surgery and 26 patients pelvic surgery. Four cases were converted to laparoscopy and six cases required at least one additional trocar outside of the single-site incision. A rectal injury occurred during a radical cystectomy, which was recognized intraoperatively and closed primarily without sequelae. Postoperative complications occurred in eight cases, including one Clavien grade IV. This outcome analysis remains preliminary because of the small sample and the limited follow-up. Nevertheless, technical nuances and learning points that the authors experienced during the study period are of interest. The Si system was preferable due to its enhanced visualization and ability to customize the console settings ergonomically. Among the several commercially available ports, the SILS Port allowed free exchange of varying cannula sizes and passage of sutures, staplers, clip appliers, and entrapment bags directly through the port. Instrument clashing, as well as gas leak and insufficient tissue retraction were experienced. To reduce clashing, crossing of the instruments was avoided by maintaining the robotic arms in parallel to the robotic camera. This subsequently required the camera lens and instruments to be moved in near unison to optimize range of motion. The incidence of gas leak was significantly reduced by tunneling the robotic trocars. Petroleum impregnated gauze or a strategically placed fascial suture can be used to overcome leakage occurring at the multichannel port due to an enlarged fascial incision. As tissue retraction can be difficult due to lack of use of the fourth robotic arm, the use of internal retraction sutures in a marionette fashion was adopted to compensate.
Another large single center cumulative experience was reported by Lee et al. [49]. They analyzed 68 consecutive robotic LESS urologic operations, including: partial nephrectomy (n = 51), nephroureterectomy (12), radical nephrectomy (2) and adrenalectomy (2), and simple nephrectomy (1). The mean operative time was 219 minutes and mean estimated blood loss 319mL. For partial nephrectomy, mean ischemia time was 27 minutes. Three intraoperative complications occurred. At a mean follow-up of eight months, there were no port-related complications, and cosmesis was excellent. The homemade single-port device provided adequate range of motion and flexibility in port placement.
In urology, the only clinical series with the use of the da Vinci Single-Site instrumentation so far has been reported by Cestari et al, who tested the technical feasibility and short-term perioperative outcomes of LESS pyeloplasty [50]. Exclusion criteria for these preliminary series were a BMI >30 kg/m2, previous abdominal and renal surgery, concomitant renal stones, an extremely large renal pelvis, pelvic kidney, and horseshoe kidney. In three cases aberrant crossing vessels were found and transposed. To ease the plasty reconfiguration, a barbed suture was used. The needles were inserted and removed under direct vision through the assistant port. Once the posterior plate of the anastomosis was completed, a DJ stent was inserted retrogradely using a flexible cystoscope in order to avoid undocking/redocking of the robotic arm system and to save time. An auxiliary 3-mm trocar was deemed necessary to retract the liver properly and expose the surgical field in one patient.
Patients’ perception of LESS
Besides a more reliable assessment of the outcomes and the need for better instrumentation, a major issue for any novel surgical procedure is represented by the patient's demand and perception of techniques [51].
Despite the limitations inherent to LESS and a paucity of evidence to support its superiority as compared to standard laparoscopy, there has been increasing public interest for this technique. The larger question then becomes the public's perception and expectations of LESS and how we as providers should address the role of these techniques during surgical counseling.
Lucas et al. surveyed patients returning to the clinic after transperitoneal laparoscopy [52]. Patients were first asked to rate certain factors and how these factors impacted their choice to pursue open surgery, laparoscopy, or LESS. Patients were likewise asked whether they preferred LESS or laparoscopy, assuming equivalence of outcomes. Respondents were thereafter asked their opinions on LESS assuming comparative surgical naivety on the part of the operating surgeon, and how increased complication rates and/or surgical failure would impact their decision to pursue LESS. In the second survey, a validated cosmesis and body image impact survey was administered. The findings of the surveys were intriguing. First, surgical success and complications were considered the driving factors among patients. Pain and convalescence were of moderate importance while scars carried less importance. Post-operative cosmesis was valued more heavily among women, younger patients, and those with benign surgical indications. Finally, the authors found a slight preference for laparoscopy (39%) as compared to LESS (30%).
Olweny et al. evaluated the importance of scarring in urology patients relative to other surgical outcomes [53]. Patients scheduled for laparo-endoscopic single-site, laparoscopic, or open kidney surgeries were recruited for the study. Overall, 90 patients completed surveys. The LESS cohort was younger and more likely to be undergoing surgery for benign indications. Before surgery, the most important surgical consideration was “surgeon reputation” while the least important factors were “delay in resuming normal diet” and “size/number of scars”. After surgery, the most important considerations were “surgeon reputation” and “no complications” while “size/number of scars” represented the second least important consideration. Among the subset of patients who completed surveys both before and after surgery, there was no significant change in median scores for any of the outcomes except “duration of hospital admission”. The median score for “size/number of scars” was significantly higher for the LESS cohort before surgery, but there was no significant difference among the cohorts after surgery. The median preoperative score for “size/number of scars” was significantly higher for younger patients and those with benign surgical conditions. Overall, the authors demonstrated that, when compared with surgeon reputation or avoidance of complications, surgical scarring was a relatively unimportant outcome for most patients before and after undergoing kidney surgery. Younger patients and those undergoing surgery for benign indications ranked scarring higher than older patients and those with oncologic indications before surgery, but these differences were insignificant after surgery. Given that patients had decided on a particular surgical approach prior to completion of the study, the primary study limitation was a response bias.
CONCLUSIONS
Significant advances have been achieved in the field of urologic LESS since the first reported clinical series in 2007. Despite unsolved challenges, LESS can be regarded as an emerging trend in minimally invasive urologic surgery and it has significantly evolved, becoming a widely applicable technique in a relatively short time. Outcomes demonstrate that a broad range of procedures can be effectively and safely preformed, given a solid laparoscopic surgical background and stringent patient-selection criteria. The recent introduction of a purpose-built instrumentation is likely to further foster the application of robotics to LESS. Further improvements are needed before this technique might reach a widespread adoption. Future advances in the field of robotic technology are expected to overcome the current limitations of LESS.
REFERENCES
- 1.Rane A, Rao P, Rao P. Single port access nephrectomy and other laparoscopic urologic procedures using a novel laparoscopic port (R-Port) Urology. 2008;72:260–264. doi: 10.1016/j.urology.2008.01.078. [DOI] [PubMed] [Google Scholar]
- 2.Raman JD, Bensalah K, Bagrodia A, et al. Laboratory and clinical development of single keyhole umbilical nephrectomy. Urology. 2007;70:1039–1042. doi: 10.1016/j.urology.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.White WM, Haber GP, Goel RK, et al. Single-port urological surgery: single-center experience with the first 100 cases. Urology. 2009;74(4):801–804. doi: 10.1016/j.urology.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Autorino R, Stein RJ, Lima E, Damiano R, et al. Current status and future perspectives in laparoendoscopic single-site and natural orifice transluminal endoscopic urological surgery. Int J Urol. 2010;17(5):410–431. doi: 10.1111/j.1442-2042.2010.02497.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaouk JH, Goel RK, Haber GP, et al. Robotic single-port transumbilical surgery in humans: initial report. BJU Int. 2009;103(3):366–369. doi: 10.1111/j.1464-410X.2008.07949.x. [DOI] [PubMed] [Google Scholar]
- 6.Rane A, Autorino R. Robotic natural orifice translumenal endoscopic surgery and laparoendoscopic single-site surgery: current status. Curr Opin Urol. 2011;21(1):71–77. doi: 10.1097/MOU.0b013e32833fd602. [DOI] [PubMed] [Google Scholar]
- 7.Autorino R, Cadeddu JA, Desai MM, et al. Laparoendoscopic single-site and natural orifice transluminal endoscopic surgery in urology: a critical analysis of the literature. Eur Urol. 2011;59(1):26–45. doi: 10.1016/j.eururo.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, White MA, Autorino R, et al. Selection of a port for use in laparoendoscopic single-site surgery. Curr Urol Rep. 2011;12:94–99. doi: 10.1007/s11934-011-0174-4. [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Park YH, Jeon HG, et al. The feasibility of laparoendoscopic single-site nephrectomy: initial experience using home-made single port device. Urology. 2010;76:862–865. doi: 10.1016/j.urology.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Ma LL, Bi H, Hou XF, et al. Laparoendoscopic single-site radical cystectomy and urinary diversion: initial experience in China using a homemade single-port device. J Endourol. 2012;26(4):355–359. doi: 10.1089/end.2011.0300. [DOI] [PubMed] [Google Scholar]
- 11.Lee SW, Lee JY. Laparoendoscopic single-site urological surgery using a homemade single port device: the first 70 cases performed at a single center by one surgeon. J Endourol. 2011;25(2):257–264. doi: 10.1089/end.2010.0445. [DOI] [PubMed] [Google Scholar]
- 12.Lin T, Huang J, Han J, Xu K, et al. Hybrid laparoscopic endoscopic single-site surgery for radical cystoprostatectomy and orthotopic ileal neobladder: an initial experience of 12 cases. J Endourol. 2011;25(1):57–63. doi: 10.1089/end.2010.0332. [DOI] [PubMed] [Google Scholar]
- 13.Seo IY, Hong HM, Kang IS, et al. Early experience of laparoendoscopic single-site nephroureterectomy for upper urinary tract tumors. Korean J Urol. 2010;51(7):472–476. doi: 10.4111/kju.2010.51.7.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SH, Hwang HK, Kang CM, Lee WJ. Transumbilical single port laparoscopic adrenalectomy: a technical report on right and left adrenalectomy using the glove port. Yonsei Med J. 2012;53(2):442–445. doi: 10.3349/ymj.2012.53.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco F, Veneziano D, Wagner S, et al. Laparoendoscopic single-site radical nephrectomy for renal cancer: technique and surgical outcomes. Eur Urol. 2012;62(1):168–174. doi: 10.1016/j.eururo.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Cindolo L, Greco F, Fornara P, et al. Nephron sparing LESS: technique and review of the current literature. Arch Esp Urol. 2012;65(3):303–310. [PubMed] [Google Scholar]
- 17.Cáceres F, Cabrera PM, García-Tello A, et al. Safety Study of Umbilical Single-port Laparoscopic Radical Prostatectomy with a New DuoRotate System. Eur Urol. 2012 May 5; doi: 10.1016/j.eururo.2012.04.043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Haber GP, Autorino R, Laydner H, et al. SPIDER surgical system for urologic procedures with laparoendoscopic single-site surgery: from initial laboratory experience to first clinical application. Eur Urol. 2012;61(2):415–422. doi: 10.1016/j.eururo.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Salas N, Gorin MA, Gorbatiy V, et al. Laparoendoscopic single site nephrectomy with the SPIDER surgical system: engineering advancements tested in a porcine model. J Endourol. 2011;25(5):739–742. doi: 10.1089/end.2010.0608. [DOI] [PubMed] [Google Scholar]
- 20.Leveillee RJ, Castle SM, Gorin MA, et al. Initial experience with laparoendoscopic single-site simple nephrectomy using the TransEnterix SPIDER surgical system: assessing feasibility and safety. J Endourol. 2011;25(6):923–925. doi: 10.1089/end.2010.0730. [DOI] [PubMed] [Google Scholar]
- 21.Haber GP, White MA, Autorino R, et al. Novel robotic da Vinci instruments for laparoendoscopic single-site surgery. Urology. 2010;76(6):1279–1282. doi: 10.1016/j.urology.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 22.Kaouk JH, Autorino R, Laydner H, et al. Robotic Single-site Kidney Surgery: Evaluation of Second-generation Instruments in a Cadaver Model. Urology. 2012 Mar 23; doi: 10.1016/j.urology.2012.02.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Kaouk JH, Autorino R, Kim FJ, et al. Laparoendoscopic single-site surgery in urology: worldwide multi-institutional analysis of 1076 cases. Eur Urol. 2011;60(5):998–1005. doi: 10.1016/j.eururo.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Irwin BH, Cadeddu JA, Tracy CR, et al. Complications and conversions of upper tract urological laparoendoscopic single-site surgery (LESS): multicentre experience: results from the NOTES Working Group. BJU Int. 2011;107(8):1284–1289. doi: 10.1111/j.1464-410X.2010.09663.x. [DOI] [PubMed] [Google Scholar]
- 25.Greco F, Cindolo L, Autorino R, et al. Laparoendoscopic single-site upper urinary tract surgery: assessment of postoperative complications and analysis of risk factors. Eur Urol. 2012;61(3):510. doi: 10.1016/j.eururo.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Best SL, Donnally C, Mir SA, et al. Complications during the initial experience with laparoendoscopic single-site pyeloplasty. BJU Int. 2011;108(8):1326–1329. doi: 10.1111/j.1464-410X.2011.10078.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramasamy R, Afaneh C, Katz M, et al. Comparison of complications of laparoscopic versus laparoendoscopic single site donor nephrectomy using the modified Clavien grading system. J Urol. 2011;186(4):1386–1390. doi: 10.1016/j.juro.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 28.Autorino R, Kaouk JH, Yakoubi R, et al. Urological laparoendoscopic single site surgery: multi-institutional analysis of risk factors for conversion and postoperative complications. J Urol. 2012;187(6):1989–1994. doi: 10.1016/j.juro.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 29.Tugcu V, Ilbey YO, Mutlu B, Tasci AI. Laparoendoscopic single-site surgery versus standard laparoscopic simple nephrectomy: a prospective randomized study. J Endourol. 2010;24(8):1315–1320. doi: 10.1089/end.2010.0048. [DOI] [PubMed] [Google Scholar]
- 30.Kurien A, Rajapurkar S, Sinha L, et al. First prize: Standard laparoscopic donor nephrectomy versus laparoendoscopic single-site donor nephrectomy: a randomized comparative study. J Endourol. 2011;25(3):365–370. doi: 10.1089/end.2010.0250. [DOI] [PubMed] [Google Scholar]
- 31.Lee SW, Lee JY, Kim KH, Ha US. Laparoendoscopic single-site surgery versus conventional laparoscopic varicocele ligation in men with palpable varicocele: a randomized, clinical study. Surg Endosc. 2012;26(4):1056–1562. doi: 10.1007/s00464-011-1997-2. [DOI] [PubMed] [Google Scholar]
- 32.Rane A, Cindolo L, Schips L, et al. Laparoendoscopic single site (LESS) adrenalectomy: Technique and outcomes. World J Urol. 2011 Apr 26; doi: 10.1007/s00345-011-0678-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Greco F, Hoda MR, Alcaraz A, et al. Laparoscopic living-donor nephrectomy: analysis of the existing literature. Eur Urol. 2010;58:498–509. doi: 10.1016/j.eururo.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Gill IS, Canes D, Aron M, et al. Single port transumbilical (E-NOTES) donor nephrectomy. J Urol. 2008;180:637–641. doi: 10.1016/j.juro.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Wang GJ, Afaneh C, Aull M, et al. Laparoendoscopic single site live donor nephrectomy: single institution report of initial 100 cases. J Urol. 2011;186(6):2333–2337. doi: 10.1016/j.juro.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Khanna R, Isac W, Laydner H, et al. Laparoendoscopic single site reconstructive procedures in urology: medium term results. J Urol. 2012;187(5):1702–1706. doi: 10.1016/j.juro.2011.12.070. [DOI] [PubMed] [Google Scholar]
- 37.Kaouk JH, Goel RK, White MA, et al. Laparoendoscopic single-site radical cystectomy and pelvic lymph node dissection: initial experience and 2-year follow-up. Urology. 2010;76(4):857–861. doi: 10.1016/j.urology.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 38.White MA, Autorino R, Spana G, et al. Robotic laparoendoscopic single-site radical nephrectomy: surgical technique and comparative outcomes. Eur Urol. 2011;59(5):815–822. doi: 10.1016/j.eururo.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Han WK, Kim DS, Jeon HG, et al. Robot-assisted laparoendoscopic single-site surgery: partial nephrectomy for renal malignancy. Urology. 2011;77(3):612–626. doi: 10.1016/j.urology.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 40.Arkoncel FR, Lee JW, Rha KH, et al. Two-port robot-assisted vs. standard robot-assisted laparoscopic partial nephrectomy: a matched-pair comparison. Urology. 2011;78(3):581–585. doi: 10.1016/j.urology.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Walz MK, Kang SW, et al. Robot-assisted posterior retroperitoneoscopic adrenalectomy: single port access. J Korean Surg So. 2011;81(Suppl 1):S21–24. doi: 10.4174/jkss.2011.81.Suppl1.S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph RA, Goh AC, Cuevas SP, et al. ‘Chopstick’ surgery: a novel technique improves surgeon performance and eliminates arm collision in robotic single-incision laparoscopic surgery. Surg Endo. 2010;24:1331–1333. doi: 10.1007/s00464-009-0769-8. [DOI] [PubMed] [Google Scholar]
- 43.Olweny EO, Park SK, Tan YK, et al. Perioperative comparison of robotic assisted laparoendoscopic single-site (LESS) pyeloplasty versus conventional LESS pyeloplasty. Eur Urol. 2012;61(2):410. doi: 10.1016/j.eururo.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Kaouk JH, Goel RK, Haber GP, et al. Single-port laparoscopic radical prostatectomy. Urology. 2008;72:1190–1193. doi: 10.1016/j.urology.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 45.White MA, Haber GP, Autorino R, et al. Robotic laparoendoscopic single-site radical prostatectomy: technique and early outcomes. Eur Urol. 2010;58(4):544–550. doi: 10.1016/j.eururo.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Fareed K, Zaytoun OM, Autorino R, et al. Robotic single port suprapubic transvesical enucleation of the prostate (R-STEP): initial experience. BJU Int. 2012 Feb 17; doi: 10.1111/j.1464-410X.2012.10954.x. [DOI] [PubMed] [Google Scholar]
- 47.Khanna R, Stein RJ, White MA, et al. Single institution experience with robot-assisted laparoendoscopic single-site renal procedures. J Endourol. 2012;26(3):230. doi: 10.1089/end.2011.0187. [DOI] [PubMed] [Google Scholar]
- 48.White MA, Autorino R, Spana G, et al. Robotic Laparoendoscopic Single Site Urological Surgery: Analysis of 50 Consecutive Cases. J Urol. 2012 Mar 14; doi: 10.1016/j.juro.2011.12.073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Won Lee J, Arkoncel FR, Rha KH, et al. Urologic robot-assisted laparoendoscopic single-site surgery using a homemade single-port device: a single-center experience of 68 cases. J Endourol. 2011;25(9):1481. doi: 10.1089/end.2010.0656. [DOI] [PubMed] [Google Scholar]
- 50.Cestari A, Buffi NM, Lista G, et al. Feasibility and Preliminary Clinical Outcomes of Robotic Laparoendoscopic Single-Site (R-LESS) Pyeloplasty Using a New Single-Port Platform. Eur Urol. 2012 Mar 28; doi: 10.1016/j.eururo.2012.03.041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Chow A, Purkayastha S, Dosanjh D, et al. Patient Reported Outcomes and Their Importance in the Development of Novel Surgical Techniques. Surg Innov. doi: 10.1177/155335061142601. [DOI] [PubMed] [Google Scholar]
- 52.Lucas SM, Baber J, Sundaram CP. Determination of Patient Concerns in Choosing Surgery and Preference for Laparoendoscopic Single-Site Surgery and Assessment of Satisfaction with Postoperative Cosmesis. J Endourol. doi: 10.1089/end.2011.0181. [DOI] [PubMed] [Google Scholar]
- 53.Olweny EO, Mir SA, Best SL, et al. Importance of cosmesis to patients undergoing renal surgery: a comparison of laparoendoscopic single-site (LESS), laparoscopic and open surgery. BJU Int. 2011 Dec 16; doi: 10.1111/j.1464-410X.2011.10784.x. [DOI] [PubMed] [Google Scholar]