Abstract
To better understand the role that water and urea fluxes play in the urine concentrating mechanism, we determined transepithelial osmotic water permeability (Pf) and urea permeability (Purea) in isolated perfused Munich-Wistar rat long-loop descending thin limbs (DTLs) and ascending thin limbs (ATLs). Thin limbs were isolated either from 0.5 to 2.5 mm below the outer medulla (upper inner medulla) or from the terminal 2.5 mm of the inner medulla. Segment types were characterized on the basis of structural features and gene expression levels of the water channel aquaporin 1, which was high in the upper DTL (DTLupper), absent in the lower DTL (DTLlower), and absent in ATLs, and the Cl-1 channel ClCK1, which was absent in DTLs and high in ATLs. DTLupper Pf was high (3,204.5 ± 450.3 μm/s), whereas DTLlower showed very little or no osmotic Pf (207.8 ± 241.3 μm/s). Munich-Wistar rat ATLs have previously been shown to exhibit no Pf. DTLupper Purea was 40.0 ± 7.3 × 10−5 cm/s and much higher in DTLlower (203.8 ± 30.3 × 10−5 cm/s), upper ATL (203.8 ± 35.7 × 10−5 cm/s), and lower ATL (265.1 ± 49.8 × 10−5 cm/s). Phloretin (0.25 mM) did not reduce DTLupper Purea, suggesting that Purea is not due to urea transporter UT-A2, which is expressed in short-loop DTLs and short portions of some inner medullary DTLs close to the outer medulla. In summary, Purea is similar in all segments having no osmotic Pf but is significantly lower in DTLupper, a segment having high osmotic Pf. These data are inconsistent with the passive mechanism as originally proposed.
Keywords: aquaporin, epithelial transport, renal medulla, urine concentrating mechanism, urea transporter-A
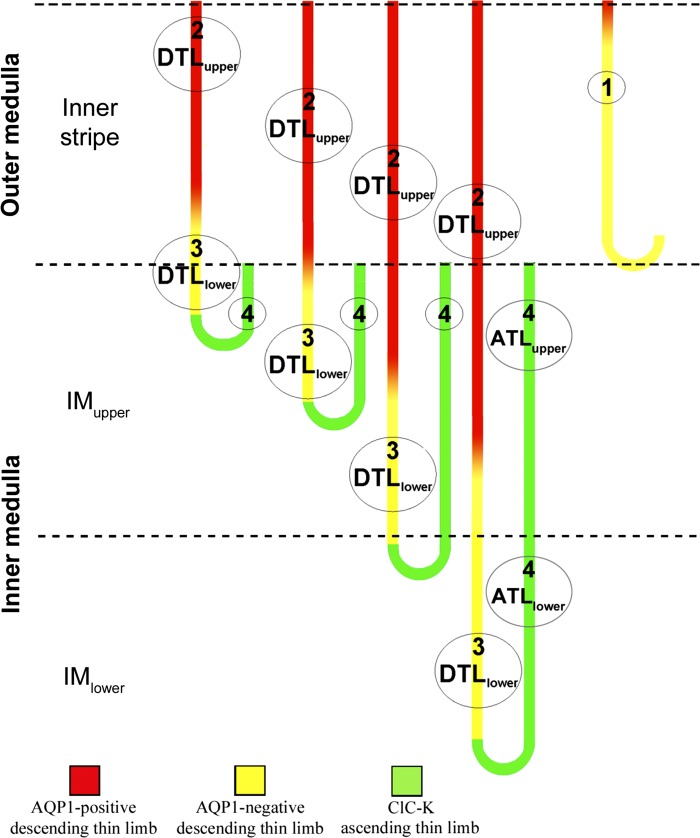
it has been long recognized, on the basis of cell and epithelial ultrastructure in a number of mammalian species, that the descending thin limbs of Henle's loops (DTLs) consist of at least three structurally distinct segments (types 1–3) (1a, 7, 15, 17, 21, 37, 38, 45). Ascending thin limbs of Henle's loops (ATLs), including a short prebend segment at the terminal end of the DTL, consist of just one morphologically distinct segment (type 4; Fig. 1).
Fig. 1.
Four structurally distinct segments of rat medullary thin limbs of Henle's loops (designated as types 1–4). Descending thin limbs (DTLs) that descend less than about 1 mm below the outer medulla (OM) express detectable aquaporin 1 (AQP1) in their OM segments and little or no AQP1 in their inner medullary (IM) segments. DTLs that descend more than about 1 mm below the OM express detectable AQP1 protein only along the initial 40% of their IM length; the terminal 60% expresses no detectable AQP1 protein (32, 34). The ascending thin limb (ATL) and prebend segment express the Cl− channel ClCK1 along their entire lengths (18, 32, 40). Urea transporter UT-A2 protein has previously been shown to be weakly expressed in some IM DTLs, but only in short portions close to the OM-IM boundary (not shown) (19, 25). For the experiments conducted in this study, the upper DTL (DTLupper) and upper ATL (ATLupper) [red and green segments, respectively, from the upper IM (IMupper)] were dissected from 0.5 to 2.5 mm below the OM; the lower DTL (DTLlower) and lower ATL (ATLlower) [yellow and green segments, respectively, from the lower IM (IMlower)] were dissected from the terminal 2.5 mm of the IM (see methods for additional details).
DTLs that form bends within the outer medulla (OM; short-loop DTLs) consist of type 1 epithelia along their entire length. DTLs that form bends at all levels throughout the inner medulla (IM; long-loop DTLs) consist of type 2 and 3 epithelia, which line two successive segments whose lengths appear to be directly proportional to loop length (31). Detectable levels of aquaporin 1 (AQP1), the principal water channel in the DTL (8), are not observed in rat inner medullary segments of long-loop DTLs forming bends within approximately the first millimeter below the OM (32, 34, 42). These DTL segments are considered to be lower DTLs (DTLlower), and their upper DTL (DTLupper) segments lie within the OM and do express AQP1 (T. L. Pannabecker, unpublished observations) (Fig. 1). DTLs of the rat that extend deeper than the first millimeter below the OM express detectable AQP1 protein only for the first 40% of their inner medullary length (DTLupper). Thus, variable lengths of DTLupper and DTLlower coexist at most levels throughout the IM, with DTLlower and prebends as the sole descending segments near the papilla tip (Fig. 1). Urea transporter UT-A2 protein is expressed in short portions of some rat inner medullary DTLs, but only in segments close to the OM (19, 25). No other known AQPs or urea transporters are expressed in inner medullary DTLs. ATLs and prebend segments consist of type 4 epithelia, express the Cl− channel ClCK1, and express no known AQPs or urea transporters.
In this study, we further characterized the structure of Munich-Wistar rat inner medullary thin limbs of Henle's loops. We also confirmed the identity of single isolated segment types by correlating AQP1 and ClCK1 mRNA expression levels with structural characteristics determined using light microscopy. We then determined water and urea transepithelial flux rates and permeabilities of structurally distinct, isolated perfused segments. The magnitudes of transepithelial fluid and solute fluxes in the thin limbs of Henle's loops are essential to understanding the role that thin limbs play in generating the high medullary osmolality that supports the urine concentrating mechanism in the mammalian kidney (9, 31).
We found, on the basis of water permeability (Pf) or urea permeability (Purea), that the DTL consists of two functionally distinct segments, whereas the ATL consists of a single functionally distinct segment. We propose that, as with chinchilla (7), these segments correspond to type 2 (DTLupper), type 3 (DTLlower), and type 4 [upper ATL (ATLupper) and lower ATL (ATLlower)] epithelia. The variable functionality along the DTL and very high Purea along the ATL are not consistent with the passive mechanism as originally proposed (20, 39) and raise further questions about solute movements across the inner medullary thin limbs.
METHODS
Animals.
Male Munich-Wistar rats (average age: ∼90 days) were purchased from Simonsen Laboratories (Gilroy, CA) or Harlan (Indianapolis, IN) or were reared in the University Animal Care facility at the University of Arizona (Tucson, AZ) and were provided with rat chow (Teklad 7001) and water ad libitum. Animals were euthanized with CO2. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and were approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Solutions.
Kidneys were bathed and dissected on ice in a solution consisting of 280 mM sucrose and 10 mM HEPES adjusted to pH 7.4 with Tris base and gassed with 100% O2 (sucrose-HEPES buffer). The solution used for perfusing and bathing the isolated tubules consisted of (in mmol/l) 118 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, and 5 urea adjusted to pH 7.4 and gassed with 95% O2-5% CO2 (bicarbonate buffer). Solution osmolalities were about 290 mosmol/kg H2O.
Tubule dissection and identification of tubule segments on the basis of structural features.
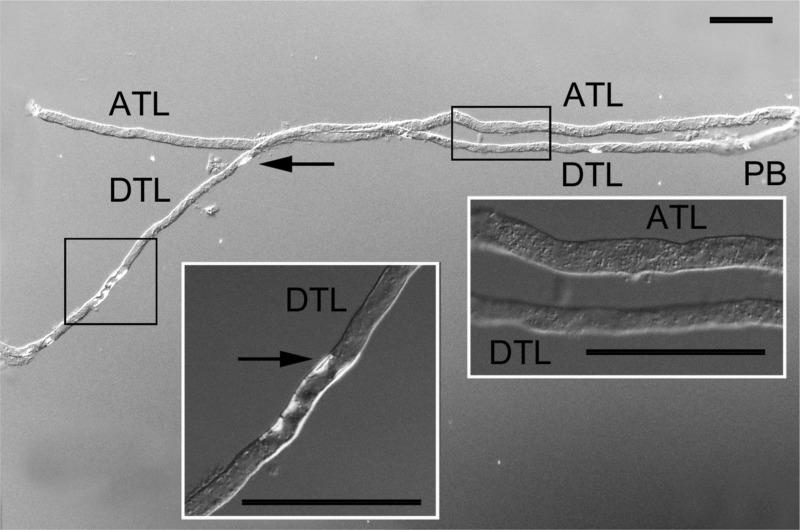
Tubule segments for perfusion and the study of osmotic Pf and Purea were teased from the isolated IM in sucrose-HEPES buffer without the aid of enzymatic agents under a stereomicroscope using reflected light below the dissection dish. First, DTLupper segments were teased from 0.5 to 2.5 mm below the OM (Fig. 1). We took care to select segments that did not include the first 0.5 mm below the OM-IM border to avoid any possibility of including UT-A2. We also chose only DTL segments from loops that extended well beyond the first 1 mm of the IM because DTLs of loops that turn within the first 1 mm lack detectable AQP1 protein and because they would have been too short to perfuse if the first 0.5 mm was not included. These selected segments would all have some AQP1 expression but could also include portions that lack AQP1 (Fig. 1). Second, DTLlower segments were teased from the terminal 2.5 mm of the IM. DTLs from this region have no detectable AQP1 protein. We were also careful to avoid the prebend region (a DTLlower segment that exhibits structural and functional characteristics of the ATL) as determined by its diameter (Fig. 2). Third, ATLupper segments were teased from 0.5 to 2.5 mm below the OM and were not from loops that turned within the first 1 mm. Fourth, ATLlower segments were teased from the lower 2.5 mm of the IM. Finally, DTLs are distinguished from ATLs by their smaller diameter and by their cell type. DTLs have nuclei that show protrusions into the lumen, whereas ATLs have round, flat nuclei (3, 33). These differences are shown most clearly when the tubule segments are viewed under a compound microscope with differential interference contrast optics (Fig. 2), but they can also be recognized under a stereomicroscope.
Fig. 2.
Differential interference contrast image of the DTLlower (DTL) and ATLlower (ATL) of a Munich-Wistar rat. Segments are recognized by their position relative to the loop bend and on the basis of structural characteristics (see methods). We have previously shown that luminal projections are abundant in DTLupper (3, 33); however, as shown in this image, luminal projections are sparse in DTLlower (arrows). The diameter of the DTLlower is less than that of the ATL and the prebend segment (PB). Diameter differences and luminal projections in DTLs are also clearly seen with a stereomicroscope. The insets show higher-magnification images of the regions within boxes. Scale bars = 100 μm.
Tubule dissection and identification of tubule segments on the basis of gene expression.
Tubule segments were also characterized by quantifying expression levels of mRNA coding for AQP1 and ClCK1. Expression of cyclophilin A served as an indicator that cDNA was successfully synthesized and amplified from each tubule. Single inner medullary thin limb segments were isolated in sucrose-HEPES buffer and selected for quantitative PCR analysis by the same structural criteria used for obtaining segments for perfusion and the study of osmotic Pf and Purea described above. Each thin limb segment was transferred with 5–10 μl buffer into a 0.5-ml microcentrifuge tube. After removal of the buffer, the tubule was lysed in 10 mM l-arginine and 1% Triton X-100 (pH 2.5, Sigma Aldrich, St. Louis, MO). cDNA was synthesized from 12.5 μl of lysate with Maxima H Minus Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA). Two microliters of cDNA were included in each 10-μl real-time PCR using SYBR Select Master Mix for CFX (Life Technologies, Austin, TX) on a Bio-Rad CFX96 Real-Time system (Bio-Rad Laboratories, Hercules, CA). Reactions were amplified for 40 cycles. AQP1 (Accession No. BC090068) was amplified with 5′-CCGAGACTTAGGTGGCTCAG-3′ (sense) and 5′-TCATGCGGTCTGTAAAGTCG-3′ (antisense), ClCK1 (Accession No. BC081761) was amplified with 5′-TATCCCTTGGTGGAGACCAG-3′ (sense) and 5′-GTCACGAACAGCGACTGAAG-3′ (antisense), and cyclophilin A (Accession No. NM017101) was amplified with 5′-CGAGCTGTTTGCAGACAAAG-3′ (sense) and 5′-GCATACAGGTCCTGGCATCT-3′ (antisense). Standard curves were used to calculate mRNA levels (in arbitrary units), and final values are expressed per millimeter of tubule length.
Perfusion of isolated tubule segments.
Tubule segments were perfused in vitro by a modification of the technique previously described by Burg et al. (4). Tubules were placed into a temperature-controlled Lucite or brass chamber with a glass bottom and observed with a stereomicroscope and inverted compound microscope. The upstream portion of the tubule was drawn into a holding pipette, which contained two concentric pipettes: a perfusion pipet, which was inserted into the tubule lumen, and an exchange pipette, for exchanging the luminal perfusate. The downstream end of the tubule was drawn into a holding pipette that had a tip of appropriate diameter to form a complete seal between perfusate and bath solution. The perfusion rate was generally between 15 and 30 nl/min. The bath temperature was held at 37°C during the collection period. Tubule dimensions were determined with an ocular micrometer.
Osmotic Pf measurements.
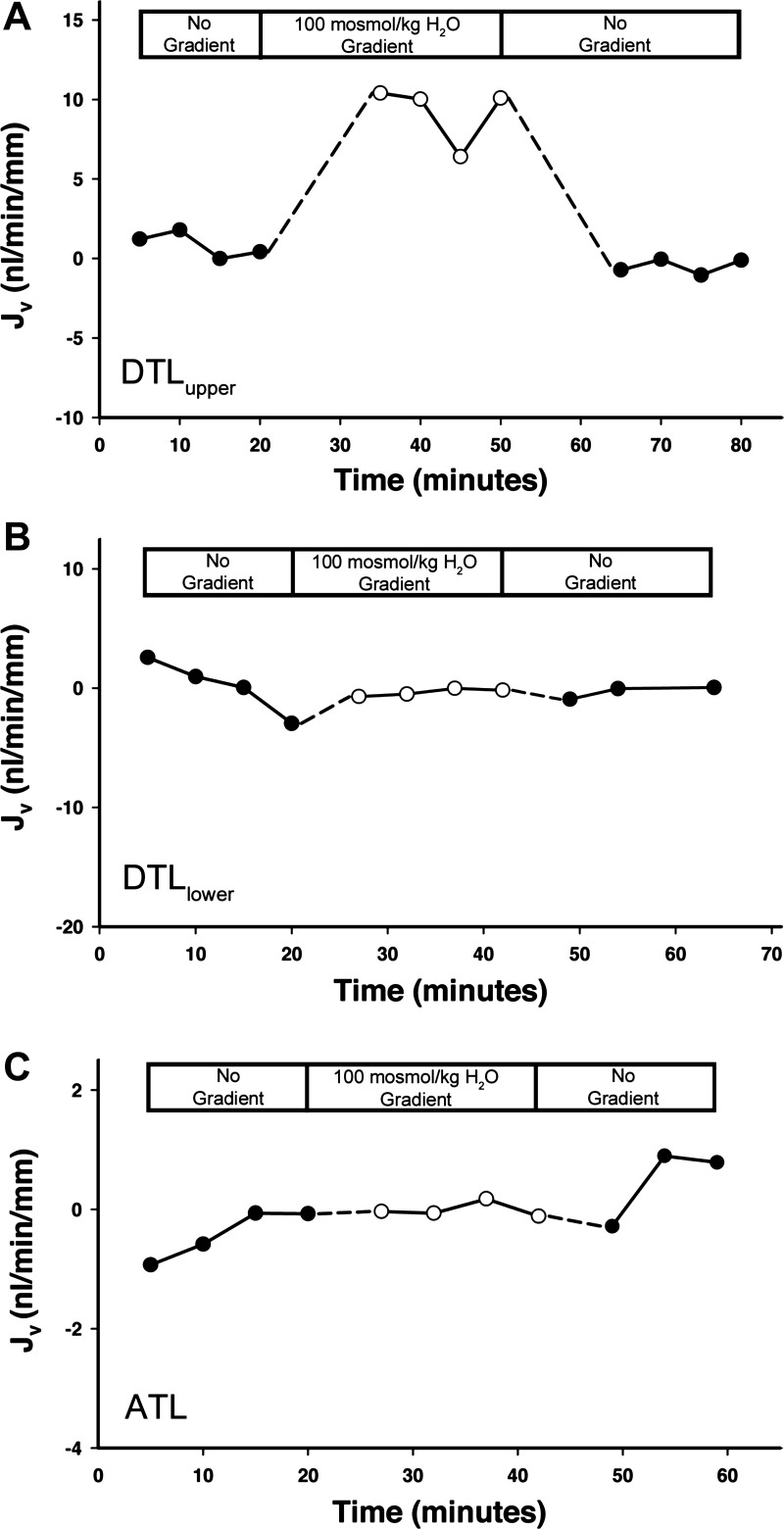
Osmotic Pf was calculated from net transepithelial fluid flux (Jv) determined in the presence of an imposed 100 mosmol/kg H2O osmolality gradient. The peritubular bath consisted of bicarbonate buffer with 100 mM sucrose added to create a 100 mosmol/kg H2O bath-to-lumen osmolality gradient. The DTL has been shown to exhibit no significant transepithelial permeability to sucrose, as shown in the hamster (14). Measurements were conducted by collecting the perfusate, which contained [14C]dextran (∼70 kDa) as the impermeant volume marker in bicarbonate buffer. The protocol for determining Jv for DTLupper, DTLlower, and ATL is shown in three representative experiments in Fig. 3. Each experiment was conducted over the course of about 60–80 min. For each tubule, the perfusion fluid in the downstream pipet was initially collected for four 5-min intervals in the absence of the osmolality gradient. The bath was then changed to bicarbonate buffer with 100 mM sucrose added, and perfusion fluid was collected for four additional 5-min intervals. Finally, the perfusion fluid was collected for another three or four 5-min intervals, again in the absence of the osmolality gradient. Jv (in nl·min−1·mm tubule length−1) was determined using the following equation: Jv = VL[(Xl/Xo) − 1] (6), where VL is the fluid collection rate per millimeter of tubule length, Xl is the [14C]dextran concentration of collected fluid, and Xo is the [14C]dextran concentration in the perfusate. Pf (in μm/s) was calculated from the following equation: Pf = Jv/[AsVw(δCosmol)] (6), where As is the tubule luminal surface area [which was calculated as πDL from the tubule inner diameter (D) and the tubule length (L)], Vw is the partial molar volume of water (18 ml/mol), and δCosmol is the log-mean transepithelial osmolality gradient along the length of the perfused tubule [calculated as δC1 − δC2/ln (δC1/δC2), where δC1 and δC2 are the transepithelial osmolality differences at the perfusion and collection ends, respectively]. Perfusate and bath osmolalities were measured with a vapor pressure osmometer (Wescor, Logan, UT). The collected fluid osmolality was estimated as follows: Op(Xl/Xo), where Op is perfusate osmolality.
Fig. 3.
Time course of change in net fluid absorption (Jv; in nl·min−1·mm tubule length−1) after the application and removal of a 100 mosm/kg H2O bath-to-lumen osmolality gradient (sucrose added to bath) in isolated perfused DTLupper, DTLlower, and ATL (44). Each experiment shown is a single representative of 5 or more replicates. Fluxes determined after the application of a gradient are shown in Table 1. Note that the abscissa and ordinate scales are not identical.
Purea measurements.
Purea was determined by measuring the unidirectional [14C]urea flux resulting from an ∼5 or ∼0.1 mM lumen-to-bath or bath-to-lumen urea concentration gradient. Because the unidirectional fluxes were nearly the same in either direction, the data were combined. The perfusate and peritubular bath consisted of bicarbonate buffer. In some experiments, 5 mM urea in either the lumen or bath was replaced with 5 mM raffinose (see results). The unidirectional flux of [14C]urea from the lumen to the bath (Jurea; in pmol·min−1·mm tubule length−1) was calculated using the following equation: Jurea = C0Vo − CLVL, where Co and CL are the urea concentrations in the perfusate and collectate, respectively, Vo is the perfusion rate per unit tubule length, and VL is the collection rate per unit tubule length. The bath-to-lumen urea flux simplifies to Jurea = −CLVL, since the perfusate urea concentration was 0 mM.
Purea (in cm/s) was calculated for lumen-to-bath fluxes from the following equation: Purea = Jurea/(πDδC), where D is the tubule diameter and δC = δC1 − δC2/ln(δC1/δC2) (where δC1 is the urea concentration gradient at the perfusion end of the tubule and δC2 is the urea concentration gradient at the collection end of the tubule) (5). Net volume flow is zero in the absence of a transepithelial osmolality gradient (see results), and tracer backflux was negligible as short tubule segments were used and tracer concentration in the trans compartment was <10% of that in the cis compartment.
Statistical analysis.
Data combined from three or more samples are reported as means ± SE; n is the number of replicates. The statistical significance of differences between means was determined with Student's paired t-test or one-way ANOVA and Duncan's post hoc test (P < 0.05).
RESULTS
Isolation of structurally distinct thin limb segments.
DTLupper and ATLs from the rat IM are distinguishable from each other on the basis of structural criteria, as we have previously reported (3, 33) (also see methods). Notably, the DTLupper has numerous cells with nuclei protruding into the tubule lumen, whereas ATLs have round, flat nuclei. In the present experiments, the DTLlower was initially identified by dissecting the descending segment along with its bend and following it to its upper descending segment. Segments from the lower IM are shown in Fig. 2 as observed with differential interference contrast optics. These segments are also apparent using a stereomicroscope with subillumination. There is also commonly a distinct thickening in diameter several hundred micrometers above the bend on the descending side of the loop (Fig. 2). This short thickened segment is similar in diameter to that of ATLlower. We generally relied on making a visual comparison of DTLlower and ATLlower tubule diameters in neighboring segments from each papilla to further confirm the identification of ATLlower segments (3, 33).
Quantitative PCR.
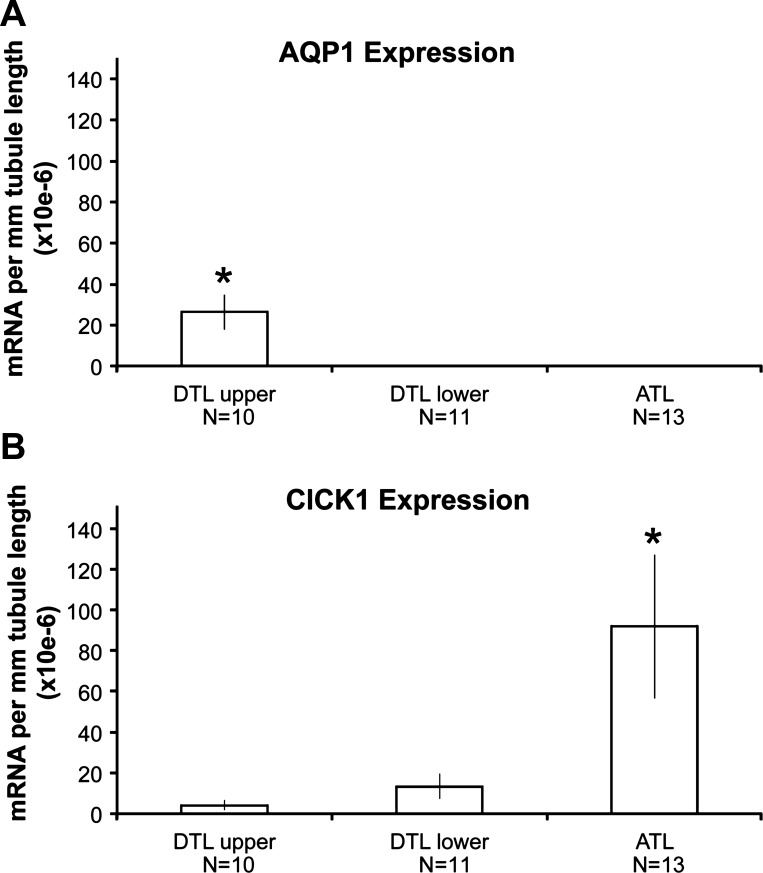
We isolated single segments of DTLupper, DTLlower, and ATLs on the basis of the structural characteristics described above and measured expression levels of mRNA encoding AQP1 and ClCK1 in each segment. Relative to other segments, DTLupper expresses high mRNA levels of AQP1 and low mRNA levels of ClCK1, DTLlower expresses essentially no AQP1 mRNA and low levels of ClCK1 mRNA, and ATL expresses essentially no AQP1 mRNA and high levels of ClCK1 mRNA (Fig. 4). These data parallel the known expression levels of AQP1 and ClCK1 protein in these segments as determined by immunohistochemistry (29, 32, 34, 41).
Fig. 4.
Expression levels of mRNA coding for AQP1 (A) and ClCK1 (B) in DTLupper, DTLlower, and ATL segments as determined with quantitative PCR. Values shown are means ± SE (in arbitrary units). Values are zero where no bars are shown. N values are numbers of tubules. *Significant difference from other groups.
Water permeabilities of isolated perfused thin limb segments.
In the absence of a transepithelial osmolality gradient, Jv was essentially zero for DTLupper, DTLlower, and ATL isolated perfused segments, as shown in representative experiments (Fig. 3). With the application of a 100 mosmol/kg H2O bath-to-lumen osmolality gradient (100 mM sucrose added to the bath), a substantial volume flux was observed in DTLupper but not in DTLlower and ATL (Fig. 3). The volume flux rapidly diminished in DTLupper upon removal of the osmolality gradient. The effects of imposing a 100 mosmol/kg H2O bath-to-lumen osmolality gradient on Jv and Pf values for all three segments are shown in Table 1. Under this condition, Jv and Pf values for DTLupper were substantially higher than values for DTLlower and for ATL, with the latter previously published by us in an earlier report (44) (Table 1). Jv and Pf values for DTLlower and ATL were not significantly different from zero.
Table 1.
Transepithelial osmotic water permeability of isolated perfused Munich-Wistar rat thin limb segments
| Segment Type | Number of Replicates | Tubule Length, μm | Collection Rate, nl·min−1·mm−1 | Collected-to-Perfusate [14C]Dextran Concentration, mM | Jv, nl·min−1·mm−1 | Pf, μm/s |
|---|---|---|---|---|---|---|
| DTLupper | 6 | 775 | 28.1 ± 1.2 | 1.31 ± 0.04 | 7.7 ± 0.9 | 3,204.5 ± 450.3 |
| DTLlower | 5 | 629 | 33.1 ± 3.2 | 1.03 ± 0.03 | 0.8 ± 0.8* | 207.8 ± 241.3* |
| ATL† | 10 | 829 | 16.0 ± 3.0 | 1.01 ± 0.02 | 0.3 ± 0.3* | 69.4 ± 72.5* |
DTLupper and DTLlower, upper and lower descending thin limbs, respectively; ATL, ascending thin limbs; Pf, water permeability; Jv, net transepithelial fluid flux.
Significantly different from DTLupper (by one-way ANOVA, P < 0.05).
Data from Ref. 44.
Urea permeabilities of isolated perfused thin limb segments.
Purea was determined by imposing a transepithelial urea gradient of about 0.1 mM for DTLupper, DTLlower, ATLupper, and ATLlower. The gradient was applied in a bath-to-lumen orientation or in a lumen-to-bath orientation. Values were similar for each orientation and were combined. Purea for DTLupper, although high, was only about one-fourth of Purea for DTLlower and both ATL segments (Table 2). Purea values for DTLlower and both ATL segments were not significantly different from each other. Purea was also determined for DTLupper and ATLupper by imposing a transepithelial urea gradient of ∼5 mM. Experiments conducted with an ∼5 mM urea gradient resulted in a Purea value that was similar to that seen with a urea gradient of 0.1 mM.
Table 2.
Transepithelial urea permeability of isolated perfused Munich-Wistar rat thin limb segments
| Segment Type | Number of Replicates | Tubule Length, μm | Collection Rate, nl·min−1·mm−1 | Transepithelial Urea Gradient, mM | Collected Urea Concentration, mM | Purea, ×10−5 cm/s |
|---|---|---|---|---|---|---|
| DTLupper | 6 | 682 ± 52 | 37.6 ± 3.8 | 5.09 ± 0.00† | 2.15 ± 0.53 | 62.9 ± 16.0‡ |
| DTLupper | 8 | 733 ± 66 | 34.4 ± 2.5 | 0.11 ± 0.00* | 0.04 ± 0.01 | 40.0 ± 7.3‡ |
| DTLlower | 9 | 551 ± 50 | 42.9 ± 6.3 | 0.09 ± 0.00* | 0.04 ± 0.01 | 203.8 ± 30.3 |
| ATLupper | 3 | 666 ± 23 | 22.3 ± 0.9 | 5.08 ± 0.01† | 3.60 ± 1.59 | 193.6 ± 52.6 |
| ATLupper | 4 | 675 ± 73 | 36.5 ± 4.3 | 0.11 ± 0.00* | 0.02 ± 0.01 | 203.8 ± 35.7 |
| ATLlower | 3 | 496 ± 82 | 49.4 ± 1.5 | 0.13 ± 0.03* | 0.03 ± 0.01 | 265.1 ± 49.8 |
Purea, urea permeability. Permeabilities of all segment types were assessed with either a bath-to-lumen or lumen-to-bath urea gradient. Permeabilities of the DTLupper and upper ATL (ATLupper) were additionally assessed with 5 mM urea in either the perfusate or bath.
Urea (5 mM) in the bicarbonate buffer was replaced with raffinose in the cis and trans compartments, so that the tubules were perfused in the presence of a gradient consisting of [14C]urea but in the absence of a significant osmolality gradient.
Urea (5 mM) in the bicarbonate buffer was replaced with 5 mM raffinose in the trans compartment but not the cis compartment, so that the tubules were perfused in the presence of a gradient consisting of [14C]urea and unlabeled urea but in the absence of a significant osmolality gradient.
Significantly different from DTLlower, ATLupper, and lower ATL (ATLlower) (by one-way ANOVA, P < 0.05).
Urea permeability of the isolated perfused DTLupper in the presence of phloretin.
Phloretin inhibits urea transport mediated by members of the solute carrier 14 (SLC14) family of UTs, including UT-A2. Because UT-A2 protein is expressed in inner medullary DTL segments lying near the OM-IM boundary (19, 25, 43), we tested effects of phloretin on urea transport in DTLupper to test if urea flux occurs by way of a member of the SLC14 family of tranporters. In the absence of phloretin, Purea was 83.2 ± 25.7 × 10−5 cm/s; in the presence of phloretin (0.25 mM), Purea was 81.9 ± 26.0 × 10−5 cm/s (means ± SE, n = 4, not significantly different by Student's paired t-test, P > 0.5).
DISCUSSION
The “passive mechanism” hypothesis of urine concentration, as originally proposed by Kokko and Rector (20) and Stephenson (39), requires high transepithelial osmotic Pf and low Purea and Na+ permeability along the DTL and low Purea along the ATL. These Pf and Purea, to a significant degree, exist neither in the isolated perfused thin limbs of the rat, as shown here, nor in the chinchilla (5, 6). The Na+ permeability of the chinchilla DTL, although high, may be sufficiently low to adhere to the Kokko and Rector and Stephenson models (6). The separation of urea from NaCl that is initiated in the outer medullary thick ascending limb and sustained and augmented by intervening cortical segments and the outer medullary collecting duct most certainly involves active transport. However, the subsequent mixing of urea and NaCl in the interstitium and vasculature of the IM could involve passive mechanisms. These mechanisms likely differ to some degree from those proposed in the Kokko and Rector and Stephenson models. Unexplained Na+ and urea imbalances in the mouse Cl− channel and UT knockout models underscore our ignorance of the mechanisms that balance inner medullary interstitial and vascular NaCl and urea composition (1, 10a, 41a). A more complete understanding of thin limb water, NaCl, and urea permeabilities and transport properties is essential to understanding a “solute-separation, solute-mixing” mechanism of urine concentration (23).
The rat inner medullary DTL consists of two structurally distinct segments that can be identified with light-level microscopy. We described structural features of the rat DTLupper in earlier publications (3, 33), which are distinct from those of the DTLlower, as shown here. Nuclear protrusions, which are abundant in DTLupper, sparse in DTLlower, and absent in ATL, enable one to identify each of these three segments with a stereomicroscope. A comparison of tubule diameters for neighboring DTLlower and ATL from each papilla also facilitates the identification of ATL segments (3, 33).
The data shown here indicate that the Munich-Wistar rat inner medullary DTLupper and DTLlower are two functionally distinct segments. In isolated perfused tubules, the DTLupper exhibits high osmotic Pf and moderately high Purea, whereas the DTLlower exhibits low osmotic Pf and very high Purea. The ATL uniformly exhibits low osmotic Pf and very high Purea along its entire length. Purea values of the rat thin limbs are substantially higher than Purea measured for free diffusion across the human red blood cell plasma membrane (1 × 10−6 cm/s) (2). The osmotic Pf and Purea values reported here are similar, in most respects, to those reported for chinchilla DTLupper, DTLlower, and ATL [in the chinchilla studies (5–7, 24), the authors used the terms middle and lower part of the long-loop descending limb to refer to the DTLs isolated from the outer 30% of the IM and from near the papilla tip, respectively]. One exception, though, is the much higher Purea for the Munich-Wistar rat DTLlower compared with chinchilla DTLlower (203 × 10−5 vs. 47.6 × 10−5 cm/s). The physiological impact of this difference remains to be determined. The DTLupper osmotic Pf values that we found for the Munich-Wistar rat are comparable to those found in DTLs isolated from near the OM-IM boundary for the Wistar rat (13), hamster (14), and mouse (8), but the DTLlower osmotic Pf values reported here are lower than those found in the Sprague-Dawley rat DTLlower (6) and are not significantly different from zero.
In the Munich-Wistar rat, the high or low osmotic Pf of each segment correspond, respectively, to high or low levels of AQP1 protein (30, 31, 34) and mRNA expression (Fig. 4). ClCK1 protein expression is high in the ATL and prebend segment with little or no expression in the DTL (18, 32, 40), corresponding to high expression of ClCK1 mRNA in the ATL and its near absence in the DTL, as shown here.
The identity of the transepithelial pathway for urea in the rat DTL and ATL remains an enigma. The absence of inhibition by phloretin in the DTLupper indicates that UT-A2 is not the pathway in this segment, the only thin limb segment known to express any of the SLC14 class of transporters in the rat IM. Likewise, phloretin did not inhibit urea fluxes in the chinchilla DTLupper, DTLlower, or ATL (5). If urea flux is transcellular, then it must involve one or more unknown UTs. Although urea and water can flow across the tight junction and through the paracellular pathway in some systems (12), there is no strong evidence to suggest that urea, a molecule similar to but larger than water, could traverse this pathway when water could not in the thin limb segments exhibiting no osmotic Pf.
Luminal fluid at the bends of the loops of the moderately antidiuretic rat and hamster that lie near the tip of the papilla is in approximate osmotic equilibrium with capillary fluid (11, 16, 27, 28, 36) and is likely in equilibrium with interstitial fluid. The high transepithelial osmotic Pf values of rat, hamster, mouse and chinchilla DTLupper thin limbs suggest that osmotic equilibration in these segments could involve relatively high water flux. This water flux must play a critical role in the urine concentrating mechanism, as mice lacking AQP1 have greatly reduced concentrating capacity (8). In contrast, because of the low Pf in rat and chinchilla DTLlower, little or no osmotic equilibration can occur via osmotic water flux. Osmotic equilibration across the DTLlower more likely occurs primarily by solute secretion, as suggested for the rat, hamster, and Psammomys (10, 26, 35). Because the DTLlower exists at all levels of the IM, equilibration by solute secretion may be important in the urine concentrating mechanism at all levels along the corticopapillary axis of the IM. A role for both osmotic water equilibration and solute secretion in thin limbs has been captured in recent mathematical models of the urine concentrating mechanism (22, 23); however, the maximum urine concentration obtained in this and all other models remains well below the maximum concentrating capacity of rats.
In summary, each inner medullary DTL, consisting of two segment types (DTLupper and DTLlower), and each ATL, consisting of a single segment type, can be distinguished on either structural grounds or on the basis of relative mRNA expression levels of AQP1 and ClCK1. Purea is only moderately high in the segment that both expresses AQP1 and has high osmotic Pf, whereas Purea is very high in all thin limb segments that lack the water channel AQP1 and have no osmotic Pf. Additional studies are needed to understand how these characteristics contribute to producing the corticopapillary osmolality gradient in the IM.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083338.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.N., K.K.E., W.H.D., and T.L.P. conception and design of research; C.M.N., K.K.E., and T.L.P. performed experiments; C.M.N., K.K.E., W.H.D., and T.L.P. analyzed data; C.M.N., K.K.E., W.H.D., and T.L.P. interpreted results of experiments; C.M.N., K.K.E., and T.L.P. prepared figures; C.M.N., K.K.E., and T.L.P. drafted manuscript; C.M.N., K.K.E., W.H.D., and T.L.P. edited and revised manuscript; C.M.N., K.K.E., W.H.D., and T.L.P. approved final version of manuscript.
REFERENCES
- 1.Akizuki N, Uchida S, Sasaki S, Marumo F. Impaired solute accumulation in inner medulla of Clcnk1−/− mice kidney. Am J Physiol Renal Physiol 280: F79–F87, 2001 [DOI] [PubMed] [Google Scholar]
- 1a.Bachmann S, Kriz W. Histotopography and ultrastructure of the thin limbs of the loop of Henle in the hamster. Cell Tissue Res 225: 111–127, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Brahm J. Urea permeability of human red cells. J Gen Physiol 82: 1–23, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brokl OH, Dantzler WH. Amino acid fluxes in rat thin limb segments of Henle's loop during in vitro microperfusion. Am J Physiol Renal Physiol 277: F204–F210, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Burg MB, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293–1298, 1966 [DOI] [PubMed] [Google Scholar]
- 5.Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: urea and NaCl permeabilities. Am J Physiol Renal Fluid Electrolyte Physiol 264: F337–F343, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F417–F426, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Chou CL, Nielsen S, Knepper MA. Structural-functional correlation in chinchilla long loop of Henle thin limbs: a novel papillary subsegment. Am J Physiol Renal Fluid Electrolyte Physiol 265: F863–F874, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Chou CL, Knepper MA, Van Hoek AN, Brown D, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J Clin Invest 103: 491–496, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dantzler WH, Layton AT, Layton HE, Pannabecker TL. Urine concentrating mechanism in the inner medulla: function of the thin limbs of Henle's loops. Clin J Am Soc Nephrol; 10.2215/CJN.08750812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rouffignac C, Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of Henle in Psammomys. J Clin Invest 48: 474–486, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Fenton RA, Chou C-L, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol 196: 927–936, 1959 [DOI] [PubMed] [Google Scholar]
- 12.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai M. Function of the thin ascending limb of Henle of rats and hamsters perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 232: F201–F209, 1977 [DOI] [PubMed] [Google Scholar]
- 14.Imai M, Taniguchi J, Yoshitomi K. Transition of permeability properties along the descending limb of long-loop nephron. Am J Physiol Renal Fluid Electrolyte Physiol 254: F323–F328, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Jamison RL, Kriz W. Urinary Concentrating Mechanism. New York: Oxford Univ. Press, 1982 [Google Scholar]
- 16.Johnston PA, Battilana CA, Lacy FB, Jamison RL. Evidence for a concentration gradient favoring outward movement of sodium from the thin loop of Henle. J Clin Invest 59: 234–240, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaissling B, Kriz W. Morphology of the loop of Henle, distal tubule, and collecting duct. In: Handbook of Physiology. Renal Physiology, edited by Windhager EE. New York: Oxford Univ. Press, 1992,. sect. 8, p 109–167 [Google Scholar]
- 18.Kieferle S, Fong PY, Bens M, Vandewalle A, Jentsch TJ. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci USA 91: 6943–6947, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol 282: F530–F540, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kokko JP, Rector FC. Countercurrent multiplication system without active transport in inner medulla. Kidney Int 2: 214–223, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Kriz W. Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol 241: R3–R16, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816–F839, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Layton HE, Knepper MA, Chou CL. Permeability criteria for effective function of passive countercurrent multiplier. Am J Physiol Renal Fluid Electrolyte Physiol 270: F9–F20, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Lim SW, Han KH, Jung JY, Kim WY, Yang CW, Sands JM, Knepper MA, Madsen KM, Kim J. Ultrastructural localization of UT-A and UT-B in rat kidneys with different hydration status. Am J Physiol Regul Integr Comp Physiol 290: R479–R492, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Marsh DJ. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol 218: 824–831, 1970 [DOI] [PubMed] [Google Scholar]
- 27.Marsh DJ, Azen SP. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol 228: 71–79, 1975 [DOI] [PubMed] [Google Scholar]
- 28.Marsh DJ, Solomon S. Analysis of electrolyte movement in thin Henle's loops of hamster papilla. Am J Physiol 208: 1119–1128, 1965 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. Chip28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371–383, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannabecker TL. Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: role of inner medullary water and urea transport pathways in the rodent medulla. Am J Physiol Regul Integr Comp Physiol 304: R488–R503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pannabecker TL. Structure and function of the thin limbs of the loops of Henle. In: Comprehensive Physiology, edited by Terjung RL. Bethesda, MD: Wiley, 2012, p. 2063–2086 [DOI] [PubMed] [Google Scholar]
- 32.Pannabecker TL, Abbott DE, Dantzler WH. Three-dimensional functional reconstruction of inner medullary thin limbs of Henle's loop. Am J Physiol Renal Physiol 286: F38–F45, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Pannabecker TL, Dahlmann A, Brokl OH, Dantzler WH. Mixed descending- and ascending-type thin limbs of Henle's loop in mammalian renal inner medulla. Am J Physiol Renal Physiol 278: F202–F208, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Pennell JP, Lacy FB, Jamison RL. An in vivo study of the concentrating process in the descending limb of Henle's loop. Kidney Int 5: 337–347, 1974 [DOI] [PubMed] [Google Scholar]
- 36.Pennell JP, Sanjana V, Frey NR, Jamison RL. The effect of urea infusion on the urinary concentrating mechanism in protein-depleted rats. J Clin Invest 55: 399–409, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz MM, Karnovsky MJ, Venkatachalam MA. Regional membrane specialization in the thin limbs of Henle loops as seen by freeze-fracture electron-microscopy. Kidney Int 16: 577–589, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MM, Venkatachalam MA. Structural differences in thin limbs of Henle: physiological implications. Kidney Int 6: 193–208, 1974 [DOI] [PubMed] [Google Scholar]
- 39.Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int 2: 85–94, 1972 [DOI] [PubMed] [Google Scholar]
- 40.Uchida S, Sasaki S, Furakawa T, Hiraoka M, Imai T, Hirata Y, Marumo F. Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in the kidney medulla. J Biol Chem 268: 3821–3824, 1993 [PubMed] [Google Scholar]
- 41.Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F. Localization and functional characterization of rat kidney-specific chloride channel. J Clin Invest 95: 104–113, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki M. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol 25: 7357–7363, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urity VB, Issaian T, Braun EJ, Dantzler WH, Pannabecker TL. Architecture of kangaroo rat inner medulla: Segmentation of descending thin limb of Henle's loop. Am J Physiol Regul Integr Comp Physiol 302: R720–R726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wade JB, Lee AJ, Liu C, Ecelbarger C, Mitchell C, Bradford AD, Terris J, Kim GH, Knepper MA. UT-A2: a 55-kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol 278: F52–F62, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Yool AJ, Brokl OH, Pannabecker TL, Dantzler WH, Stamer WD. Tetraethylammonium block of water flux in aquaporin-1 channels expressed in kidney thin limbs of Henle's loop and a kidney-derived cell line. BMC Physiol 2: 4, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77–88, 2006 [DOI] [PubMed] [Google Scholar]