Abstract
The adult kidney is derived from the interaction between the metanephric blastema and the ureteric bud. Platelet-derived growth factor (PDGF) receptor β is essential for the development of the mature glomerular tuft, as mice deficient for this receptor lack mesangial cells. This study investigated the role of Src tyrosine kinase in PDGF-mediated reactive oxygen species (ROS) generation and migration of metanephric mesenchymal cells (MMCs). Cultured embryonic MMCs from wild-type and PDGF receptor-deficient embryos were established. Migration was determined via wound-healing assay. Unlike PDGF AA, PDGF BB-induced greater migration in MMCs with respect to control. This was abrogated by neutralizing an antibody to PDGF BB. Phosphatidylinositol 3-kinase (PI3K) inhibitors suppressed PDGF BB-induced migration. Conversely, mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) inhibitors had no effect. Src inhibitors inhibited PDGF-induced cell migration, PI3K activity, and Akt phosphorylation. Adenoviral dominant negative Src (AD DN Src) abrogated PDGF BB-induced Akt phosphorylation. Hydrogen peroxide stimulated cell migration. PDGF BB-induced wound closure was inhibited by the antioxidants N-acetyl-l-cysteine, tiron, and the flavoprotein inhibitor diphenyleneiodonium. These cells express the NADPH oxidase homolog Nox4. Inhibiting Nox4 with antisense oligonucleotides or small interfering RNA (siRNA) suppressed PDGF-induced wound closure. Inhibition of Src with siRNA reduced PDGF BB-induced ROS generation as assessed by 2′,7′-dichlorodihydrofluorescein diacetate fluorescence. Furthermore, PDGF BB-stimulated ROS generation and migration were similarly suppressed by Ad DN Src. In MMCs, PDGF BB-induced migration is mediated by PI3K and Src in a redox-dependent manner involving Nox4. Src may be upstream to PI3K and Nox4.
Keywords: mesenchyme, metanephric, migration, Nox4, platelet-derived growth factor
cellular migration is requisite for embryogenesis. The adult, permanent, or metanephric kidney is derived from a metanephric diverticulum and the metanephric mesoderm (reviewed in Ref. 63). In mammals, the initiation of metanephric kidney development is defined when a portion of epithelium from the mesonephric, or Wolffian, duct invades the intermediate mesoderm, inducing the formation of a metanephric blastema comprised of the adjacent metanephric mesenchyme (6). The epithelium is termed the ureteric bud and ultimately forms the urinary tracts from the connecting segments, collecting ducts, renal pelvices, and ureters in the adult (69). Uninduced mesenchyme will develop into the stroma of the kidney, and mesenchymal condensations will mature into most of the nephron, ranging from Bowman's capsule to the distal tubule. Development of the mature nephron from the metanephric mesenchyme and ureteric bud is dependent on a number of genes for normal development.
There are four known platelet-derived growth factor (PDGF) chains that comprise five dimeric isoforms found in vivo: AA, AB, BB, CC, and DD. There are clearly at least two transcellular PDGF receptors, α and β, the latter being capable of recognizing the B chain of the PDGF heterodimer AB or homodimer BB. Cultured metanephric mesenchymal cells express functional PDGF receptor β, which has been demonstrated by autophosphorylation and activation of phosphatidyl-3-kinase in response to PDGF. Immunohistochemical studies have documented that PDGF receptor β is expressed in the metanephric mesenchyme (1). It is well established that the PDGF B chain is requisite for mesangial cellular development (9). Mesangial cell precursors utilize PDGF BB and PDGF receptor β signaling for migration and DNA synthesis during metanephrogenesis (4).
Phosphatidylinositol 3-kinase mediates PDGF BB-induced migration in a number of cells (11, 67) and has been shown to be required in the formation of the epicardium (62). Notably, PDGF BB also leads to the generation of reactive oxygen species (ROS) via the PDGF receptors (77). Because we recently reported that PDGF-induced DNA synthesis was mediated by Nox4 in the metanephric mesenchyme (82), the involvement of ROS in PDGF-induced migration was explored in cultured metanephric mesenchymal cells isolated from 11.5 gestational day mouse embryos.
MATERIALS AND METHODS
Materials.
Recombinant PDGF AA and BB were purchased from R&D Systems (Minneapolis, MN). A neutralizing antibody to PDGF BB (0.1 μg/ml) and the isotype control, polyclonal goat IgG, were from Abcam (Cambridge, MA). The PDGF receptor β (958) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against PDGF receptor α, p-Akt (Ser473), Akt, p-ERK, ERK, p-Src, and Src were from Cell Signaling Technology (Danvers, MA). Phosphotyrosine and actin antibodies were from Upstate Cell Signaling Solutions and Sigma-Aldrich (Waltham, MA and St. Louis, MO), respectively. Protein Sepharose beads were from Pierce (Rockford, IL). The inhibitors AG1296, LY294002, PD98059, U0126, PP2, and SU6656 were purchased from Calbiochem (Darmstadt, Germany). Ad GFP and Ad DN Src were provided by Goutam Ghosh Choudhury. Hydrogen peroxide, tiron, N-acetyl-l-cysteine (NAC), and diphenyleneiodonium chloride (DPI) were purchased from Sigma. Lipofectamine 2000 was from Invitrogen (Grand Island, NY). Small interfering RNA (siRNA) was from Dharmacon (Lafayette, CO).
Mouse metanephric mesenchymal cell culture, cell transfection, and infection.
Immortalized metanephric mesenchymal cells previously established from wild-type C57 black gestational day 11.5 mouse embryos were used (82). All animal protocols were reviewed by the Alexion Institutional Animal Care and Use Committee. Phosphothiolated S and AS oligonucleotides for Nox1 and Nox4 were used for transfection experiments. AS oligonucleotides were designed near the ATG start codon of native Nox4 (5′-AGCTCCTCCAGGACAGCGCC-3′). The Nox1 S and AS oligonucleotide sequences were 5′-GGGAAACTGGCTGGTTAACC, and 5′-GGTTAACCAGCCAGTTTCCC, respectively (Integrated DNA Technologies, Coralville, IA). Twenty-four-well dishes were seeded with 50,000 cells/well with 1 μM of the AS or S oligonucleotides and incubated for 48 h, as described. Monolayers were washed and incubated for another 48 h in serum-deprived media containing 0 or 1 μM S or AS oligonucleotides. Infection with Ad DN Src or Ad GFP control was performed as described previously (10, 20, 27).
Nox4 siRNA.
Cells were cultured in antibiotic-free media until 30% confluent. Cells were treated with 400 nM of NT or Nox4 siRNA (Dharmacon) using X-tremeGENE transfection reagent (Roche). When confluent, monolayers were serum-deprived and treated with the indicated doses of siRNA.
Cell migration assays.
The wound-healing assay was performed similarly to that described for fibroblasts (26) and vascular smooth muscle cells (31). Cells were grown to near-confluence and deprived of serum overnight. Monolayers were “wounded” with a plastic 200-μl pipette tip, washed with PBS, and incubated in serum-free media at 37°C and 5% CO2. The plates were photographed with an inverted phase-contrast microscope (×10, Nikon) at 0 and 4.5 h with a digital camera (Nikon D50). Migratory rates were determined for cells by measuring the distance of wound closure in millimeters. The photographs from time 0 and 4.5 h were overlaid, and the cell paths were determined between leading-edge cells at six uniformly spaced points along the wound edge.
PDGF receptor tyrosine kinase assay.
Wild-type cells were treated for 15 min with PDGF AA (100 ng/ml) or PDGF BB (10 ng/ml). Cells were lysed in radioimmunoprecipitation assay buffer with 1 mmol/l sodium orthovanadate (82) at 4°C. Protein concentrations were determined for the cleared supernatants using Bio-Rad protein assay dye reagent. One hundred micrograms of protein were immunoprecipitated (17, 21, 32, 61) with 1 μg of anti-PDGF receptor β or α with protein G-Sepharose beads, and the mixture was rotated at 4°C overnight. Samples were washed and labeled with [γ-32P]ATP as described (32). Samples were incubated in at 30°C for 15 min. Reactions were quenched with 850 μl of RIPA, and samples were spun, washed, and boiled with 20 μl of Laemmli sample buffer. Proteins were separated on a 7.5% SDS-PAGE (1.5 mm), and the assay was conducted as described (18).
Phosphatidylinositol 3-kinase assay.
Monolayers were lysed in radioimmunoprecipitation assay buffer (38). One hundred micrograms of protein were immunoprecipitated with 1 μg monoclonal phosphotyrosine antibody (4G10, Upstate) with 40 μl of protein G beads, rotating for 2 h at 4°C as previously described (19). Fifteen microliters of protein A-Sepharose beads (50% vol/vol slurry) were added and rotated at 4°C for 2 h. The immunobeads were washed 3× with RIPA, 1× with PBS, 1× with buffer A (0.5 mM LiCl, 0.1 M Tris·HCl, pH 7.5, 1 mM Na3VO4), 1× with double-distilled water, and 1× with buffer B (0.1 M NaCl, 0.5 mM EDTA, 20 mM Tris·HCl, pH 7.5). The immunobeads were then resuspended in 50 μl of PI3-K assay buffer (20 mM Tris·HCl, pH 7.5, 0.1 M NaCl, and 0.5 mM EGTA). Next, 0.5 μl of 20 mg/ml phosphatidylinositol was added and incubated at 25°C for 10 min. A cocktail of 1 μl of 1 M MgCl2 and 10 μCi of [γ-32P]ATP was added and incubated at room temperature for 10 min. The reaction was stopped with 150 μl of chloroform, methanol, and 11.6 N HCl (50:100:1). The reaction was extracted with 100 μl of chloroform. The organic layer was washed with methanol and 1 N HCl (1:1). The reaction product was dried overnight and re-suspended in 10 μl of chloroform. The samples were separated by thin layer chromatography and developed with CHCl3/methanol/28% NH4OH/H2O (129:114:15:21). The spots were visualized on film by autoradiography (19).
Immunoblotting.
Cells were incubated in 10% serum-containing DMEM for 3 days, grown to confluence, washed twice with Dulbecco's PBS, and incubated in serum-free media overnight. Cells were pretreated with inhibitors for 1 h before treatment with 10 ng/ml of recombinant human PDGF-BB (R&D Systems) for 10 min. Monolayers were frozen at −80°C, and later freeze-thawed in RIPA buffer containing 0.1 TIU aprotinin, 50 nM leupeptin, and 1 mM PMSF. Protein (15 μg) from lysates with Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 5% β-mercaptoethanol was loaded onto precast Tris-glycine gels (Invitrogen) and separated by electrophoresis. This protein was transferred to nitrocellulose membranes, and these were blocked overnight with 5% dry milk in TBS. For blots analyzed with the infrared imaging system (Odyssey, Li-Cor, Lincoln, NE), membranes were blocked overnight with 5% bovine serum albumin (Sigma). Membranes were incubated with primary antibodies overnight. Displayed are representative immunoblots.
Detection of ROS generation.
Cells were seeded on chambered no. 1 coverglass, grown to 70–80% confluence, and then serum-deprived overnight. The peroxide-sensitive fluorescent probe 5-(and-6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (DCF) was used to assess intracellular ROS generation (37). This peroxide-sensitive fluorescent probe is converted to the highly fluorescent 2′,7′-dichlorodihydrofluorescein by intracellular esterases and intracellular peroxides (including hydrogen peroxide and peroxynitrites). Cells were loaded with DCF (10 μM) for 30 min, washed, and then stimulated with PDGF BB (20 ng/ml). Images were taken over 0–60 min using an Olympus inverted microscope with a ×40 APlanoFluo objective with an Olympus Fluoview confocal laser-scanning attachment. Fluorescence was measured at an excitation wavelength of 488 nm, with emission detected using a 510- 550-nm band-pass filter (37, 39).
Statistical analyses.
Means ± SE were analyzed by one-way ANOVA with Tukey's post hoc test unless otherwise specified.
RESULTS
PDGF BB is a potent chemoattractant for wild-type metanephric mesenchymal cells.
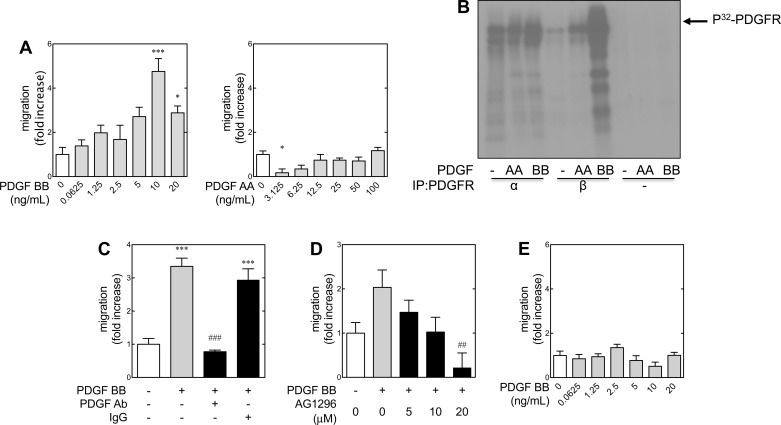
Cell types vary in their chemotactic response to the different PDGF isoforms (reviewed in Ref. 72). Metanephric mesenchymal cells isolated from 11.5-day-old embryonic kidneys were employed for these experiments (82). In vitro wound healing or scratch assays were used to assess time-dependent migration in response to PDGF AA or BB in wild-type and PDGF receptor β-deficient cells (Fig. 1A). PDGF BB induced migration of wild-type cells from 10 to 20 ng/ml (Fig. 1A, left). PDGF AA had very little effect even up to 100 ng/ml (Fig. 1A, right). If anything, the 3.125-ng/ml dose appeared to suppress migration with respect to control (Fig. 1A, right, lane 2). This is consistent with our previously published work (71), showing that PDGF receptor α can modulate chemotactic signaling, which was particularly evident in metanephric mesenchymal cells devoid of the β receptor. These results suggest that microenvironmental concentrations of the PDGF AA ligand may have a role in nephrogenesis, particularly with regard to glomerular development.
Fig. 1.
Platelet-derived growth factor (PDGF) BB induces migration of metanephric mesenchymal cells via PDGF receptor (PDGFR) β. In vitro scratch (or “wound healing”) assays were used to assess PDGF-induced cell migration under various conditions. Starting with a confluent monolayer of quiescent cells, a linear “wound” was created with a 200-μl plastic pipette tip. Migration was quantified by the distance of wound closure with respect to time relative to an untreated control. A: PDGF BB induces migration of wild-type (+/+) cells (left). PDGF AA did not demonstrate a promigratory effect at high doses (right). In fact, a small dose (3.125 ng/ml) of PDGF AA appeared to have an inhibitory effect. B: immune complex kinase assay using immunoprecipitates for PDGFR α (C-20) or β (958). Confluent monolayers were lysed in RIPA buffer with protease inhibitor tablets (Roche). The assay was performed with immunoprecipitates from 100 μg lysate from control, PDGF AA (100 ng/ml)-, or PDGF BB (10 ng/ml)-stimulated cells (15-min duration) as described in materials and methods. Lanes 7–9 represent the immunoprecipitation with negative immunoglobulin controls (IgG control). C: neutralizing PDGF BB antibody (PDGF Ab) abrogates PDGF BB-induced migration with respect to PDGF BB-treated cells and those pretreated with an isotype IgG control. D: PDGFR inhibitor A1296 abrogates PDGF BB-induced migration in a dose-dependent manner. E: PDGF BB does not elicit migration in PDGFR β null metanephric mesenchymal cells. *P < 0.05, ***P < 0.001 with respect to controls. ##P < 0.01, ###P < 0.001 with respect to PDGF BB only groups by 1-way ANOVA and Tukey's post hoc analysis.
PDGF signaling is complex because a number of different ligand dimers act with different affinities on two receptors that induce homo- and/or heterodimerization (43). Both PDGF receptor α and β may be activated by PDGF BB, although this ligand binds the β receptor with much greater affinity (81). Therefore, PDGF BB can activate its respective receptors in a number of combinations: α/α, α/β, and β/β (43). During renal development, the PDGF AA and BB ligands are thought to act in an autocrine manner to stimulate the proliferation and differentiation of glomerular cells (4). We have demonstrated that these metanephric mesenchymal cells express both α and β receptors and that PDGF receptor α mediates PDGF AA-induced migration when PDGF receptor β is knocked down (71). Given that PDGF BB elicits cell migration, the degree of contribution of PDGF receptor β to PDGF BB-induced migration was explored.
Therefore, given the promiscuity of PDGF BB as a ligand, (i.e., capable of stimulating either PDGF receptor α or β), a tyrosine kinase assay (18) was used to determine the relative activation of these PDGF receptors under these experimental conditions (Fig. 1B). PDGF BB led to a very mild increase in PDGF receptor α phosphorylation with respect to control (lanes 3 and 1, respectively) and the PDGF AA-treated group (lane 2). This observation may reflect coimmunoprecipitation of heterodimerized α-β receptors (43). Nonetheless, PDGF β receptor immunoprecipitates from PDGF BB-treated cells demonstrated significantly higher degrees of phosphorylation (lane 6) with respect to either PDGF AA or control. These data implicate PDGF receptor β as a significant mediator (relative to PDGF receptor α) in PDGF BB-induced migration. To substantiate that migration was ligand induced rather than a secondary (paracrine) effect, experiments were conducted with a neutralizing antibody against PDGF BB (Fig. 1C). PDGF BB-induced migration was abrogated by preincubation with 0.1 μg/ml of the neutralizing antibody against the ligand, whereas the isotype control had no effect.
Cellular migration can be a result of several different converging pathways. To demonstrate that this migration was a PDGF receptor-mediated effect, cells were pretreated with a PDGF receptor inhibitor, AG1296. This compound decreased PDGF BB-induced migration in a dose-dependent manner (Fig. 1D). We have previously described the isolation and characterization of metanephric mesenchymal cells that are deficient in PDGF receptor β (82). These PDGF receptor β-deficient cells did not demonstrate increased migration in response to PDGF BB (Fig. 1E), similar to what our laboratory has previously reported using modified Boyden chamber studies (71). For all subsequent studies, the maximal dose of PDGF BB for migration (i.e., 10 ng/ml) was used. Overall, these data demonstrated that PDGF BB was more potent at inducing migration in wild-type metanephric mesenchymal cells than PDGF AA, and this was likely mediated by PDGF receptor β.
PDGF BB-induced migration in MMCs is mediated via phosphatidylinositol 3-kinase.
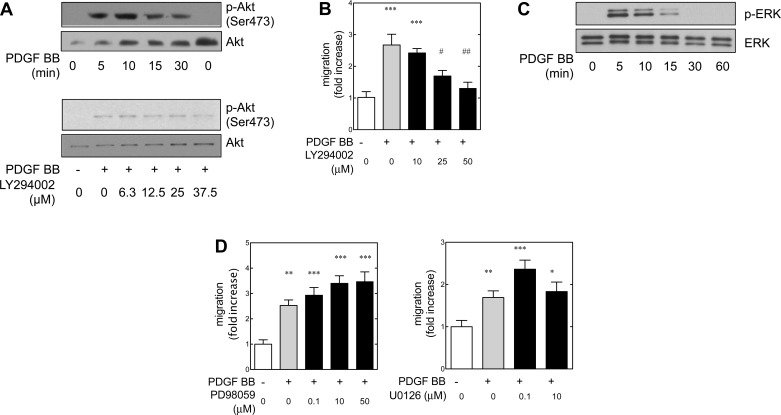
There are a number of studies on chemotaxis that implicate the phosphatidylinositol 3-kinase system in migration (72). Phosphatidylinositol turnover is associated with the reorganization of the cytoskeleton that is often requisite for migration (12). Phosphatidylinositol 3-kinase is activated near the migratory front of the cell membrane inner surface, allowing for vectorial gradient-directed migration. The involvement of phosphatidylinositol 3-kinase in PDGF receptor β-mediated chemotaxis is cell type dependent (72). PDGF BB-induced migration in cultured human (19) and rat (70) metanephric mesenchymal cells is also phosphatidylinositol 3-kinase dependent. The serine/threonine kinase Akt is immediately downstream to phosphatidylinositol 3-kinase and is often used as a surrogate marker of phosphatidylinositol 3-kinase activation. Furthermore, in certain cells Akt is involved in membrane ruffling and migration (49). Therefore, the phosphorylation of Akt was examined. PDGF BB led to a time-dependent phosphorylation of Akt (Fig. 2A) at the activation site Ser473. It is well known that Akt is among the downstream targets of phosphatidylinositol 3-kinase (29). Mouse metanephric mesenchymal cells that are deficient in PDGF receptor β that stably express an “add-back” mutant β receptor capable of activated phosphatidylinositol 3-kinase demonstrate that PDGF BB-stimulated Akt phosphorylation is phosphatidylinositol 3-kinase dependent (82). Predictably, attenuation of Akt phosphorylation was confirmed when cells were pretreated with the phosphatidylinositol 3-kinase inhibitor LY294002.
Fig. 2.
In metanephric mesenchymal cells, PDGF BB-induced migration is independent of the mitogen-activated protein kinase/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade. A: immunoblot demonstrating time-dependent phosphorylation of Akt (Ser473) in response to PDGF BB (top). Phosphatidylinositol 3-kinase (PI3K) inhibitor Ly294002 suppressed Akt phosphorylation in a dose-dependent manner (bottom). B: inhibition of PI3K dampened PDGF BB-induced migration in a dose-dependent manner. C: time course of PDGF BB-induced ERK phosphorylation in metanephric mesenchyme. D: neither MEK inhibitor PD98059 nor U0126 inhibit PDGF BB-induced migration. Cells were pretreated with the indicated inhibitors for 30 min. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. #P < 0.05, ##P < 0.01 vs. PDGF BB-treated group by 1-way ANOVA and Tukey's post hoc analysis.
In rat metanephric mesenchymal cells, it is known that PDGF BB-induced chemotaxis is dependent on phosphatidylinositol 3-kinase (4). Given the role of phosphatidylinositol 3-kinase as a key mediator in many instances of PDGF BB-induced chemotaxis, we examined the effect of phosphatidylinositol 3-kinase inhibitors on PDGF BB-induced wound healing in cultured mouse metanephric mesenchymal cells. Metanephric mesenchymal cells were pretreated with the phosphatidylinositol 3-kinase inhibitor LY294002, and Akt phosphorylation at Ser473 was assessed after 15 min of PDGF BB treatment. Analogous to the suppression of Akt phosphorylation by LY294002, this inhibitor reduced PDGF BB-stimulated cell migration in a dose-dependent manner (Fig. 2B). Overall, these results demonstrated that phosphatidylinositol 3-kinase was involved in PDGF BB-stimulated migration in metanephric mesenchymal cells.
Many signals converge on the mitogen activated protein (MAP) kinases, and these also can be involved in cell migration (44). Our laboratory has previously reported that PDGF BB-induced migration in cultured human mesangial cells is dependent on phosphatidylinositol 3-kinase and the MAP kinase/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway (19). PDGF BB and PDGF AA lead to activation of the Ras-Raf-MAPK kinase kinase (MEK)-ERK pathway in rat metanephric mesenchymal cells (70). Some PDGF BB-induced migration is mediated by the MEK-ERK pathway (70). Our experiments demonstrated that PDGF BB activated phosphatidylinositol 3-kinase via PDGF receptor β, and we have demonstrated that phosphatidylinositol 3-kinase can regulate MAP kinase activation (19, 82). Therefore, the role of the MEK-ERK pathway was investigated in the cultured mouse metanephric mesenchymal cells. Quiescent monolayers demonstrated a time-dependent phosphorylation of ERK in response to PDGF BB (Fig. 2C). However, the MEK inhibitors PD98059 and U0126 did not have an effect on PDGF BB-induced migration (Fig. 2D). These data demonstrated that migration in metanephric mesenchymal cells was independent of PDGF BB-induced activation of the MEK-ERK pathway.
PDGF BB-induced migration is mediated by Src in metanephric mesenchymal cells.
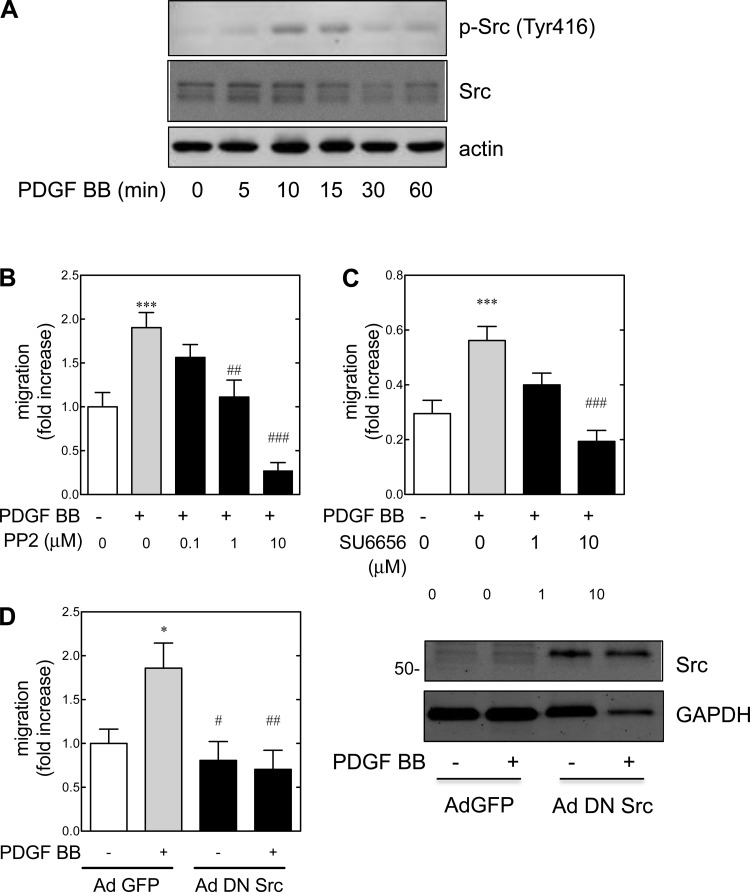
The Src family kinases associate with the juxtamembrane portion of PDGF receptor β (64). However, the role of Src in PDGF-induced chemotaxis in various cell types is controversial (72). PDGF-stimulated migration in vascular smooth muscle cells is dependent on Src activation (65, 83). Given that PDGF BB-induced migration is only partially dependent on the MEK/ERK pathway in rat metanephric mesenchymal cells, and this pathway did not appear to play a role in PDGF BB-induced migration in wild-type mouse metanephric mesenchymal cells, the involvement of Src as a mediator of PDGF BB-induced migration was examined (Fig. 3). Treatment with PDGF BB led to a time-dependent phosphorylation of Src at Tyr416 (an indicator of upregulated enzyme activity) (52) that peaked at 10 min (Fig. 3A). Given that several different downstream effectors can mediate cell chemotaxis in various cell types (72), the dependency on the Src pathway was examined with pharmacological inhibitors. Pretreatment of metanephric mesenchymal cells with either PP2 (Fig. 3B) or SU6656 (Fig. 3C) suppressed PDGF BB-induced migration in dose-dependent manners. To confirm that Src was involved in PDGF BB-mediated migration, metanephric mesenchymal cells were infected with adenoviruses containing a dominant negative Src mutant (Ad DN Src) or control [Ad green fluorescent protein (GFP)], and migration was assessed (Fig. 3D). Compared with control, Ad DN Src completely abrogated PDGF BB-induced migration. Immunoblotting of infected metanephric mesenchymal cells demonstrated successful infection with the adenovirus (Fig. 3D, right). It was concluded that PDGF BB led to Src phosphorylation at Tyr416, and Src mediated PDGF BB-induced migration.
Fig. 3.
PDGF BB-induced migration is mediated by Src in metanephric mesenchymal cells. A: PDGF BB leads to time-dependent phosphorylation of Src (Tyr 416). Quiescent +/+ metanephric mesenchymal cells were treated with PDGF BB, and immunoblotting was performed as described. B and C: Src is involved in PDGF BB-induced migration. Wild-type cells were pretreated with the Src inhibitors, and migration was assayed as described. *P < 0.05, ***P < 0.001 vs. control. ##P < 0.01, ###P < 0.001 vs. PDGF BB-treated group by 1-way ANOVA and Tukey's post hoc analyses. D, left: dominant negative Src adenovirus (ad DN Src) infection abrogates PDGF BB-induced migration. Right: immunoblot for Src in control green fluorescent protein adenovirus (ad GFP)-infected metanephric mesenchymal cells with respect to Ad DN Src group.
Src is upstream of the phosphatidylinositol 3-kinase/Akt pathway in PDGF BB-stimulated metanephric mesenchymal cells.
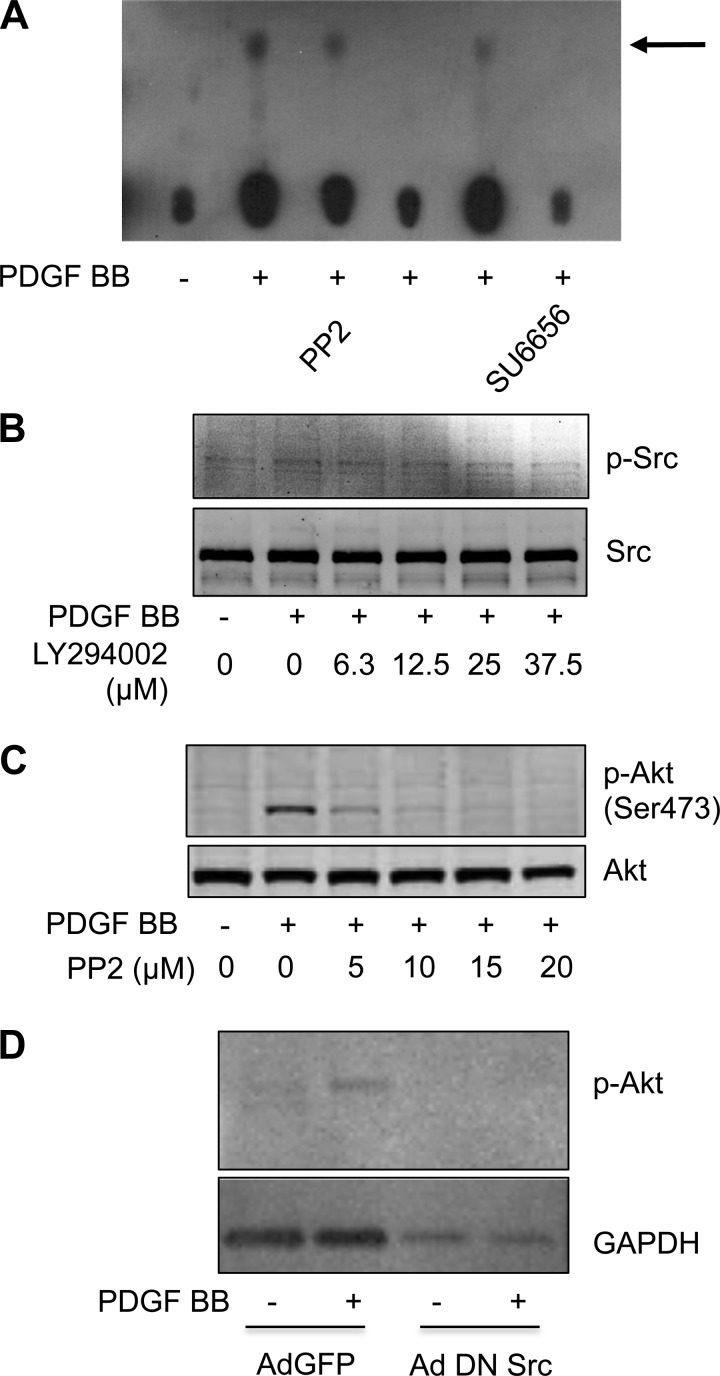
Both phosphatidylinositol 3-kinase and Src are requisite for PDGF BB-induced migration in metanephric mesenchymal cells. Therefore, the role of Src in phosphatidylinositol 3-kinase activation and downstream activation of Akt were examined. PDGF BB-treated monolayers demonstrated decreased phosphatidylinositol (3,4,5)-trisphosphate generation when pretreated with the Src inhibitors PP2 or SU6656 by phosphatidylinositol 3-kinase assay (Fig. 4A). Conversely, pretreatment of cells with the phosphatidylinositol 3-kinase inhibitor LY294002 had little effect on Src phosphorylation (Fig. 4B). Cells pretreated with the Src inhibitors PP2 demonstrated suppressed PDGF BB-induced phosphorylation of Akt (Fig. 4C). Furthermore, cells infected with Ad DN Src failed to demonstrate an increase in Akt phosphorylation in response to PDGF BB (Fig. 4D). Therefore, in metanephric mesenchymal cells, PDGF BB-induced activation of Src was upstream of phosphatidylinositol 3-kinase.
Fig. 4.
Src is upstream of the PI3K/Akt pathway in metanephric mesenchymal cells. A: Src inhibitors PP2 and SU6656 inhibit PDGF BB-induced PI3K activity. Serum-deprived monolayers were pretreated with the indicated inhibitors and stimulated with PDGF BB. A PI3K assay was conducted as described in materials and methods. Thin-layer chromatography plates were activated in chambers containing saturation buffer and dried overnight. Immunoprecipitates of 100 μg protein were employed in a PI3K assay as described in the materials and methods. Reactions were separated by thin-layer chromatography. The arrow indicates phosphatidylinositol 3-phosphate. B: PI3K inhibitor LY294002 has minimal effect on Src phosphorylation in PDGF BB-treated metanephric mesenchymal cells. C: Src inhibitor PP2 abrogates PDGF BB-induced Akt phosphorylation in metanephric mesenchymal cells. D: adenoviral dominant negative (Ad DN) Src abrogates PDGF BB-induced Akt phosphorylation. Metanephric mesenchymal cells were infected with Ad DN Src or Ad green fluorescent protein (GFP) as a control. Quiescent cells were stimulated with PDGF BB, and immunoblotting was performed as described in materials and methods.
In metanephric mesenchymal cells, PDGF BB-induced migration is redox dependent.
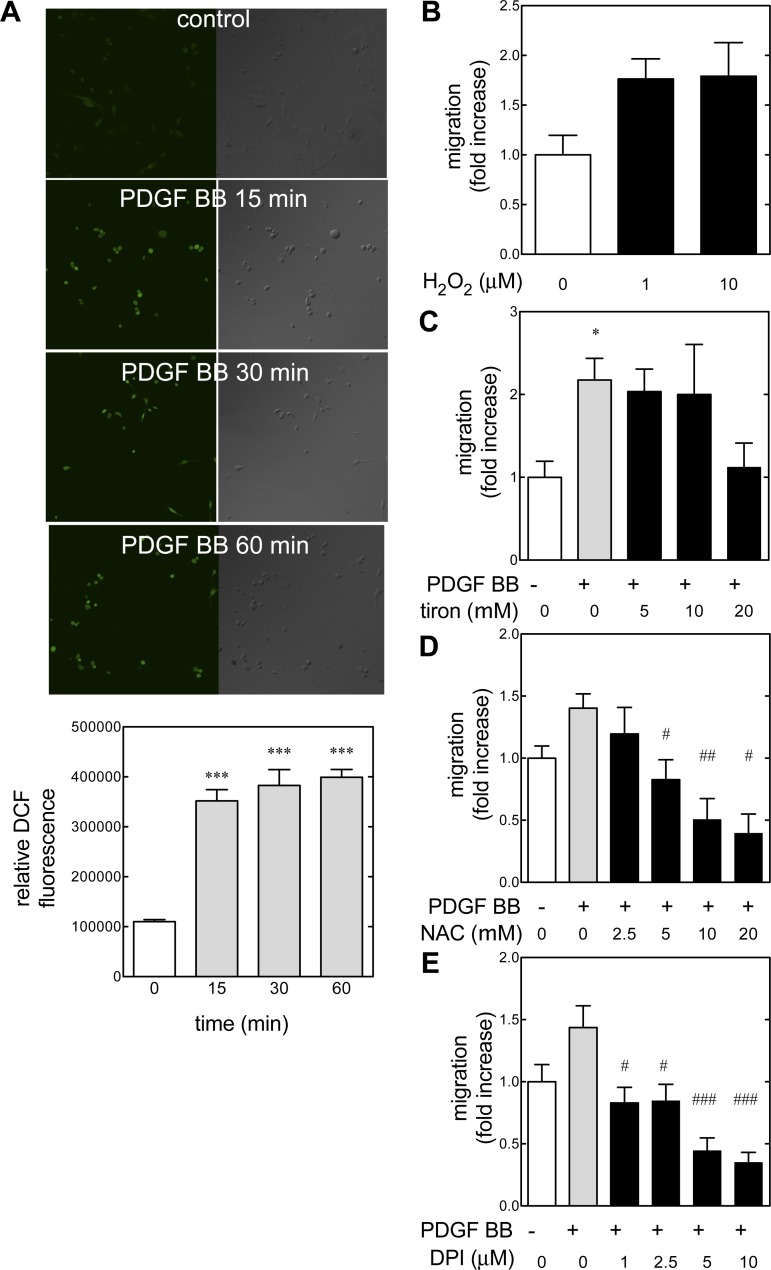
PDGF triggers the generation of ROS in a number of cell types, and this generation of ROS may be a mediator in cell signaling (reviewed in Ref. 48). Several intracellular effectors are redox dependent, such as c-Src and Akt (40). In smooth muscle, PDGF BB-stimulated migration proceeds via ROS generation and subsequent Src activation (83). Because we have found that generation of ROS is an important mediator of DNA synthesis in these mouse metanephric mesenchymal cells (82), the role of redox signaling in migration was explored (Fig. 5). Consistent with our prior findings, PDGF BB treatment led to the generation of ROS as detected by loading quiescent cells with the peroxide-sensitive fluorescent probe DCF (Fig. 5A). Treatment with 1–10 μM hydrogen peroxide increased wound closure rates in quiescent cells (Fig. 5B). The antioxidant tiron (1,2-dihyrodxybenzene-4,5-disulfonate) (53) suppressed PDGF BB-induced DNA synthesis in a dose-dependent manner (Fig. 5C). NAC quenches the effect of ROS by increasing cellular reduced glutathione. In pretreated metanephric mesenchymal cells, NAC demonstrated a dose-dependent reduction of PDGF BB-induced migration (Fig. 5D). Because we have previously demonstrated that an NAD(P)H oxidase homolog (Nox) is involved in PDGF BB-induced Akt and ERK activation, the effect of pretreatment with the flavoprotein inhibitor DPI on PDGF BB-stimulated migration was assessed (Fig. 5E). DPI abrogated PDGF-induced migration even at 1 μM concentration. In total, these data demonstrated that PDGF BB induced ROS in cultured metanephric mesenchymal cells and that subsequent stimulation of migration was redox sensitive. Furthermore, PDGF BB-induced migration could be quenched with a Nox inhibitor.
Fig. 5.
PDGF BB mediates migration by reactive oxygen species (ROS) generation. A: quiescent metanephric mesenchymal cells were loaded with 5-(and-6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF; 10 μM) for 30 min before treatment with PDGF BB (20 ng/ml). Graph depicts the intensity (integrated densities) of fluorescence. ***P < 0.001 by 1-way ANOVA and Tukey's post hoc analysis. B: hydrogen peroxide induces migration in metanephric mesenchymal cells. Quiescent monolayers were wounded and treated with 1–10 μM H2O2. Migration was assessed as described. C: tiron inhibits PDGF BB-induced migration. Migration was assessed as described. *P < 0.05 by 1-way ANOVA and Tukey's post hoc analysis. D: effect of N-acetyl-l-cysteine (NAC) on PDGF BB-induced migration. Cells were pretreated with 2.5–20 mM NAC, and migration was assessed as described. E: effect of diphenyleneiodonium (DPI) on PDGF BB-induced migration was measured in metanephric mesenchymal cells. Monolayers were pretreated with 0.1–20 μM DPI. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. PDGF BB-treated group by 1-way ANOVA and Tukey's post hoc analysis.
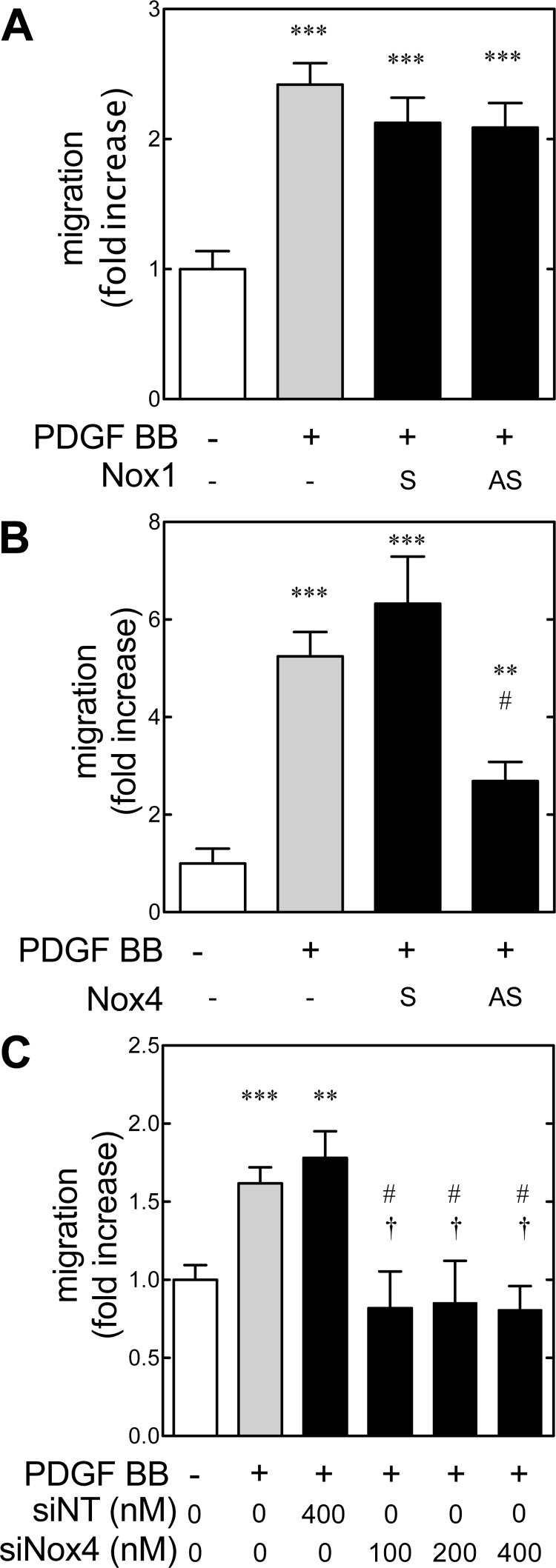
PDGF BB-induced migration is mediated by Nox4.
PDGF AA has been shown to lead to ROS generation via phosphatidylinositol 3-kinase and the Nox subunit p47phox (8). We have previously shown that metanephric mesenchymal cells express Nox4 and that PDGF BB-induced DNA synthesis in metanephric mesenchymal cells is mediated by Nox4-generated ROS. The role of Nox1 and 4 in PDGF-stimulated migration was therefore examined. Metanephric mesenchymal cells were seeded with 1 μM sense (S) and antisense (AS) oligonucleotides for the start sequence of Nox4 or Nox1 for control (82). Neither S nor AS Nox1 suppressed PDGF BB-induced migration significantly (Fig. 6A). Conversely, AS Nox4 treatment significantly suppressed PDGF BB-induced migration compared with S treatment (Fig. 6B). To validate these findings, monolayers of wild-type cells were grown with siRNA for Nox4 (siNox4) or a nontargeting (siNT) siRNA as a control, and migration was assessed (Fig. 6C). PDGF BB-induced migration was abrogated by siNox4 treatment. Collectively, these data demonstrated that ROS-dependent PDGF BB-induced migration was mediated by Nox4 in metanephric mesenchymal cells.
Fig. 6.
NAD(P)H oxidase homolog 4 (Nox4) mediates PDGF BB-induced migration in metanephric mesenchymal cells. A and B: cells were seeded in the presence of 1 μM sense (S) and antisense (AS) Nox1 or Nox4, serum-deprived with the same concentrations of oligonucleotides, and PDGF BB-induced migration was assessed as described. Values are from 2 independent experiments, 3 wells each. C: PDGF BB-induced migration is abrogated by small interfering (si) RNA for Nox4. Results were compared with 1-way ANOVA and Tukey's post hoc analysis. **P < 0.01, ***P < 0.001 with respect to the control group. #P < 0.05 vs. PDGF BB-treated group. †P < 0.05 with respect to the PDGF BB- and si nontargeting (siNT) group.
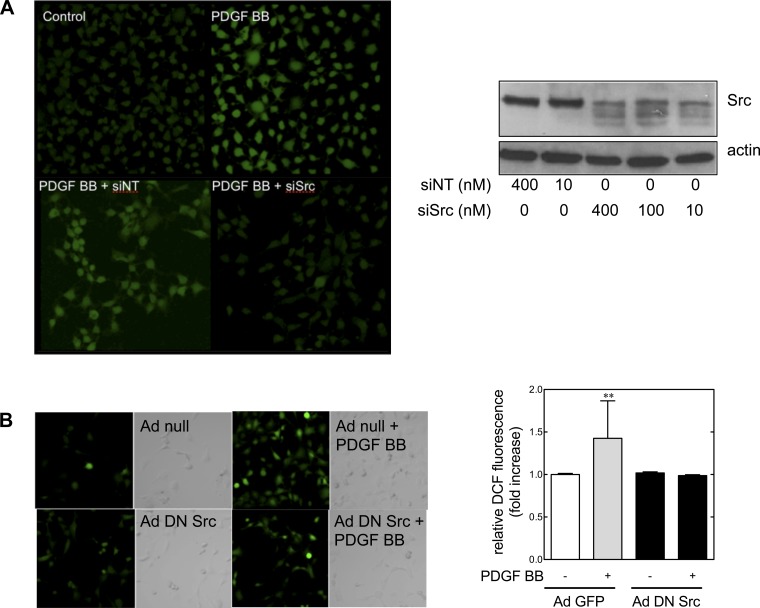
Src is required for PDGF BB-induced ROS generation in metanephric mesenchymal cells.
Experiments in metanephric mesenchymal cells have demonstrated that Src, phosphatidylinositol 3-kinase, Nox4, and ROS generation are involved in PDGF BB-induced migration. Therefore, the role of Src in PDGF BB-induced ROS generation was examined in wild-type cells cultured with siRNA for Src (siSrc, using siNT as a control) preloaded with DCF (Fig. 7A). Treatment with siSrc dampened PDGF BB-induced ROS generation (top). An immunoblot of siSrc-treated cells demonstrated effective suppression of Src (bottom). To confirm the role of Src in ROS generation, cells were infected with DN adenoviral Src (Ad DN Src) or a null adenovirus (Ad null) as a control, and PDGF BB-induced ROS generation was examined (Fig. 7B). Again, inhibition of Src suppressed PDGF BB-induced ROS generation. Treatment with Ad DN Src did not have an effect on apoptosis as assessed by flow cytometry for annexin V (data not shown). These results demonstrated that PDGF BB-induced Src activation was required for ROS generation in metanephric mesenchymal cells.
Fig. 7.
PDGF BB-induced ROS generation is mediated by Src in metanephric mesenchymal cells. A: Src mediates PDGF BB-induced ROS generation. Top: cells were seeded with siRNA (10 nM) (82), loaded with DCF (10 μM) for 30 min, stimulated with PDGF BB, and fluorescence was detected by laser-scanning confocal microscopy (71). Bottom: cells were seeded and grown to confluence with siRNA for Src (siSrc) or control, and levels of Src protein were assessed by immunoblotting. B: Ad DN Src cells suppress PDGF BB-induced ROS generation. Metanephric mesenchymal cells were grown on coverslips and treated with Ad DN Src or Ad null as a control, and DCF fluorescence was assessed. **P < 0.01 with respect to the control group by 1-way ANOVA and Tukey's post hoc analysis.
DISCUSSION
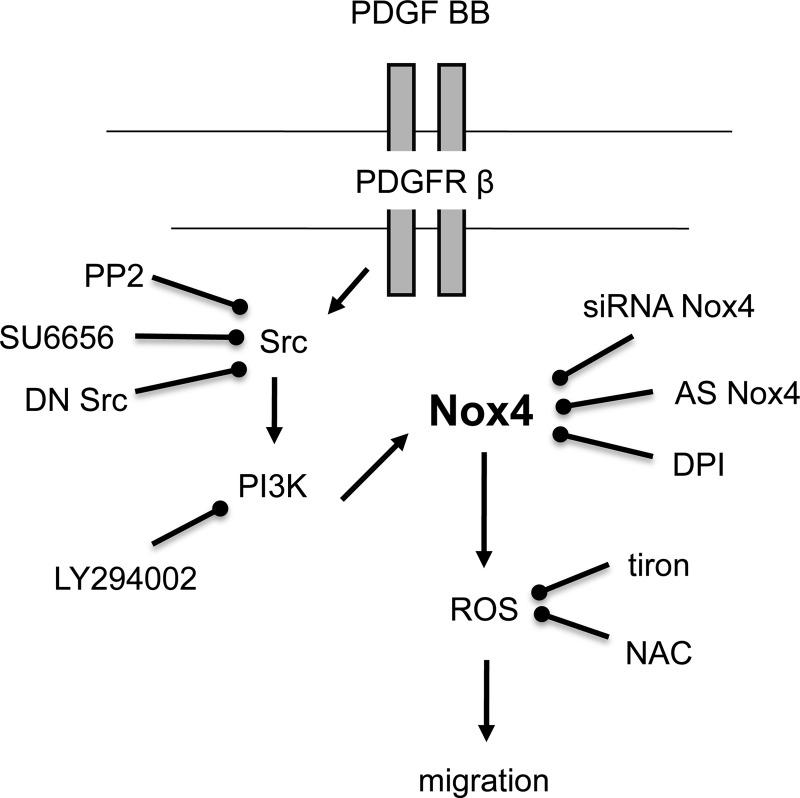
PDGF receptor β plays an essential role in mesangial cell development and in glomerular pathology. Mice deficient for PDGF B-chain and PDGF receptor β lack mesangial cells and kidney glomerular tufts (58). The receptor is localized to undifferentiated metanephric mesenchymal cells in rat embryonic kidneys, and at later stages is expressed in mesangial cell precursors in the cleft of the comma-shaped and S-shaped bodies and in more mature glomeruli in a mesangial distribution (4). Cultured metanephric mesenchymal cells express functional PDGF receptor β, which has been demonstrated by autophosphorylation and activation of phosphatidyl-3-kinase in response to PDGF. Migration and proliferation of metanephric mesenchymal cells is induced by PDGF, as it acts in a paracrine manner to stimulate the migration and proliferation of mesangial cell precursors from undifferentiated metanephric mesenchyme to the mesangial area (4). Once these cells are within the maturing glomerulus, PDGF acts in an autocrine or short-loop paracrine manner to stimulate their proliferation and differentiation (4). Hemizygous mouse embryos that express a chimeric PDGF receptor with an extracellular β domain and an intracellular α domain have a similar phenotype as PDGF receptor β-null mice: the glomeruli lack mesangial cells (51). Interestingly, α and β PDGF receptors trigger similar signaling cascades and yet have markedly different functions during embryogenesis (51). The present study demonstrates 1) that PDGF receptor β is requisite for PDGF-mediated migration in metanephric mesenchymal cells, 2) this is dependent on phosphatidylinositol 3-kinase and Src, and 3) this is mediated by ROS mediated by Src-induced activation of NAD(P)H oxidase Nox4 (Fig. 8).
Fig. 8.
Src-induced ROS generation mediates PDGF BB-induced migration.
PDGF BB stimulated migration in a dose-dependent manner in mouse metanephric mesenchymal cells (Fig. 1A). The importance of Src in growth factor receptor-induced migration has been well detailed (31). PDGF-mediated migration in cultured rat vascular smooth muscle cells was mediated via ROS and subsequent activation of Src and other redox-dependent effectors (83). In human arterial smooth muscle cells, PDGF BB-induced activation of the MAP kinase cascade may not be requisite for chemotaxis, as IGF-I (a weak activator of the MAP kinase cascade) still elicits migration (12). We have found that PDGF BB stimulates chemotaxis in rat metanephric mesenchymal cells, measured by a modified Boyden chamber technique. Contrary to what was found in the current study, chemotaxis was partially dependent on the MEK1/ERK pathway (70). That PDGF BB-induced migration in mouse metanephric mesenchymal cells is mediated by Src may account for the subtotal abrogation of chemotaxis in rat metanephric mesenchyme during MEK/ERK inhibition.
That ROS mediate pathological effects during embryogenesis is increasingly reported. For instance, high glucose has been reported to induce morphogenic effects in ureteric bud branching ex vivo via an oxidative-dependent mechanism (91). However, there is scant information on the role of ROS in mediating cell signals in normal organogenesis. The generation of ROS is involved in the signaling pathways of specific biological processes such as mitogenesis and migration in vascular smooth muscle cells and fibroblasts (41).
PDGF-induced migration in certain cells is redox dependent. Scavengers of ROS have suppressed 12-O-tetradecanoylphorbol-13-acetate-induced migration of HepG2 cells (86). That ROS stimulate migration has been demonstrated in dedifferentiated epithelial cells, but not embryonic mesenchymal cells to our knowledge. The reactive oxygen scavenger NAC, the antioxidant catalase, and the flavin-containing oxidase [e.g., Nox NAD(P)H oxidases] inhibitor DPI all inhibit PDGF-induced migration in vascular smooth muscle cells (77, 83). It is possible that embryonic stem cells generate ROS via an NA(P)DH oxidase in cardiac development to stimulate cell proliferation (74).
There is growing evidence that the NAD(P)H oxidases of the Nox family are important sources of ROS in a wide range of cell types, ranging from vascular smooth muscle to numerous renal cells (7, 35, 36, 55, 56). Moreover, the Nox enzymes have been reported to play a key role in the redox-sensitive signaling pathway triggered by most of the agonists regulating the function of these cells (7, 35, 36, 55, 56). It has been documented that NAD(P)H oxidase-derived ROS are required for the propagation of PDGF BB- or PDGF AA-induced signals in vascular and renal cells (7, 13, 35, 36, 55, 56).
NAD(P)H oxidase catalytic subunits (including Nox1, Nox4, or Nox5) as well as Nox-regulatory subunits (such as p22phox, p47phox, p67phox, or Rac1) have been proposed to be part of the oxidases activated upon stimulation with PDGF (7, 13, 35, 36, 55, 56). Interestingly, the nature of the Nox subunit appears to differ depending on the cell type. The isoforms Nox1 and Nox5 contribute to PDGF BB-induced cell proliferation in vascular smooth cells (46, 57), whereas the isoform Nox4 but not Nox1 is required for the mitogenic effect of PDGF BB in metanephric mesenchymal cells (82). In adult mesangial cells, Nox1 rather than Nox4 seems to be implicated in PDGF-mediated oxidative stress (68). The role of ROS in PDGF-induced migration has been described in vascular smooth muscle cells (8, 14, 45, 78, 83). The antioxidant NAC suppresses PDGF-induced migration in vascular smooth muscle cells (76). Furthermore, it has been recently demonstrated that PDGF BB mediates vascular smooth muscle migration in a Rac1- and Nox1-dependent manner (60, 83). PDGF AA-mediated chemotaxis also requires a p47phox- and Rac1-based NADPH oxidase in the same cells (8). In the present work, we demonstrate for the first time that Nox4 is implicated in metanephric mesenchymal cell migration toward PDGF BB. Our data are consistent with the recent report showing that Nox4 participates to lung fibroblast chemotaxis in response to PDGF (2). Moreover, Nox4 has been implicated in angiogenesis in ovarian cancer cells (89) as well as in endothelial angiogenesis (24, 25). Nox4 is a primary mediator of adipose-derived stem cell migration under hypoxic conditions (50) as well as in vascular smooth muscle cells stimulated by IGF-I. Chemotaxis in cultured monocytes (THP-1) may also be promoted in a Nox4-dependent manner (80). These findings strongly reinforce the idea that Nox4 is a critical mediator of cell migration. A role for the Nox oxidases in chemotaxis is further supported by the fact that there are a number of agonists known to increase Nox activity, including, but not limited to, tumor necrosis factor-α, vascular endothelial growth factor (VEGF), angiotensin II, basic fibroblast growth factor (bFGF), thrombin, and oxidized LDL that are potent chemotactic agents (7, 13, 35, 36, 55, 56). For instance, a Rac1- and Nox2-containing oxidase has been implicated in VEGF-induced migration in endothelial cells (7, 13, 35, 36, 55, 56), whereas Nox1 promotes the chemotactic effects of bFGF in vascular smooth muscle cells (75).
Chemotactic processes are requisite for kidney development and the formation of the glomerulus. Nox4 seems to play a key role in metanephric mesenchymal cell migration. Therefore, it is highly relevant to identify the upstream and downstream effectors of the oxidase. The present work provides new insights related to the signaling pathway linking PDGF receptor β to the redox-dependent activation of Akt and subsequent migration in metanephric mesenchymal cells. We have previously shown that ROS generation by Nox4 is dependent on phosphatidylinositol 3-kinase and that Nox4-derived ROS modulate Akt phosphorylation upon stimulation of these cells with PDGF BB (82). The present finding that Src is positioned upstream of phosphatidylinositol 3-kinase in the signaling cascade elicited by PDGF BB indicates that Src-mediated phosphatidylinositol 3-kinase activation is probably involved in Nox4-dependent ROS production. The sequence of events described here is particularly noteworthy because Src is often reported to be a downstream target of Nox4-derived ROS rather than a distal activator of the oxidase (10, 15, 87). The position of Src as being both an upstream modulator and downstream effector of Nox oxidase is seen in the case of Nox1 (34, 47, 73). Src is known to contribute to the activation of Nox2 or Nox1 via phosphorylation of cytosolic components such as p47phox and NoxA1 or through activation of small GTPase Rac1, leading to the formation of an active enzymatic complex (8, 22, 34, 42, 47, 79). Similar to Src, phosphatidylinositol 3-kinase also mediates growth factor-dependent ROS production by facilitating the recruitment of p47phox and Rac1 to the cell membrane, thereby assembling the active NADPH oxidase complex (5, 8, 30, 90). Because Nox4 does not require recruitment of cytosolic subunits for its activity, it is likely that Src is not acting through these mechanisms. Therefore, the mechanism by which Src-mediated phosphatidylinositol 3-kinase activation triggers Nox4-dependent ROS production remains to be elucidated. A possibility is that proteins recently identified as modulators of Nox4 such as Poldip2 or p47phox-related protein Tks5 (28, 59) are regulated by Src and phosphatidylinositol 3-kinase. Interestingly, Tks5 is a substrate of Src that is necessary for cancer and embryonic cellular migration (23, 28, 66). Moreover, a homolog of Tks5, Tks4, has been shown to be phosphorylated by Src during Nox1 activation (23, 33). Very recently, it has been shown (in vascular smooth muscle cells) that recruitment of Nox4 to a membrane scaffold SHP-1/Grb is required for ROS generation and Src activation in response to IGF-1 (88). This is consistent with the data herein; i.e., there is a close functional interplay between Src and Nox4. The difference in our system is that Src may regulate Nox4-dependent ROS generation. Interestingly, the interaction of Nox4 with the scaffold is dependent on its tyrosine phosphorylation and on Pyk2 activation, a known target of Src.
Another interesting observation obtained in the present study is the finding that ERK activation is not involved in PDGF-induced metanephric mesenchymal cell migration. This is surprising since we have previously reported that Nox4 promotes ERK phosphorylation and subsequent mitogenesis after stimulation of these cells by PDGF (82). In some cells, the generation of ROS via an NADPH oxidase is a mechanism of sustaining ERK activation, and this results in cell adhesion and migration (85, 86). That inhibition of MEK did not abrogate PDGF BB-induced migration in our experiments indicates that such a redox-activated MEK/ERK pathway for migration is cell type specific.
In addition to cell specificity, it appears that there is specificity of the cell signaling pathway in metanephric mesenchymal cells. ERK and Akt are both regulated by Nox4 and contribute to PDGF-induced mitogenesis, yet PDGF-induced migration is independent of ERK. This specificity needs to be further investigated. We suspect that the mechanism is downstream of MEK/ERK and could be due to specific compartmentalization in specific redox-dependent or -independent signaling domains (3, 16). This concept of kinase-specific biological effects of PDGF or the specificity of redox signaling was previously suggested and is likely to be critical for the roles of the protein kinases in regulating various cellular processes (3, 16).
If endothelial cell migration is MEK/ERK dependent and metanephric mesenchymal cell migration is not, then this may be a mechanism by which a single ligand acts on different cell types to recruit each cell type to specific areas, e.g., the developing glomerular cleft. Our results for the involvement of Nox4 in metanephric mesenchymal cells are similar to those found in human endothelial cells (25). Nox4 may be involved in PDGF receptor β autophosphorylation (14). Indeed, PDGF receptor β phosphorylation has been shown to be redox dependent (14), and in endothelial cells overexpressing Nox4, PDGF receptor α phosphorylation is increased and associated with increased migration (25). Therefore, a feedback loop linking the ROS generated by Nox4 to PDGF receptor β phosphorylation and leading to its maximal activation may take place in metanephric mesenchymal cells. It is important to note that Src has been proposed to be part of such a redox circuit upon PDGF stimulation in fibroblasts (14).
PDGF-induced cellular migration depends on different signaling pathways specific to the type of cell (54, 84). In this study, PDGF BB induced migration of wild-type mouse metanephric mesenchymal cells via Src activation. Migration was dependent on Nox4-derived ROS and phosphatidylinositol 3-kinase/Akt activation, but independent of the MEK/ERK pathway. Given how cell specific the effects are, these results suggest cell type-specific mechanisms for differential effects of growth factors on cell migration. Understanding the functional interplays between the various redox-sensitive pathways is relevant in the context of defining the redox regulation of the processes involved in the maturation of the glomerulus and organ development.
GRANTS
This work was supported by a Fellow-to-Faculty Transition Award from the American Heart Association (no. 121052). B. Wagner is supported by a Department of Veterans Affairs Career Development Award and a Department of Veterans Affairs Merit Award. Y. Gorin is supported by a Juvenile Diabetes Research Foundation Grant and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-079996.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.W. provided conception and design of research; B.W. performed experiments; B.W. analyzed data; B.W. interpreted results of experiments; B.W. prepared figures; B.W. drafted manuscript; B.W. and Y.C.G. edited and revised manuscript; B.W. and Y.C.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Hanna E. Abboud, Goutam Ghosh Choudhury, and Jill Ricono for materials used for some of these experiments.
REFERENCES
- 1.Alpers CE, Hudkins KL, Ferguson M, Johnson RJ, Rutledge JC. Platelet-derived growth factor A-chain expression in developing and mature human kidneys and in Wilms' tumor. Kidney Int 48: 146–154, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 28: 1347–1354, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Arar M, Xu YC, Elshihabi I, Barnes JL, Choudhury GG, Abboud HE. Platelet-derived growth factor receptor beta regulates migration and DNA synthesis in metanephric mesenchymal cells. J Biol Chem 275: 9527–9533, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am J Physiol Cell Physiol 298: C679–C692, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Bard J. The metanephros. In: The Kidney: From Normal Development to Congenital Disease, edited by Vize PD, Woolf AS, Bard J. San Diego, CA: Elsevier, 2003, p. 139–148 [Google Scholar]
- 7.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 283: 7864–7876, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Betsholtz C, Lindblom P, Bjarnegard M, Enge M, Gerhardt H, Lindahl P. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr Opin Nephrol Hypertens 13: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283: 24061–24076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann NY Acad Sci 766: 416–430, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest 93: 1266–1274, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, Vincenzini MT. Redox regulation of platelet-derived-growth-factor-receptor: role of NADPH-oxidase and c-Src tyrosine kinase. Biochim Biophys Acta 1745: 166–175, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Chen JK, Harris RC. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol 32: 981–991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury GG, Ghosh-Choudhury N, Abboud HE. Association and direct activation of signal transducer and activator of transcription1alpha by platelet-derived growth factor receptor. J Clin Invest 101: 2751–2760, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int 57: 908–917, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am J Physiol Renal Physiol 273: F931–F938, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Choudhury GG, Mahimainathan L, Das F, Venkatesan B, Ghosh-Choudhury N. c-Src couples PI 3 kinase/Akt and MAPK signaling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal 18: 1854–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Choudhury GG, Wang LM, Pierce J, Harvey SA, Sakaguchi AY. A mutational analysis of phosphatidylinositol-3-kinase activation by human colony-stimulating factor-1 receptor. J Biol Chem 266: 8068–8072, 1991 [PubMed] [Google Scholar]
- 22.Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem 280: 20700–20711, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans 40: 129–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol 27: 2319–2324, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 159: 1087–1096, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey N, Ghosh-Choudhury N, Das F, Li X, Venkatesan B, Barnes JL, Kasinath BS, Ghosh Choudhury G. PRAS40 acts as a nodal regulator of high glucose-induced TORC1 activation in glomerular mesangial cell hypertrophy. J Cell Physiol 225: 27–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal 2: ra53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10: 262–267, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem 281: 16128–16138, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ghosh-Choudhury G, Kim YS, Simon M, Wozney J, Harris S, Ghosh-Choudhury N, Abboud HE. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J Biol Chem 274: 10897–10902, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal 2: ra54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell 21: 4287–4298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorin Y, Block K. Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free Radic Biol Med 61C: 130–142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci (Lond) 125: 361–382, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J 15: 1909–1920, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J 381: 231–239, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol 296: H1048–H1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell 80: 213–223, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 117: 4619–4628, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Ishizawa K, Izawa-Ishizawa Y, Dorjsuren N, Miki E, Kihira Y, Ikeda Y, Hamano S, Kawazoe K, Minakuchi K, Tomita S, Tsuchiya K, Tamaki T. Angiotensin II receptor blocker attenuates PDGF-induced mesangial cell migration in a receptor-independent manner. Nephrol Dial Transplant 25: 364–372, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, Kamata T. A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. FASEB J 26: 2049–2059, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Kang SW. Two axes in platelet-derived growth factor signaling: tyrosine phosphorylation and reactive oxygen species. Cell Mol Life Sci 64: 533–541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim EK, Tucker DF, Yun SJ, Do KH, Kim MS, Kim JH, Kim CD, Birnbaum MJ, Bae SS. Linker region of Akt1/protein kinase Balpha mediates platelet-derived growth factor-induced translocation and cell migration. Cell Signal 20: 2030–2037, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Song SY, Park SG, Song SU, Xia Y, Sung JH. Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev 21: 2212–2221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell 7: 343–354, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49: 65–73, 1987 [DOI] [PubMed] [Google Scholar]
- 53.Krishna CM, Liebmann JE, Kaufman D, DeGraff W, Hahn SM, McMurry T, Mitchell JB, Russo A. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch Biochem Biophys 294: 98–106, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, Zetter BR. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature 367: 474–476, 1994 [DOI] [PubMed] [Google Scholar]
- 55.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, San Martin A. Platelet-derived growth factor (PDGF) regulates Slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem 286: 35430–35437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marra F, Choudhury GG, Abboud HE. Interferon-gamma-mediated activation of STAT1alpha regulates growth factor-induced mitogenesis. J Clin Invest 98: 1218–1230, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res 103: 1393–1401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev 19: 484–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori S, Ronnstrand L, Yokote K, Engstrom A, Courtneidge SA, Claesson-Welsh L, Heldin CH. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J 12: 2257–2264, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mureebe L, Nelson PR, Yamamura S, Lawitts J, Kent KC. Activation of pp60c-src is necessary for human vascular smooth muscle cell migration. Surgery 122: 138–144, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, Stewart RA, Izpisua-Belmonte JC, Courtneidge SA. A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS One 6: e22499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peppel K, Zhang L, Orman ES, Hagen PO, Amalfitano A, Brian L, Freedman NJ. Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc Res 65: 674–682, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Pleskova M, Beck KF, Behrens MH, Huwiler A, Fichtlscherer B, Wingerter O, Brandes RP, Mulsch A, Pfeilschifter J. Nitric oxide down-regulates the expression of the catalytic NADPH oxidase subunit Nox1 in rat renal mesangial cells. FASEB J 20: 139–141, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Ribes D, Fischer E, Calmont A, Rossert J. Transcriptional control of epithelial differentiation during kidney development. J Am Soc Nephrol 14, Suppl 1: S9–S15, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Ricono JM, Arar M, Choudhury GG, Abboud HE. Effect of platelet-derived growth factor isoforms in rat metanephric mesenchymal cells. Am J Physiol Renal Physiol 282: F211–F219, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Ricono JM, Wagner B, Gorin Y, Arar M, Kazlauskas A, Choudhury GG, Abboud HE. PDGF receptor-β modulates metanephric mesenchyme chemotaxis induced by PDGF AA. Am J Physiol Renal Physiol 296: F406–F417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ronnstrand L, Heldin CH. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int J Cancer 91: 757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Sancho P, Fabregat I. NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J Biol Chem 285: 24815–24824, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J 20: 1182–1184, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27: 1736–1743, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Shimizu H, Nakagawa Y, Murakami C, Aoki N, Kim-Mitsuyama S, Miyazaki H. Protein tyrosine phosphatase PTPεM negatively regulates PDGF β-receptor signaling induced by high glucose and PDGF in vascular smooth muscle cells. Am J Physiol Cell Physiol 299: C1144–C1152, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 78.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 981–987, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vassbotn FS, Ostman A, Siegbahn A, Holmsen H, Heldin CH. Neomycin is a platelet-derived growth factor (PDGF) antagonist that allows discrimination of PDGF alpha- and beta-receptor signals in cells expressing both receptor types. J Biol Chem 267: 15635–15641, 1992 [PubMed] [Google Scholar]
- 82.Wagner B, Ricono JM, Gorin Y, Block K, Arar M, Riley D, Choudhury GG, Abboud HE. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. J Am Soc Nephrol 18: 2903–2911, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res 94: 1219–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Wennstrom S, Siegbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene 9: 651–660, 1994 [PubMed] [Google Scholar]
- 85.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 25: 695–705, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Wu WS, Tsai RK, Chang CH, Wang S, Wu JR, Chang YX. Reactive oxygen species mediated sustained activation of protein kinase C alpha and extracellular signal-regulated kinase for migration of human hepatoma cell Hepg2. Mol Cancer Res 4: 747–758, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Xi G, Shen X, Maile LA, Wai C, Gollahon K, Clemmons DR. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCzeta-dependent manner in vascular smooth muscle cells. Diabetes 61: 104–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xi G, Shen XC, Wai C, Clemmons DR. Recruitment of Nox4 to a plasma membrane scaffold is required for localized ROS generation and sustained Src activation in response to IGF-I. J Biol Chem 288: 15641–15653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67: 10823–10830, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Xia M, Li G, Ma J, Ling W. Phosphoinositide 3-kinase mediates CD40 ligand-induced oxidative stress and endothelial dysfunction via Rac1 and NADPH oxidase 2. J Thromb Haemost 8: 397–406, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Zhang SL, Chen YW, Tran S, Chenier I, Hebert MJ, Ingelfinger JR. Reactive oxygen species in the presence of high glucose alter ureteric bud morphogenesis. J Am Soc Nephrol 18: 2105–2115, 2007 [DOI] [PubMed] [Google Scholar]